Research Article - Biomedical Research (2017) Volume 28, Issue 9
HPLC determination of chlorogenic acid in Verbena officinalis L. extract and its in-vitro antibacterial activity
Zhonghao Zhang1 and Taowen Pan2*1College of Life Science and Technology, Huazhong Agricultural University, Wuhan City, Hubei Province, China
2Institute (College) of Integrative Medicine, Dalian Medical University, Dalian City, Liaoning Province, China
- *Corresponding Author:
- Taowen Pan
Institute (College) of Integrative Medicine
Dalian Medical University, China
Accepted on February 1, 2017
Abstract
To investigate the method for quantitatively determining chlorogenic acid in Verbena officinalis L. extract; and to determine its content and in-vitro antibacterial activity. Cylinder-plate method and MIC assay were employed to compare the antibacterial activity between different concentrations of Verbena officinalis L. extracts; and to evaluate the antioxidant activity of various extracts in the DPPH radical system, hydroxyl radical system and anti-lipid peroxidation system. Verbena officinalis L. extracts exhibited inhibitory zone diameters all reaching above 15 mm against Escherichia coli, Proteus vulgaris and Bacillus subtilis at a mass concentration of 100 mg/ml, with MICs all ≤ 100 mg/ml. In various radical systems, different concentrations of Verbena officinalis L. extracts all exhibited weak scavenging effect on superoxide anion radicals, but manifested strong effect in scavenging DPPH and hydroxyl radicals and in inhibiting lipid peroxidation. With mass concentration IC50 of sample solution corresponding to a 50% scavenging rate as a comparative index, IC50 of high dose extract for these three free radicals were calculated to be 0.20, 0.15 and 2.69 mg/ml, respectively. Chlorogenic acid content in Verbena officinalis L. extract was 30.23%. The present method is accurate, sensitive and highly specific, which can be used for determination of chlorogenic acid content in Verbena officinalis L. and its extract. Verbena officinalis L. extract has strong antibacterial activity against Gram-negative bacteria, and further study is needed to clarify its antibacterial mechanism. Verbena officinalis L. extract is suitable for in-depth exploitation as a botanical antioxidant.
Keywords
Verbena officinalis L., Chlorogenic acid, Quantitative determination, HPLC, Antibacterial activity, Antioxidant activity.
Introduction
Verbena officinalis L. is a plant in the Verbenaceae family, which is cool, slightly bitter and has blood-cooling, stasis-dissipating, menstruation-stimulating, heat-clearing, detoxifying, itching-relieving, parasite-expelling and swelling-eliminating functions. China has abundant Verbena officinalis L. resources. The herb contains volatile oils, iridoids, phenylpropionic acid, flavonoids, chlorogenic acid, etc. [1-3]. Preliminary experiment has found that the effective constituent of Verbena officinalis L. extract was chlorogenic acid, which has been confirmed to possess antibacterial and anti-inflammatory activities [4]. The authors established a method for determining chlorogenic acid content in Verbena officinalis L. extract and performed quantitative analysis on the herb, thereby providing a fast, accurate determination method for quality control of Verbena officinalis L. and its extract. A variety of phenolic substances in Verbena officinalis L. have anticancer activity [5]. In the present experiment, 30%, 60% and 95% ethanol were used as extracting solvents to comparatively analyse the in-vitro antibacterial and antioxidant activities of various extracts, thereby providing a scientific basis for the extraction, component analysis and bioactive functional development of Verbena officinalis L. extract.
Instruments and Reagents
Instruments
Agilent 1260 HPLC system; G1314F-VWD UV detector; Chemstation chromatography data system; FA2015 electronic analytical balance (Qiyamei Co., Ltd., Nanjing); KQ-500 ultrasonic cleaner (Shangxin Ultrasonic Equipment Co., Ltd., Jiangsu); DSN-S16 electro-thermostatic water bath (Kaituo Instruments Co., Ltd., Tianjin).
