Research Article - Biomedical Research (2017) Volume 28, Issue 4
How much should we observe patients with mad honey poisoning?
Ozlem Bilir1*, Gokhan Ersunan1, Ozcan Yavasi1, Kamil Kayayurt2, Barıs Giakoup3 and Mehmet Bostan41Department of Emergency Medicine, Recep Tayyip Erdogan University, Rize, Karadeniz, Turkey
2Department of Emergency Medicine, Acibadem University, Turkey
3Department of Emergency Medicine, Rize State Hospital, Turkey
4Department of Cardiology, Tayyip Erdogan University, Turkey
- *Corresponding Author:
- Ozlem Bilir
Department of Emergency Medicine
Recep Tayyip Erdogan University, Turkey
Accepted date: August 9, 2016
Abstract
The aim of this study was to understand better the pathophysiology of this intoxication by evaluating the effect of mad honey ingestion on Inferior Vena Cava (IVC) diameters and IVC Collapsibility Index (IVC-CI) and develop an objective algorithm for the duration of fluid replacement and observation. The patients with the medical history of mad honey ingestion and admitted to the emergency service due to the signs of mad honey poisoning were analysed. Their data concerning age, gender, admission symptoms and the time of onset of these symptoms, the vital signs during admission, the administered treatment, the post-treatment recovery time and vital signs were all recorded. The inferior vena cava diameter and the IVC collapsibility index were assessed by ultrasonography. Of 29 patients included in the study, 79.31% were male, the average age was 52.76 ± 17.52 years, and the most common cause of admission was dizziness. While 0.9% saline solution was administered to all patients, in 82.75% intravenous atropine was started. Significant differences were determined between the vital signs, the inferior vena cava diameters, and the collapsibility indexes of the pre and post treatment periods. The ingestion of mad honey should be questioned in the medical history in patients who were admitted to the emergency services due to hypotension, bradycardia, and syncope. The assessment of the vital signs and the measurement of the inferior vena cava diameters of the patients should be the parts of the follow-up. The monitoring of the responses to the administered atropine and/or normal saline solutions should be made by the ultrasonographic assessment of the inferior vena cava diameter and the IVC Collapsibility Index (IVC-CI), in addition to monitoring the vital signs.
Keywords
Mad honey, Inferior vena caval diameters, Collapsibility index
Introduction
Mad honey poisoning is a clinical state resulting from the ingestion of honey produced from the flower and nectar of Rhododendron ponticum type of plants that are mainly found in the natural flora of the Black Sea region of Turkey [1]. Grayanotoxin, which is a kind of neurotoxin responsible for this toxicity, taken from these plants cannot be detoxified by bees, and is directly blended into the honey, resulting in intoxication. Grayanotoxin binds to voltage-gated sodium channels and triggers cardio inhibitory Bezold-Jarisch Reflex (BJR), characterized by bradycardia, continued hypotension and peripheral vasodilatation by inhibition of central vasomotor centers with a reduced sympathetic output and a reduced peripheral vascular resistance through stimulation of unmyelinated afferent cardiac branches of the vagus nerve [2-6]. Thus, the clinical picture of mad honey poisoning mimics cholinergic syndrome [7]. Hypotension and bradycardia are the most commonly seen signs of this toxicity [8-11]. Although bradycardia responds well to intravenous atropine, the decrease in blood pressure is partially attenuated and complete restoration takes a period of time despite intravenous fluid replacement. This time period for fluid replacement and observation varies in different studies between 2-24 hours according to local practices [8-12]. In this prospective cohort study, we aimed to better understand the pathophysiology of this intoxication by evaluating the effect of mad honey ingestion on Inferior Vena Caval (IVC) diameters and Inferior Vena Cava Collapsibility Index (Inferior Vena Cava-CI) and developed an objective algorithm for duration of fluid replacement and observation.
Methods
Study design
We conducted a prospective cohort study from April 2013 to April 2014. Our local ethics committee approved the study protocol, and written informed consent was obtained from the participants before ultrasonographic examination.
Study setting and population
This study was conducted at an academic, adult tertiary care center Emergency Department (ED) in northern part of eastern coast of Turkey, where Rhododendron ponticum type plants are among natural flora. This emergency department serves more than 150000 adult patients annually. All patients presenting to emergency department with the primary diagnosis of mad honey poisoning were enrolled. The diagnosis of mad honey poisoning was based on a history of ingestion of mad honey and typical signs. Exclusion criteria included age less than 18 years, pregnancy, liver transplantation, acute abdomen, renal failure, patients who were transferred from other hospitals after the administration of atropine or intravenous fluid, vasoactive medicines, or an inability to consent for the study.
