Research Article - Journal of Fisheries Research (2023) Volume 7, Issue 1
History, present, and future of the shellfish fishery in Panama: an update
Juan A Gomez H1, Janzel R Villalaz G2 and Italo Goti2*1Department of Natural, Exact and Technology, Panama University, Panama City, Panama
2Department of Marine Biology and Limnology, Panama University, Panama City, Panama
- *Corresponding Author:
- Italo Goti
Department of Marine Biology and Limnology
Panama University, Panama City, Panama
E-mail: italo.goti@up.ac.pa
Received: 02-Dec-2022, Manuscript No. aajfr-22-82122; Editor assigned: 05-Dec-2022, PreQC No. aajfr-22-82122(PQ); Reviewed: 23-Dec-2022, QC No. aajfr-22-82122; Revised: 09-Jan-2023, QC No. aajfr-22-82122; Published: 17-Nov-2022, DOI:10.35841/aajfr-7.1.131
Citation: Gomez HJA, Villalaz GJR, Goti I. History, present, and future of the shellfish fishery in Panama: An update. J Fish Res. 2023;7(1):131
Abstract
Mollusks represent an essential food resource for communities settled in the coastal zone, especially in the Pacific of Panama due to the high density of inhabitants. From pre-Columbian times to the present, its importance has not diminished, especially the species that have commercial value that has served as a livelihood for coastal dwellers at the national level, as well as internationally for export, contributing to the gross domestic product (GDP) from the country. Five families stand out both in the Pacific and in the Caribbean of Panama. Of those captured in the Pacific, the Pinctada mazatlanica pearl oyster has been marketed since pre- Columbian times for the high value of the pearls it produces. Currently, this resource has been decimated to the point that the species no longer produces pearls. Another species of economic importance is Argopecten ventricosus, which during the 1980s generated economic income for the country of approximately 10 million dollars until the population was reduced to the point that it is no longer of commercial interest. In the Panamanian Caribbean, mollusks have been preferred as a food source and some species, such as the Strombus, are sold as ornaments due to their showiness. The effect of the problem of contamination in the coastal zone on the filtering mollusks that incorporate these contaminants and affect human health when consumed is alert; this explains why some of these are used as biological indicators.
Keywords
Venus clam, Mangrove cockle, Pacific Panama, CO2 capture, Aquaculture.
Introduction
In Panama, mollusks have represented a source of food since pre-Columbian times, 8,000 years ago, and these represent the second marine group with the greatest diversity. In addition, due to the resplendent colour of their shells, they were used by the indigenous people in burials and subsequently incorporated into the diet of humans as the Hexaplex gastropods (Prince murex spiny snail), the Pacific Calico Scallop, Argopecten cockle, the Frilled Venus, Chione clam and later it has become a source of income for the villagers and the country's economy [1].
Pre-Columbian History
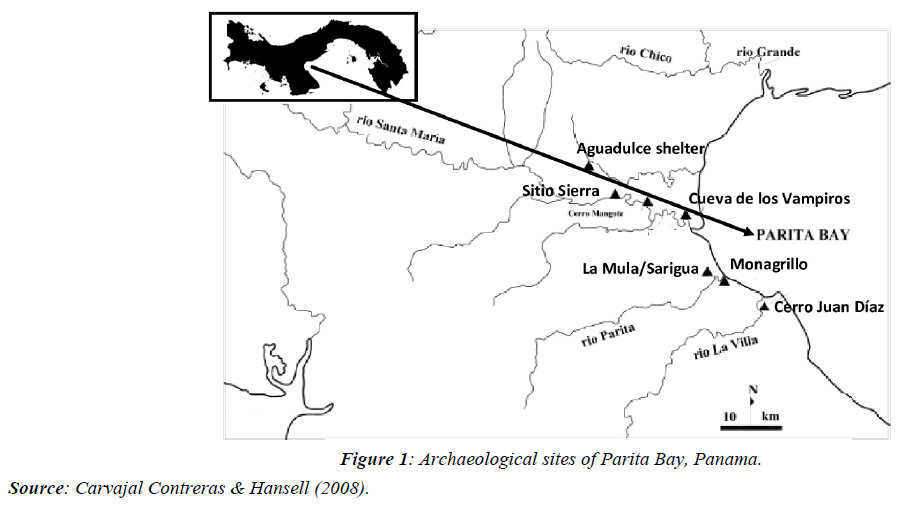
In various archaeological studies in Panama, there are records of mollusks in all the coastal settlements evaluated around the Azuero dry arch, from 5 thousand years BP until the arrival of the Spaniards, in places such as Monagrillo, Cerro Mangote, La Mula/Sarigua, Sierra site, Cerro Juan Diaz, Aguadulce shelter, Cueva de los Ladrones [2,3] (Figure 1). In most of these localities, there is abundant evidence of the use of Strombus galeatus, Conus patricius, and Melongena patula, among gastropods and bivalves: Spondylus spp, Pinctada mazatlanica, and Anadara grandis, in the use of ornaments, years before the presence of Spondylus sp. and Pinctada sp. as dominant species in the vestiges of decorations [4,5]. Due to the absence of these two groups in the shell middens of Isla de Las Perlas, it was concluded that they were used exclusively for bartering [6].