Reagents
Methanol, acetonitrile (HPLC grade, ACS, Reag. Ph Eur.); phosphoric acid and other reagents (chemic grade, Jintianli Chemical Plant, Tianjin); double-distilled water (self-prepared); chlorogenic acid reference (batch No. 201605012B, National Institute for the Control of Pharmaceutical and Biological Products); Verbena officinalis L. (purchased from the Tasly Pharmacy), identified by the Associate Professor Zhang Qixin of the Chengdu University of TCM as the whole plant of Verbenaceae plant Verbena officinalis L. Nutrient agar (Microbial Reagents Co., Ltd., Kunshan); 1,1-DPPH (Sigma, USA); crystal violet (Fine Chemical Co., Ltd., Nanjing).
Escherichia coli, Proteus vulgaris and Bacillus subtilis were provided by the Jiangsu Institute of Microbiology.
Preparation of Verbena officinalis L. extract
Verbena officinalis L. powder was Soxhlet extracted with 30% ethanol till the thimble was colorless. The extracted liquid was collected, concentrated in vacuo on a rotary evaporator and dried at low temperature to give the extract. Extraction yield was calculated to be 15.6%.
Methods
Chromatographic conditions: column
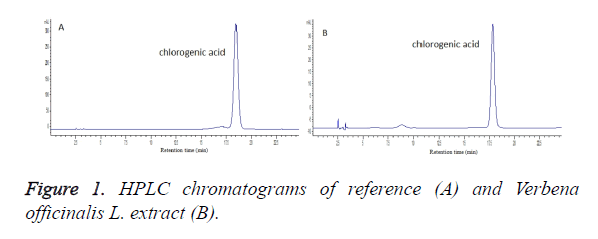
Waters C18 (250 mm × 4.6 mm, 5 μm); mobile phase: acetonitrile-0.4% phosphoric acid solution (14:86); flow rate: 1.0 ml/min; column temperature: room temperature; detection wavelength: 330 nm; and injection volume: 10 μL. Quantification was performed by external standard method, while number of theoretical plates was calculated to be not less than 3000 based on the peak of chlorogenic acid. Chromatograms of reference and sample are shown in Figure 1.
Preparation of sample solution
About 0.5 g of Verbena officinalis L. extract was accurately weighed, placed in a flask, added precisely with 50 ml of 50% (volume fraction) methanol, weighed, ultrasonicated for 30 min, then weighed again, compensated for the weight loss with 50% methanol and shaken well. 1 ml of the solution was accurately weighed, placed in a 100 ml flask, diluted to the mark with 50% methanol and shaken well. An appropriate amount of subsequent filtrate was then taken and filtered through microporous membrane (0.45 μm) to give the sample solution.
Plotting of standard curve
5.60 mg of chlorogenic acid reference was accurately weighed, placed in a 10 ml brown volumetric flask, diluted to the mark with methanol and shaken well to prepare the stock reference solution. 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, and 2.00 ml of the stock reference solution were accurately drawn, placed separately into 10 ml brown volumetric flasks, diluted to the mark with methanol and shaken well to give the chlorogenic acid reference solutions. Each 10 μl of the reference solutions were taken and determined separately under the “chromatographic conditions” for measurement of peak area values.
Precision test
10 μl of chlorogenic acid stock reference solution was accurately drawn and injected six consecutive times under the “chromatographic conditions” for calculation of RSD for chlorogenic acid based on the peak area.
Reproducibility test
Sample solution was prepared as per the method in the “Preparation of sample solution”. After filtering through 0.45 μm microporous membrane, 10 μl of sample solution was accurately drawn and injected six consecutive times according to the “chromatographic conditions” for calculation of RSD for chlorogenic acid based on the peak area.
Stability test
Sample solution was prepared as per the method in the “Preparation of sample solution”. After filtering through 0.45 μm microporous membrane, 10 μl of sample solution was accurately drawn at a 4 h interval and injected at 0, 4, 8, 12, 20 and 24 h under the “chromatographic conditions” for calculation of RSD for chlorogenic acid based on the peak area.