Study protocol
On admission, patients' ages and sex, time of honey ingestion, signs and symptoms, pulse rate and blood pressure were recorded. All patients were put on a cardiac monitor and 12- lead electrocardiogram was obtained. Two emergency physicians, who were blinded to the above results and were not responsible for the treatment of the patient, performed the ultrasonographic inferior vena cava measurements without disrupting the therapy. Measurements were taken in the semisupine position using a Fazone CB model ultrasound machine with a 3.5 MHz convex transducer (Fazone CB, Fujifilm, USA). Care was taken to record the maximal inferior vena cava diameter seen during an entire respiratory cycle. The maximum (IVCexp) and minimum (IVCins) inferior vena cava diameters were measured distal to the confluence of hepatic veins in M-mode. The images showing the obtained measurements were stored. The inferior vena cava collapsibility index was calculated as (IVCexp-IVCins)/(IVCexp) × 100. The patients were treated by the decision of treating physician. Following administration of atropine or intravenous fluid, pulse rate and blood pressure were continuously recorded. Once the heart rate and blood pressure remained stable during a half hour period and the treating physician made the decision to discharge the patient from emergency department or to admit the patient to the intensive care unit, ultrasonographic measurements were repeated. Pulse rate, blood pressure measurement and electrocardiogram were also repeated. All patients were followed on cardiac monitor for at least 6 hours after the normalisation of heartbeat and hypotension as advised in the literature. Outpatient follow-up were advised to all patients. If a patient did not follow-up as recommended, the patient was contacted by telephone to determine if there was morbidity or mortality.
Data analysis
Data were entered using a form prepared by the researchers that was completed during initial presentation and at the time of patient discharge. All statistical analyses were performed using Statistical Package for the Social Sciences version 17.0 (SPSS IBM, Armonk, NY). Categorical data are presented as frequencies and percentages, normally distributed continuous data as the mean ± SD with minimum and maximum values. Non-normally distributed data was presented as median and Interquartile Ranges (IQR). Normality analysis was checked by Shapiro-Wilk test. Inferior Vena Cava (IVC) values at admission and after normalization of blood pressure were compared with paired t-test. A P-value of <0.05 was considered statistically significant.
Results
During the study period, 29 patients were admitted to the emergency department due to the honey poisoning. Demographic characteristics of the patients are shown in Table 1. The vital signs and ultrasonographic Inferior Vena Cava (IVC) measurements before and after the treatment were shown in Table 2.
| Variables | Findings (n, %) |
|---|---|
| Age , mean ± SD (range) | 52.76 ± 17.52 (18-87) |
| Sex, male | 23 (79.31%) |
| Duration of symptom onset (hours) mean ± SD (range) | 2.91 ± 1.75 ( 0.5-6.5) |
| Causes of admission | |
| Dizziness | 15 (51.72%) |
| Syncope | 9 (31%) |
| Nausea-vomiting | 8 (27.58%) |
| ECG | |
| Sinus bradycardia | 20 (68.96%) |
| Normal sinus rhythm | 5 (17.24%) |
| Complete AV block | 3 (10.34%) |
| AF with slow ventricular response | 1 (3.44%) |
| Treatment | |
| 0.9% NaCl 100 ml/h | 29 (100%) |
| Atropine | 24 (82.75%) |
| Follow-up | |
| Discharged | 24 (82.75%) |
| Admitted to coronary ICU | 5 (17.24%) |
| Recovery time, minutes, median (IQR) | 210 (IQR=80-385.50) |
| Outcome death | None |
| SD: Standard Deviation; IQR: Interquartile Range); AF: Atrial Fibrillation; ICU: Intensive Care Unit); ECG: Electro Cardio Graph. | |
Table 1. The characteristics of patients with mad honey intoxication.
| Before treatment | After treatment | p |
|---|---|---|
| Systolic blood pressure (mmHg) | 80.38 ± 12.50 (50-110) | 124.62 ± 16.70 (90-160) |
| Diastolic blood pressure (mmHg) | 49.10 ± 11.79 (20-70) | 72.83 ± 9.42 (52-90) |
| Pulse rate (beats/minute) | 43.79 ± 8.50 (30-66) | 72.72 ± 16.06 (35-114) |
| Mean arterial pressure (mmHg) | 59.56 ± 11.38 (30-83.33) | 90.09 ± 10.37 (66-106.66) |
| IVC inspiratory (cm) | 1.63 ± 0.39 (0.97-2.41) | 1.14 ± 0.33 (0.62-2.15) |
| IVC expiratory (cm) | 2.00 ± 0.43 (1.10-2.87) | 1.76 ± 0.38 (1.16-2.68) |
| IVC-CI (%) | 18.93 ± 8.21 (2.01-34.24) | 35.60 ± 9.71 (18.59-52.30) |
| Values are presented as mean ± SD (range; minimum, maximum). IVC: Inferior Vena Cava; IVC-CI: Inferior Vena Cava Collapsibility Index. | ||
Table 2. Vital signs and Inferior Vena Cava (IVC) measurements before and after the treatment.