In addition to the mollusks used in ornamentation, the species Natica sp., Iphigenia sp. and Polymesoda sp. stand out. As a food source due to the proximity of the settlements above to areas of the muddy substrate adjacent to the mangroves [7]. On the other hand, in the archaeological remains the presence of species such as Donax sp. and Tivela sp. of sandy sediment and, Polymesoda sp. of estuarine waters for human consumption [3].
During the pre-Columbian occupation period, the distance to the coast varied, concerning the current level, more than 15km, 1000BP, due to transgression processes up to 1km, 5000BP, by marine progression and, finally, in recent years occupation, the sea moved away up to 4.2km to the present [8], the use of the mollusk species indicated above did not vary significantly during the pre-Columbian period [2], however, due to the time that has elapsed, there may have been variation in the density and size of the organisms.
With the arrival of the colonizing Spaniards in America, a specific selectivity was observed for the consumption of mollusks from the intertidal zone, decreasing over time, particularly the bivalves of the Family Ostreidae and Donacidae [9].
Current History
At present, the exponential growth of the world population has forced the search for alternative sources that guarantee food security through the development of aquaculture as an alternative, through easily domesticated marine species that represent a food option for man, or by the capture of edible species extracted directly from the environment [10].
In the marine environment, one of the most important groups is the species that constitute the Phylum Mollusca, because they represent a large biomass in terms of invertebrates [11], constituting an important line in fishery production with great demand in the market local and international.
Because Panama has two coasts, in the Pacific and the Caribbean, it can be pointed out that it has great marine wealth, however, there is no basic knowledge about the rational and sustained exploitation of some of its natural resources, such This is the case of bivalve mollusks, whose manual extraction has been permanently uncontrolled, causing their depletion, whose fishery is concentrated on the capture of species that have served as a source of commercialization and food by the settled fishing communities in the coastal zone of the Panamanian Pacific and Caribbean sector [12].
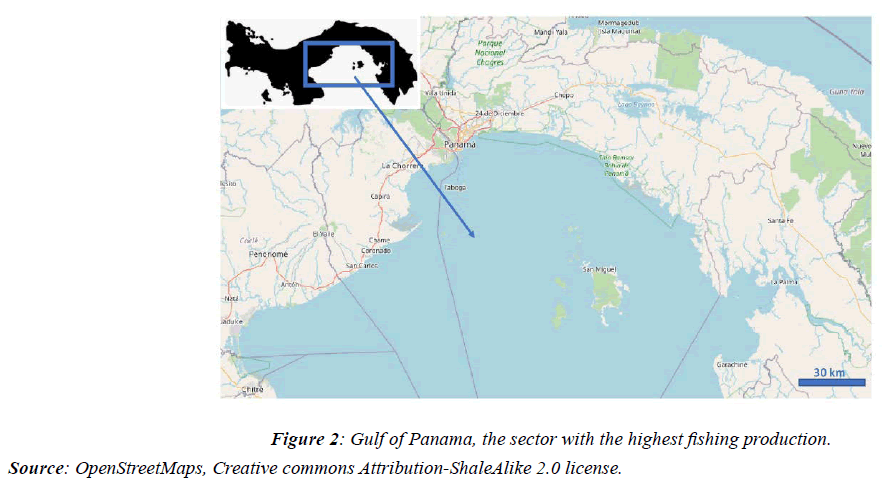
The Pacific of the Isthmus of Panama, with a tidal range of 16 to 18 feet and a great extension of 1,780km (1,100miles), and in particular the Gulf of Panama, has been the most exploited sector of preference in the country, and where the Most of the fishing for fish, shellfish, squid, shrimp, and anchovies is important for its contribution to the economy and livelihood of communities living near the coast. These resources show yields that vary significantly in time and space [13]. They are generally influenced by the oceanographic conditions of the sector, which vary during the dry and rainy seasons, which affects the life cycles of many organisms that are of commercial importance [14].