Recovery test
Six aliquots of 0.5 g of samples with known contents were accurately weighed, added separately with appropriate amount of chlorogenic acid reference solution and prepared into solutions as per the method in the “Preparation of sample solution”. Then, the sample solutions were injected and analysed according to the “chromatographic conditions”, peak areas were measured, and amounts of various constituents were calculated, as well as recoveries.
Sample content determination
Three aliquots of 1 g of Verbena officinalis L. samples from six different batches were accurately weighed, prepared into solutions as per the method in the “Preparation of sample solution”, then injected and analysed under the “chromatographic conditions”. Peak areas were measured, and chlorogenic acid contents were calculated. Data were expressed as ͞x ± SD.
Determination of in-vitro antibacterial activity
Bacterial suspensions with final concentration of 0.5 × 107 CFU/ml were prepared by plate count method [6].
Bacteriostatic tests were performed using cylinder-plate method and MIC assay, with three replicate plates being tested per bacterial species. Assessment criteria of antibacterial effect: inhibition zone diameter>20 mm (extremely sensitive); inhibition zone diameter between 15-20 mm (highly sensitive); inhibition zone diameter between 10-14 mm (moderately sensitive); inhibition zone diameter<10 mm (lowly sensitive); no inhibition zone 6 mm (resistant) [7]. MIC of extract was defined by the sample concentration corresponding to the plate with no colony growth.
Determination of in-vitro antioxidant activity
Tests were performed according to DPPH radical scavenging assay [8]; hydroxyl radical scavenging assay [9]; and lipid peroxidation inhibition assay [10] with 2, 6-BHT, PG and ascorbic acid as the positive controls. Three parallel samples were used in all the above tests.
Data processing
Data were statistically analysed and processed using Origin 8.1 and SPSS Statistics V17.0 software.
Results
Linearity was investigated as per the method in the “Plotting of standard curve”, where standard curve was plotted with reference concentration as the abscissa and peak area as the ordinate. Results showed that the linear regression equation for chlorogenic acid was Y=27698.52X+2215.98, r=0.9998, indicating good linearity of the chlorogenic acid within a 14.0-112.0 mg/L range.
Precision test was operated as per the method in the “Precision test”. RSD for chlorogenic acid was calculated to be 0.98%, indicating qualified precision of the test instrument.
Reproducibility test was operated as per the method in the “Reproducibility test”. RSD for chlorogenic acid was calculated to be 1.21%, indicating good reproducibility of the method.
Stability test was operated as per the method in the “Stability test” method. RSD for chlorogenic acid was calculated to be 1.46% based on the peak area, indicating good stability of the sample solution within 24 h.
Recovery test
As shown in Table N, average recovery of chlorogenic acid in Verbena officinalis L. extract determined by the present method was 99.76%, with a RSD of 2.3% (n=9), indicating that the method was accurate and reliable for quantitative determination of chlorogenic acid in Verbena officinalis L.
Sample content determination
Sample solution was prepared as per the method in the “Preparation of sample solution”, and then chlorogenic acid contents in 7 batches of Verbena officinalis L. extracts were determined. Table 1 lists the results.
| Extract No. | Average chlorogenic acid content (%) |
|---|---|
| MBC201605A | 34.2 |
| MBC201605B | 33.5 |
| MBC201605C | 38.7 |
| MBC201605D | 35.6 |
| MBC201605E | 40.9 |
| MBC201605F | 58.2 |
| MBC201605G | 30.2 |
| Mean | 38.76 |
Table 1. Chlorogenic acid quantification results in Verbena officinalis L. extracts (n=3).