In all patients, 100 ml/h normal saline solution and in 82.75% of patients, intravenous atropine was administered as the treatment. No pathological findings were present in the hematologic and biochemical tests, and also in the obtained chest x-rays. The median regression period of the symptoms following the emergency service admission Interquartile Ranges (IQR) was 210 minutes (range 80-385). While 82.75% (n=24) of the patients were discharged uneventfully after their follow-up in the emergency department, 17.24% (n=5) were hospitalized in the coronary intensive care unit as shown in Table 1. In one patient pacemaker was implanted due to the developing complete Atrioventricular (AV) block, whereas the remaining four patients (13.79%) were discharged after the follow-up.
Discussion
Besides being used as a nutrient, mad honey was used for centuries in the complementary and alternative medicine due to the substances it contains, such as amino acids, proteins, and antioxidants. Particularly, its use in the gastrointestinal system disorders, hypertension, diabetes, arthritis, and dysfunctional sexual disorders are at the forefront [1,13]. Of the patients included in this study, 79.31% were middle-aged men. This finding was similar to the literature and was considered to originate from the use of the mad honey in alternative medical treatments. The most common causes of admission were dizziness and syncope, which are due to bradycardia and hypotension. The time period following the onset of the symptoms and their admission to the emergency department was 2.91 ± 1.75 hours and the median emergency department follow-up period was 210 minutes. While the time of patients’ full recovery was found as 23.4 ± 10.5 hours in the study conducted by Demircan et al., there are differences among studies concerning the follow-up period [8]. While implementation of cardiac monitoring for 2- 6 hours was recommended by Gunduz et al. in their study conducted in 2006, in another study of theirs carried out in 2009, they stated that this period should be 6 hours following normalisation of the arterial blood pressure and pulse values [1,14]. Their result is similar to the result of our study.
Honey, produced by bees from the nectar of mountain roses which belong to the Rhododendron family, is contaminated with a neurotoxin named grayanotoxin. This biological toxin binds to the voltage-dependent sodium channels, leaving them in a depolarized state [15]. The neurotoxins such as veratridine, batrachotoxin, and grayanotoxin are within the lipid-soluble toxin group, and they are bound to the neurotoxin receptor-2 sites in the sodium channels [2]. They create their selective effects by activation through hyperpolarization. The stimulation of the demyelinated afferent cardiac branches of the vagus nerve leads to the reduced sympathetic output in the central vasomotor center and the peripheral vascular resistance. By this means, grayanotoxin causes bradycardia, hypotension, and peripheral vasodilation by stimulating the cardioinhibitory Bezold-Jarisch Reflex (BJR) [5]. The identification of this cardioinhibitory reflex, which is manifested particularly in mad honey poisoning, is an important issue because the observed symptoms are not the signs of a structural heart disorder, but related to the activation of the vagal afferent nerve by grayanotoxin. Atropine was given to 82.75% of the patients included in the study and the rapid improvement observed in the pulse rate (p<0.01) was the indicator of the activation of this cardioinhibitory reflex. Although bradycardia is the most common electrocardiographic manifestation of this ingestion, it may also mimic more severe cardiac conditions by causing specific electrocardiographic changes [12]. Complete atrioventricular block was identified in 10.34% and atrial fibrillation with slow ventricular response in 3.44% of our patients. Only one patient who had developed complete Atrioventricular (AV) block necessitated pacemaker implantation during the intensive care follow-up. In this patient, the most significant cause was considered as the underlying structural cardiac disorder.
The hypotension manifested due to the neuroaxonal blockade caused by grayanotoxin is the indicator of the sympathetic blockade [5,16]. Following the blockade, the reduced systemic vascular resistance and the decreased myocardial contractility lead to peripheral vasodilation, together with the centrally directed circulation of the lower extremity and the spleen [17]. This results in both expiratory and inspiratory inferior vena cava diameters to enlarge and collapsibility to decrease, such as in the case of congestion. The hypotension, not fully restored following atropine administration improves with the administration of saline solution, by triggering a cardio protective reflex, called the Bain bridge reflex. In this major cardiac reflex, which was first described in dogs under anaesthesia in 1915, as the result of fluid administration, the low-pressure receptors located in the atrium are activated, and the bradycardia is eliminated together with the hypotension [18,19]. This results in both inferior vena cava diameters to decrease and collapsibility to increase.