Particularly in the months of January-March corresponding to the dry season, a mass of deep and cold water, with a temperature that fluctuates between 17 and 20°C, rich in nutrients, replaces the warm surface water, generating the phenomenon known of upwelling, typically of the Gulf of Panama [15], an event that changes the stratified condition of the water column and stimulates planktonic production [16-19], favouring the reproduction of organisms due to changes in temperature and salinity of seawater typical of the time [20,21]. However, from April to December (rainy season) the temperature of the seawater reaches 30°C, the density of phytoplankton is reduced and the nutrient inputs come from continental erosion that is discharged into the rivers that flow into the coastal zone (Figure 2).
In the Pacific area of Panama, the natural populations of mollusks are exploited for local consumption by traditional fishing communities, due to their high commercial value and their great international and national demand, particularly in the provinces of Chiriquí (Pedregal), Veraguas (Mariato, Gulf of Montijo), Los Santos (Albina Grande and Guarare), Panama (Bique Beach) and Darién (Playon de Garachine- Taimati) [22].
Mollusks as a food source
Among the mollusks captured in the Pacific area that is most frequently used as a food source, the following stand out:
Family Pectinidae: Argopecten ventricosus (Sowerby II, 1842), former Argopecten circularis, also known as “conchuela”, Pacific calico scallop, Catarina clam or Pacific calico scallop.
Family Veneridae: Leukoma asperrima (Sowerby I,), formerly Protothaca asperrima, called little-necked clam, Venus clam.
Family Arcidae: Anadara tuberculosa known as “concha prieta” or “concha negra” or mangrove cockle and Larkinia grandis previously classified as Anadara grandis, commonly known as donkey helmet, donkey paw, cocalica or mangrove cockle, grand ark.
Family Ostreidae: Striostrea prismatic, former Ostrea iridescens, Crassostrea columbiensis, former Ostrea columbiensis and Crassostrea iridescens, common oyster known as stone oyster, rock oyster, rocky oyster.
Family Pteridae: Pinctada mazatlanica, called the pearl oyster, is used for food and in pearl farming.
Mollusks Fisheries
Argopecten ventricosus (Pacific calico scallop)
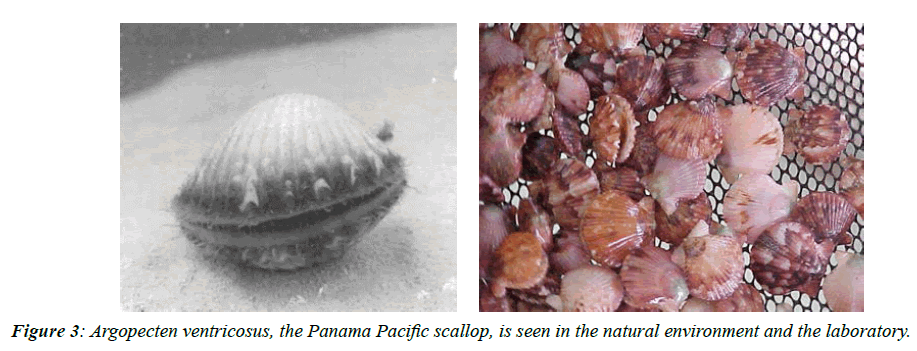
It extends from Isla Cedros in Baja California to Puerto Paita in Peru. In Panama, it occurs only in the Gulf of Panama, located in the coastal zone at depths of 1 to 135m, in muddy environments and bottoms that have large amounts of scallop shells that have served as a substrate for the larval settlement of the species. Its lifespan is 2 years and the shell can reach a maximum height of 6.0cm (2.3in) [26].