In-vitro antibacterial activity of Verbena officinalis L. extract
As shown in Table 2, no inhibition zone was present in all the blank control tests. Aqueous extracts showed no inhibition zone against the three bacterial species within a mass concentration range of 25-100 mg/ml. Inhibition zone diameters of 95%, 60% and 30% ethanol extracts against the three bacterial species enlarged with increasing mass concentration of sample solution. However, the bacterial species exhibited differing sensitivities to these three extracts. At a mass concentration of 100 mg/ml, three species of bacteria tested were highly or extremely sensitive to the 95% ethanol extract; highly sensitive to the 60% ethanol extract; and overall moderately sensitive to the 30% ethanol extract. MIC assay results showed that none of the plates containing 100 mg/ml 30% ethanol extract had colony growth, indicating that the 30% ethanol extract' MIC against various bacteria was ≤ 100 mg/ml. In comparison, plates containing the same mass concentration of 60% and 95% ethanol extract all exhibited individual or partial colony growth, suggesting that the two extracts’ MICs against various bacteria were>100 mg/ml. Thus presumably, the order of antibacterial potency of Verbena officinalis L. extracts was 30% ethanol extract>60% ethanol extract>95% ethanol extract. Extraction solvent had a very significant influence on the antibacterial activity of extract (P<0.01), which was probably due to differences in the type or amount of active antibacterial constituents dissolved from Verbena officinalis L. in different solvent systems.
| Test bacteria | Mass concentration (mg/ml) of 30% ethanol extract | Mass concentration (mg/ml) of 60% ethanol extract | Mass concentration (mg/ml) of 95% ethanol extract | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 100 | 50 | 25 | 100 | 50 | 25 | |
| Escherichia coli | 21.12 ± 1.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 11.51 ± 1.49 | 6.00 ± 0.00 | 6.00 ± 0.00 | 19.23 ± 2.09 | 13.00 ± 2.01 | 6.00 ± 0.00 |
| Proteus vulgaris | 16.33 ± 2.09 | 13.04 ± 1.95 | 6.00 ± 0.00 | 11.34 ± 2.09 | 6.00 ± 0.00 | 6.00 ± 0.00 | 15.67 ± 1.53 | 10.33 ± 1.52 | 6.00 ± 0.00 |
| Bacillus subtilis | 15.32 ± 1.28 | 6.00 ± 0.00 | 6.00 ± 0.00 | 14.67 ± 1.00 | 10.54 ± 1.15 | 9.34 ± 0.43 | 15.32 ± 1.05 | 11.32 ± 2.08 | 6.00 ± 0.00 |
Table 2. Inhibition zone diameters of three Verbena officinalis L. extracts (͞x ± s, n=3).
In-vitro antioxidant activity of Verbena officinalis L. extract
DPPH radical scavenging capacity: As shown in Table 3, DPPH radical scavenging capacities of the three extracts were enhanced with increasing mass concentration of sample solution. At a mass concentration of 1.0 mg/ml, DPPH radical scavenging rate was 85.32% for 30% ethanol extract, 78.27% for 60% ethanol extract and 74.53% for 95% ethanol extract, showing fairly strong scavenging capacities. The dose-response relationship equation between the mass concentration and scavenging rate was fitted using Origin8.1 software to calculate the IC50 of each sample (mass concentration of sample required for 50% scavenging rate), based on which the antioxidant levels of various extracts were defined and evaluated. According to calculation, IC50 for scavenging DPPH radicals was 0.20 mg/ml for aqueous extract, 0.30 mg/ml for 95% ethanol extract, 0.36 mg/ml for acetone extract and 0.60 mg/ml for ethyl acetate extract. Clearly, 30% ethanol extract possessed the strongest DPPH radical scavenging capacity among three ethanol extracts, followed by the 60% ethanol extract, while 95% ethanol extract had the weakest capacity.