The determination of the fluid requirement of the body during evaluation of the efficacy of the treatment, which takes place in every clinical assessment in the emergency department, is also essential in mad honey poisoning. In this situation, the evaluation of the inferior vena cava diameters can be used as a practical and non-invasive method [20-22]. Various methods have been used in conducted studies for measurement of the inferior vena cava diameter. However, among these methods, M-mode and the measurement during the inspiratory phase were stated to be effective in monitoring the body fluids [23].
Inferior Vena Cava Collapsibility Index (IVC-CI) measurement is a method that can be used in monitoring the response to treatment in mad honey poisoning. Non-invasively assessed inferior vena cava dimensions, together with the respirophasic changes, show the volume and pressure status of the inferior vena cava [24]. CVP (Central Venous Pressure), which is a simple but useful indicator of the preload showing the ventricular functions, can be noninvasively estimated with the help of these parameters in adults [20,25]. In conducted studies, the presence of a correlation between central venous pressure and inferior vena cava diameter, and also between central venous pressure and Inferior Vena Cava Collapsibility Index (IVC-CI) were demonstrated. The reason for this is the Inferior Vena Cava Collapsibility Index (IVC-CI) being based upon the physiological relationship between the venous pressure and the volume, which is a reflection of central venous pressure. Thus, due to the intrathoracic negative pressure occurring during the inspiration, the inferior vena cava collapses more, leading to the differences in Inferior Vena Cava Collapsibility Index (IVC-CI). The presence of significant differences between the respirophasic changes in the inferior vena cava dimensions before and after the treatment in our study supports this situation.
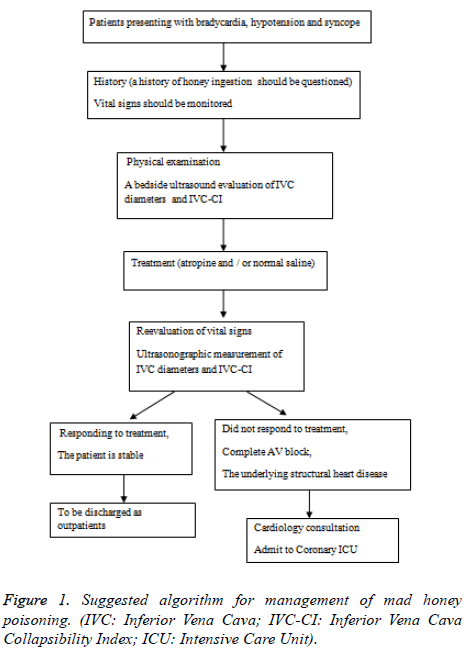
The progression of the transnational trade together with the increasing interest in natural products has led to the mad honey poisoning being seen in the countries other than Turkey [26]. Therefore, for treatability of the patients admitted to the emergency department with hypotension, bradycardia, and syncope, ingestion of mad honey should be questioned in medical history. The assessment of the vital signs and measurement of the inferior vena cava diameters should also be the part of the follow-up. Monitoring the response to the atropine and/or normal saline solutions administered to the patients should be made by ultrasonographic measurement of the inferior vena cava diameters and Inferior Vena Cava Collapsibility Index (IVC-CI) evaluation, in addition to monitoring the vital signs. Patients, whose current complaints have regressed and vital signs stabilized, can be discharged from the emergency department to outpatient follow-up. However, consultation with cardiology should be made for patients who have complete atrioventricular block, or whose bradycardic rhythm has not improved. When necessary, these patients should be followed-up in the coronary intensive care units. Moreover, in mad honey intoxications seen in patients having structural cardiac disorders, the follow-up periods should be prolonged, and when necessary, they should be hospitalized for treatment as shown in Figure 1.
References
- Gunduz A, Turedi S, Uzun H, Topbas M. Mad honey poisoning. Am J Emerg Med 2006; 24: 595-598.
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 2005; 57: 397-409.
- Maejima H, Kinoshita E, Seyama I, Yamaoka K. Distinct sites regulating grayanotoxin binding and unbinding to D4S6 of Na (v) 1.4 sodium channel as revealed by improved estimation of toxin sensitivity. J Biol Chem 2003; 278: 9464-9471.