In the Gulf of Panama, artisanal fishing has historically been sustained and considered of economic importance for coastal communities; however, between 1980 and 1985, the natural banks began to disappear, due to overfishing and the El Nino phenomenon [25].
The history of “conchuela” fishing dates back to the 1960s, with the first official statistics from the Panamanian government recording those vessels harvested around 300 metric tons of scallops per year (Figure 3) [27]. The information was not collected again until 1975 when the Directorate of Marine Resources at the time reported that 6.9t of adductor muscle, was worth U.S $5,699.
In 1976 exports totalled 143 tons worth of U.S $351,026, and the value per ton tripled, but the impact of harvesting was severe and there were no scallops available for harvest in 1977 [28]. Fishing resumed in 1982 when 26 tons were harvested, but in 1983 and 1984, exports fell to 3.9 tons and 1.5tons, respectively. Later, in 1985 and 1986, the scallop fishery expanded dramatically towards the sectors of the Veracruz beach up to the Farallon beach, province of Panama, where they were harvested at depths of 3 to 20m and in that same year (1985) beds were found of calico scallops in the Gulf of Panama, near San Miguel (Rey) Island, Tortola Island, Tortolita Island, Veracruz Beach, Farallon Beach and in Parita Bay [27].
At that time, twenty shrimp boats had licenses to fish scallops, which used large nets to harvest (Figure 4) using boats 13m (42ft) long in 1986 (Figure 5).
Due to the good oceanographic conditions, the great production of “conchuela” scallops in 1985 and 1986 resulted in a large settlement of mollusk seeds and a low density of predators. However, in 1985 predation increased rapidly, and after 1986 most scallops were decimated [29].
Again in 1987, there was an increase in the scallop population that introduced strong foreign currency into the national treasury, with exports reaching 41mt. During the first 6 months of that year, exports were 2,050mt, worth US$1 $10 million [30].
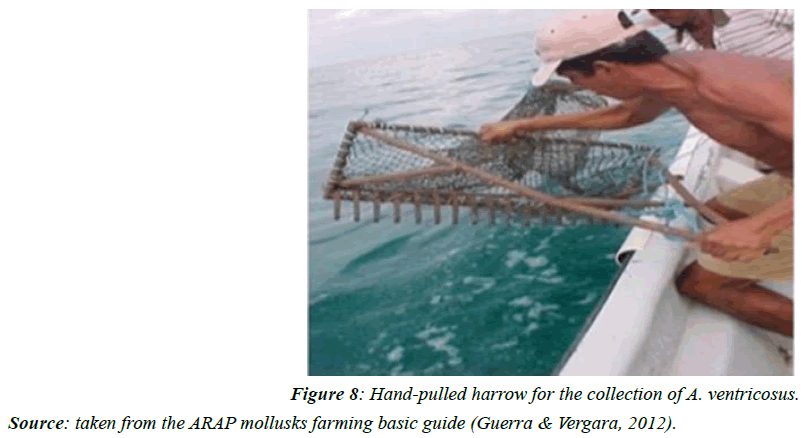
This fishery since its inception has been very fluctuating, during the years 1986-87; its exports represented currency in the order of 25million dollars, exported to the North American market, where the product is known as "bay scallop", which had great acceptance and demand [24]. In addition, 5m (16ft) long vessels powered by 25 or 40hp outboard motors (Figure 6) and small boats with crews of three, (Figure 7) using dredges and dredges (Figure 8) pulled by hand, which allowed him to harvest approximately half a barrel of scallops in 20 minutes of dredging, collecting approximately 20 barrels a day. A 20-bushel catch produced approximately 300pounds of muscle.