| Sample solution | Mass concentration (mg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.05 | 0.1 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | |
| 30% ethanol extract | 6.93 ± 0.52 | 19.08 ± 1.58 | 27.00 ± 0.31 | 42.09 ± 0.48 | 59.50 ± 0.32 | 74.50 ± 1.55 | 83.21 ± 1.67 | 85.32 ± 1.03 |
| 60% ethanol extract | 6.75 ± 0.26 | 10.57 ± 0.54 | 19.92 ± 1.21 | 29.22 ± 1.00 | 58.45 ± 0.33 | 69.13 ± 0.56 | 73.12 ± 0.45 | 78.27 ± 0.66 |
| 95% ethanol extract | 6.48 ± 1.02 | 13.09 ± 1.31 | 18.82 ± 1.76 | 22.01 ± 1.54 | 34.81 ± 0.88 | 48.55 ± 1.42 | 63.91 ± 1.72 | 74.53 ± 1.02 |
| Ascorbic acid | 13.54 ± 1.12 | 21.49 ± 1.24 | 40.66 ± 1.23 | 67.76 ± 0.76 | 87.21 ± 0.42 | 93.21 ± 0.59 | 94.52 ± 0.21 | 94.72 ± 0.12 |
Table 3. DPPH radical scavenging capacities of three Verbena officinalis L. extracts and ascorbic acid (͞x ± s, n=3).
Hydroxyl radical scavenging capacity
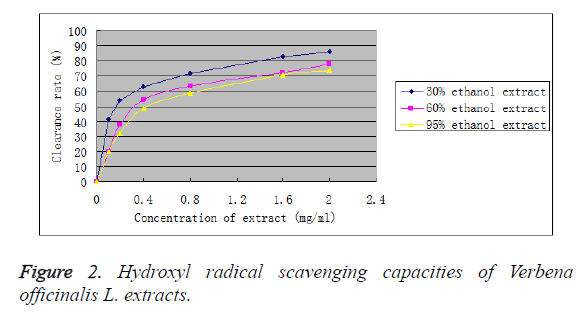
As can be seen from Figure 2, hydroxyl radical scavenging capacities of the three extracts increased overall with increasing mass concentration of sample. However, at low mass concentrations (below 0.4 mg/ml), scavenging rate presented an obvious variation trend. Afterwards, with the rise of mass concentration, variation of scavenging rate mitigated. At a 2.0 mg/ml concentration, hydroxyl radical scavenging rate was 85.82% for 30% ethanol extract, 77.99% for 60% ethanol extract and 73.67% for 95% ethanol extract. After equation fitting of dose-response relationship using Origin 8.1 software, IC50 was calculated to be 0.17 mg/ml for 30% ethanol extract, 0.21 mg/ml for 60% ethanol extract and 0.41 mg/ml for 95% ethanol extract. Clearly, all three extracts exhibited very strong hydroxyl radical scavenging capacities, which were far stronger than the synthetic antioxidants BHT (IC50: 0.60 mg/ml) and PG (IC50: 0.89 mg/ml). Among them, 30% ethanol extract possessed the strongest hydroxyl radical scavenging capacity, followed by 60% ethanol extract.
Anti-lipid peroxidation activity
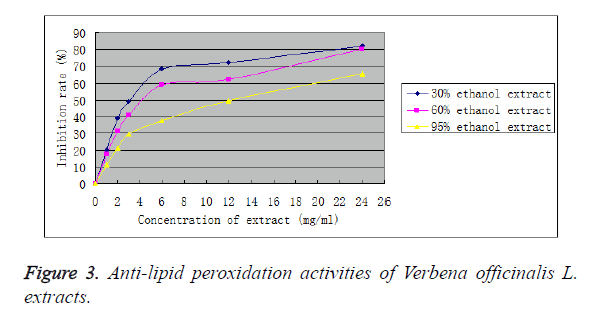
As shown in Figure 3, three extracts all had strong inhibitory effects on lipid peroxidation, which generally increased with increasing mass concentration of sample. At a mass concentration of 24 mg/ml, peroxidation inhibition rates were 82.14% for 30% ethanol extract, 80.09% for 60% ethanol extract and 65.34% for 95% ethanol extract. After equation fitting via Origin8.1 software, IC50 was calculated to be 2.69 mg/ml for 30% ethanol extract, 3.20 mg/mL for 60% ethanol extract and 13.25 mg/ml for 95% ethanol extract. Thus, 30% ethanol extract possessed the strongest anti-lipid peroxidation activity, followed by the 60% ethanol extract, while 95% ethanol extract had the weakest activity. Nevertheless, their anti-lipid peroxidation activities were far weaker than BHT (IC50: 0.01 mg/ml).