- Yilmaz O, Eser M, Sahiner A, Altintop L, Yesildag O. Hypotension, bradycardia and syncope caused by honey poisoning. Resuscitation 2006; 68: 405-408.
- Campagna JA, Carter C. Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology 2003; 98: 1250-1260.
- Onat F, Yegen BC, Lawrence R, Oktay A, Oktay S. Site of action of grayanotoxins in mad honey in rats. J Appl Toxicol 1991; 11: 199-201.
- Gunduz A, Turedi S, Russell RM, Ayaz FA. Clinical review of grayanotoxin/mad honey poisoning past and present. Clin Toxicol (Phila) 2008; 46: 437-442.
- Demircan A, Keleay A, Bildik F, Aygencel G, Doayan NO. Mad honey sex: therapeutic misadventures from an ancient biological weapon. Ann Emerg Med 2009; 54: 824-829.
- Bostan M, Bostan H, Kaya AO, Bilir O, Satiroglu O. Clinical events in mad honey poisoning: a single centre experience. Bull Environ Contam Toxicol 2010; 84: 19-22.
- Eroaylu SE, Urgan O, Onur OE, Denizbaaya A, Akoaylu H. Grayanotoxin (mad honey)-on-going consumption after poisoning. Balkan Med J 2013; 30: 293-295.
- Saritas A, Kandis H, Baltaci D, Erdem I. Paroxysmal atrial fibrillation and intermittent left bundle branch block: an unusual electrocardiographic presentation of mad honey poisoning. Clinics (Sao Paulo) 2011; 66: 1651-1653.
- Alemdar R, Aslantas Y, Caglar O, Celer A, Ekinou I, Ozhan H. An unusual case of mad honey poisoning presented to the emergency clinic with ST-wave elevation. Konuralp Tip Dergisi 2013; 5: 48-50.
- Lue TF. Erectile dysfunction. N Engl J Med 2000; 342: 1802-1813.
- Gunduz A, Meria ES, Baydin A, Topbaay M, Uzun H. Does mad honey poisoning require hospital admission? Am J Emerg Med 2009; 27: 424-427.
- Maejima H, Kinoshita E, Seyama I, Yamaoka K. Distinct sites regulating grayanotoxin binding and unbinding to D4S6 of Na (v) 1.4 sodium channel as revealed by improved estimation of toxin sensitivity. J Biol Chem 2003; 278: 9464-9471.
- Rooke GA, Freund PR, Jacobson AF. Hemodynamic response and change in organ blood volume during spinal anaesthesia in elderly men with cardiac disease. Anesth Analg 1997; 85: 99-105.
- Saada M, Catoire P, Bonnet F, Delaunay L, Gormezano G, Macquin-Mavier I, Brun P. Effect of thoracic epidural anaesthesia combined with general anaesthesia on segmental wall motion assessed by transesophageal echocardiography. Anesth Analg 1992; 75: 329-335.
- Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol 1915; 50: 65-84.
- Crystal GJ, Salem MR. The Bainbridge and the reverse Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg 2012; 114: 520-532.
- Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20: 857-861.
- Kosiak W, Swieton D, Piskunowicz M. Sonographic inferior vena cava/aorta diameter index, a new approach to the body fluid status assessment in children and young adults in emergency ultrasound-preliminary study. Am J Emerg Med 2008; 26: 320-325.
- Yavasi O, Unluer EE, Kayayurt K, Ekinci S, Saglam C, Surum N, Koseoglu MH, Yesil M. Monitoring the response to treatment of acute heart failure patients by ultrasonographic inferior vena cava collapsibility index. Am J Emerg Med 2014; 32: 403-407.
- Celebi Yamanoglu NG, Yamanoglu A, Parlak I, Pinar P, Tasun A, Erkuran B, Aydinok G, Torlak F. The role of inferior vena cava diameter in volume status monitoring: the best sonographic measurement method? Am J Emerg Med 2015; 33: 433-438.
- Yoichi I, Akiko T, Kaduki K, Satoshi M, Naoko O, Hideaki S. Usefulness of respiratory variation of inferior vena cava diameter for estimation of elevated central venous pressure in children with cardiovascular disease. Circ J 2011; 75: 1209-1214.
- Simonson JS, Schiller NB. Sonospirometry: A new method for non-invasive estimation of mean right atrial pressure based on two dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol 1988; 11: 557-564.
- von Malottki K, Wiechmann HW. Acute life-threatening bradycardia: food poisoning by Turkish wild honey. Dtsch Med Wochenschr 1996; 121: 936-938.