Puerto Caimito, a province of Panama, at that time was an important landing point for small scallop boats, with approximately 300 of them [27]. About 400 people, including fishermen, divers, cleaners, intermediaries, drivers, and assistants, worked in this fishery.
In the past decade, the scallops A. ventricosus kept the national market with annual landing rates of 20,000mt, but serious disturbances related to the capture of the resource drastically reduced production and in mid-1986 it disappeared again.
Between the years 1985-86, the shrimp boats brought the whole scallops to the Port of Vacamonte to sell them to large companies, whose workers extracted the muscle in the processing plants. On the contrary, the small fishermen in artisanal boats took them to the beach or some nearby ports such as Caimito, fished them, and packed them in 3.5kg plastic bags. Another group, the middlemen, sold the meat to exporting companies through the U.S for $6.30/kg. The fishery provided almost 35,000 jobs.
The distribution of income among the different workers was 70% for fishermen, 17.5% for peelers, and 12.5% for intermediaries [31]. Most of the scallop meat was sold to the United States; air shipments were made from Panama docks to US retail outlets and took no less than 48hours.
At the time, the scallop fishery brought fishing conflict between the shrimp boats and the small boats, the crews of these boats affirmed that the shrimpers were exhausting the beds of “conchuelas”. To resolve the conflict, the Directorate of Marine Resources ruled that shrimp boats be excluded from fishing for scallops within 4.5km (3 miles) of the coast [32].
Currently, the situation of the scallop fishery in the Gulf of Panama has completely collapsed, and the fishermen have resorted to commercially valuable fish and shrimp. The collapse is attributed to various causes, including the short useful life of the scallops, predation, overfishing, and the lack of a good management plan for the resource, the internal oceanographic variations of El Niño that negatively affected recruitment and the deterioration of the environment caused by pollutants [7].
The predictable future of the scallop fishery would again reach the production that was obtained in the period 1985 to 87 when the environmental conditions of the time offer conditions that can promote a high density of plankton, similar to that of 1985 and that guarantees the presence of scallop’s larvae in large quantities, in addition to the presence of predators that functioned as species of ecological balance at the time.
On the other hand, if a high density of scallops occurs again, the Aquatic Resources Authority (ARAP) must take immediate actions, among which the quality control of the product can be pointed out, which must include good sanitary conditions, of the processing plants, in addition to the storage area in the boats for the commercialization and innocuous marketing that must include new preparation techniques to taste it.
Regarding artificial farming, the future has a good prognosis, due to the economic value that the commercialization of its adductor muscle represents, however, the use of some techniques applied in aquaculture related to the reproductive stages controlled in its feeding, allows obtaining progeny of quality [33], in addition to the advantages of repopulating natural banks, which have been affected by natural phenomena and overfishing [31]. On the other hand, it is important to be careful about infections caused by certain marine pathogens that can generate effects in the early stages of the development of the species. In this sense [34] certain pathogens produce high mortality in the larvae and juveniles of the scallop, particularly Vibrio alginolyticus [35], for which the use of probiotics that help the immune system of bivalve mollusks is recommended, even when studies of these organisms are quite incipient [36,37].
Bivalves have always been subject to the invasion of parasites and predation, it is necessary to ensure the health of the resource, due to the implications it has as a food resource. In 1977, a heavy infestation of a larval stage of a digenetic trematode and a minor infestation of a cestode in the adductor muscle of scallops were reported, but the parasites turned out not to be harmful to humans, for this reason, it is recommended to establish a plan of permanent sanitary monitoring to guarantee the commercialization of the species from the zoo sanitary point of view [38].
It is necessary to carry out monitoring of the scallops fishery that includes the collection of oceanographic and fishing data, an ecological study of the natural banks and new tests of the reproductive cycle, and the application of possible modern aquaculture techniques that can provide seeds to repopulate the depleted beds of the scallops specimens, in addition to establishing fishing licenses for boats, fishing gear and types of nets and creating a legal regulation of temporary prohibition of captures or closed seasons, either by weight, size of the specimens and stage of the periods in specific breeding areas, in addition to changes in early reproductive stage and organism size [39,40].