Discussions
Determination of chlorogenic acid and mobile phase solution found that the maximum absorption wavelength was 330 nm within a scanning wavelength range of 200-500 nm. Taking into account the sensitivity and detection of impurity peaks, 330 nm was selected as the monitoring wavelength, which was consistent with the reference [11]. After comparison, acetonitrile-0.4% phosphoric acid solution (14:88) was picked as the mobile phase. Chlorogenic acid exhibited good peak shape and resolution. Peak appearance time was desirable as well. So in this study, acetonitrile-0.4% phosphoric acid solution (14:88) was used as the mobile phase. Chlorogenic acid is quantitatively determined mainly by HPLC, TLC-scanning, capillary zone electrophoresis and spectrophotometry at present [12]. In view of multiple interference constituents in Verbena officinalis L., HPLC was selected for its quantification.
Existing studies have shown that Verbena officinalis L. contains a variety of chemical constituents, mainly flavonoids, phenols, volatile oil substances, etc. Phytochemicals have different dissolution properties in different solvents. In general, water is suitable for extracting hydrophilic constituents such as proteins, saccharides, tannins and glycosides. Hydrophilic constituents are mostly soluble in ethanol as well. Some of the lipophilic constituents also have great solubility in 95% ethanol, thus resulting in differing yields of Verbena officinalis L. extracts obtained with different concentrations of ethanol, as well as type and content discrepancies of chemical constituents. Among various chemical constituents, phenolic hydroxyl-containing compounds have attracted wide attention owing to their anti-cancer, anti-aging, anti-inflammatory and ROS-scavenging pharmacological functions [13-15]. Preliminary experiment found that different concentrations of ethanol were the suitable extraction solvent for Verbena officinalis L.
Antibacterial activities of the three Verbena officinalis L. extracts were investigated, which might be subject to the combined effects of a variety of chemical constituents. However, some non-active substances probably interfered with each other to affect the antibacterial effect, some of which could also become excellent bacterial medium components to promote the growth of pathogenic bacteria. All the three extracts exerted antibacterial activities against three test bacteria at a mass concentration of 100 mg/ml, of which the 30% ethanol extract exhibited the strongest antibacterial capacity. It is appropriate to use 30% ethanol as the extraction solvent in the development of Verbena officinalis L. extract as botanical pesticides or plant-derived antibacterial agents. Chemical component separation and activity screening on the extract are still needed, in order to clarify and obtain its active constituents.
Three Verbena officinalis L. extracts exhibited differing scavenging capacities against different radials in different antioxidant systems. Overall, DPPH and hydroxyl radical scavenging abilities were stronger than the anti-lipid peroxidation activity. This may be related to the differing sensitivities and mechanisms of antioxidant constituents in Verbena officinalis L. to different free radicals. If the antioxidant level was defined and evaluated according to the IC50 for DPPH and hydroxyl radical scavenging and anti-lipid peroxidation, the antioxidant potency order of the three extracts would be 30% ethanol extract, 60% ethanol extract and 95% ethanol extract in a descending order. IC50 of 30%, 60% and 95% ethanol extracts in DPPH radical, hydroxyl radical and anti-lipid peroxidation systems were 0.20, 0.15 and 2.69 mg/ml, respectively. These three Verbena officinalis L. extracts possessed scavenging capacities against the most active, most toxic hydroxyl radicals that were far stronger than the synthetic antioxidants BHT and PG. Tannins are a class of reducing compounds containing phenolic hydroxyl groups. In the complex biochemical reaction process, self-oxidation of phenolic hydroxyl-containing compounds allows them to have strong antioxidant activity [15]. There ought to exist a doseresponse relationship between the content and the antioxidant potency of phenolic hydroxyl-containing compounds.
Conclusion
Methodological research revealed that the present method was simple, accurate and reproducible, which can be used for quantitative determination of chlorogenic acid in Verbena officinalis L. and its extract. Three Verbena officinalis L. extracts exhibited differing scavenging capacities against different radials in different antioxidant systems. Overall, DPPH and hydroxyl radical scavenging abilities were stronger than the anti-lipid peroxidation activity. Further chemical component separation targeting the 30% ethanol extract and antioxidant activity assay on each component are the necessary path for screening various effective lead antioxidant substances in Verbena officinalis L.
References
- Shu J, Chou G, Wang Z. Two new iridoids from Verbena officinalis L. Molecules 2014; 19: 10473-10479.
- Schönbichler SA, Bittner LK, Pallua JD, Popp M, Abel G. Simultaneous quantification of verbenalin and verbascoside in Verbena officinalis by ATR-IR and NIR spectroscopy. J Pharm Biomed Anal 2013; 84: 97-102.
- Makino Y, Kondo S, Nishimura Y, Tsukamoto Y, Huang ZL, Urade Y. Hastatoside and verbenalin are sleep-promoting components in Verbena officinalis. Sleep Biological Rhythms 2009; 7: 211-217.
- Sheyla R, Olman H, Mikel GIC, Icigo N, Iciar A, Diana A, Rita YC, Maria IC. Chemical composition, mineral content and antioxidant activity of Verbena officinalis L. LWT-Food Sci Technol 2011; 44: 875-882.
- Manuel AE, Sheyla R, Diana A, Iciar A, Rita YC, María IC. Antiproliferative effect of phenylethanoid glycosides from Verbena officinalis L. on colon cancer cell lines. LWT Food Sci Technol 2015; 63: 1016-1022.
- Kolibab K, Yang A, Parra M, Derrick SC, Morris SL. Time to detection of mycobacterium tuberculosis using the MGIT 320 system correlates with colony counting in preclinical testing of new vaccines. Clin Vaccine Immunol 2014; 21: 453-455.
- Davis CE, Hunter WJ, Ryan JL, Braude AI. Simple method for culturing anaerobes. Appl Microbiol 1973; 25: 216-221.
- Piang-Siong W, de Caro P, Marvilliers A, Chasseray X, Payet B. Contribution of trans-aconitic acid to DPPH scavenging ability in different media. Food Chem 2017; 214: 447-452.
- Olugbami JO, Gbadegesin MA, Odunola OA. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacognosy Research 2015; 7: 49-56.
- Tsuchiya H. Lipid peroxidation-inhibitory effects of perioperatively used drugs associated with their membrane interactions. Oxidants Antioxidants Med Sci 2014; 3: 91-98.
- Craig AP, Fields C, Liang N, Kitts D, Erickson A. Performance review of a fast HPLC-UV method for the quantification of chlorogenic acids in green coffee bean extracts. Talanta 2016; 154: 481-485.
- Chung DM, Chung YC, Chun HK. Spectrophotometric assay for determination of chlorogenic acid using green pigment formation and quantitative analysis of chlorogenic acid in Blueberry leaf. J Life Sci 2011; 21: 610-612.
- Shao P, Zhang JF, Chen XX, Sun PL. Microwave-assisted extraction and purification of chlorogenic acid from by-products of Eucommia Ulmoides Oliver and its potential anti-tumor activity. J Food Sci Technol 2015; 52: 4925-4934.
- Guan F, Wang H, Shan Y, Chen Y, Wang M. Inhibition of COX-2 and PGE2 in LPS-stimulated RAW264.7 cells by lonimacranthoide VI, a chlorogenic acid ester saponin. Biomed Rep 2014; 2: 760-764.
- Feng Y, Yu YH, Wang ST, Ren J, Camer D, Hua YZ, Zhang Q, Huang J, Xue DL, Zhang XF, Huang XF, Liu Y. Chlorogenic acid protects d-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharmaceutical Biology 2016; 54: 1027-1034.