Regarding biodiversity, observations made in situ report that species of portunid crabs, gastropods, octopuses, starfish, and rays are natural predators that generate risks in the juvenile and adult stages of the scallops. Exposure to these predators affects the thickness of the shell and the adductor muscle, which increase, further developing these structures as a survival strategy [41,42], which is why it is proposed to carry out monitoring studies on the condition of natural banks, especially in the periods of reproduction that occur as a result of seasonal variations in the Gulf of Panama [16] and low temperatures accompanied by high densities of phytoplankton during the dry season [17], conditions that explain the relationship between the environmental factors and reproductive status of A. ventricosus [26].
Leukoma asperrima (Venus clam)
The white clam L. asperrima, formerly Protothaca asperrima, has been a food source since the last century, distributed along the American Pacific coast, from Baja California to Peru [43]. It is a food resource that dates back to the last century, but the problem of contamination and heavy extraction has caused a strong reduction, which is endangering the banks of this species [10].
The exploitation of this clam, typical of sandy beaches and mangroves, has constituted a resource of great nutritional and economic importance in some countries of the region, including ours, which has been extracted daily and permanently, stored in large quantities for sale, due to the attractiveness of its meat (Figure 9).
Records of collections of L. asperrima indicate that it reaches average sizes between 29 and 33mm, and its growth is subject to seasonal changes in the area [38], with a predominance of the smaller ones between March to June [39]. Its shell is rough and has a sub-circular to sub-ovate shape, characterized by a striking coloration, with fine edges, ribs, and bright chocolate brown spots with a chalky white or cream background, where rays and dark patches predominate with a protruding chocolate lunule [23].
Based on the characteristics of the shell, [46] two subspecies of L. asperrima and L. histrionica are distinguished. The latter has a generally white ground colours, with three or more discontinuous radial rows of brown finger-like markings. It is mainly found in areas of the heterogeneous substrate, made up of remains of shells and fine mangrove roots mixed with sand and mud [43]. The species mentioned above inhabit muddy sandy beaches, in particular, Playa Bique (Arraijan), Playa Leona (Chorrera), Chepo, Punta Chame, and Puerto Caimito, located in the province of Panama and the beaches of Garachine and Taimiti in Darién, and Farallon in Cocle [47].
The fishermen of the communities mentioned above are made up of low-income inhabitants from the interior of the Republic, who have dedicated themselves to manually harvesting L. asperrima during periods of low tide, where other clams that grow in these sectors, as is the case of L. staminea, Iliocchione subrugosa (formerly Chione subrugosa) and Donax sp., making extraction a non-selective practice. These last three species are captured in smaller quantities, compared to L. asperrima, they have traditionally been used as a source of cheap protein for their diet; In addition to representing the economic income for the sustenance of the fishing families in the area, as it is a product of high consumption by the Panamanian population and quoted in local restaurants [1].
The first report of the capture of this product was made for Playa Bique, where the Bique River flows, which exerts its pollutant discharges in the coastal zone [46], one of the points of greatest collection where it was observed how the inhabitants of the place, they extracted large quantities of this mollusk [48]. Since then, studies have continued in the area, on topics such as sexual maturation, vertical migration, seasonal growth, and relationship with phytoplankton [49- 54], in addition to reproduction [38] and biomass [46], which has continued to report the exploitation of this product on said beach. These investigations lead us to suggest that the population of L. asperrima, despite this exploitation, has remained stable.
Over time, the extraction of this clam has become a traditional fishery of the fishing communities of the sector and to date, there have been no reports of controls or records of the extraction. Currently, the harvests continue to be carried out daily and are transported for sale to local markets. The sites with the largest catches par excellence are Puerto Caimito and Playa Bique, the latter being the sector where the activity occurs with more intensity. The population extracts large amounts of this clam to meet consumer demand and its sale is reflected in the economic income of the communities in the sector.
Some sector research is still being carried out in the area, but aspects of the biology of this species, such as knowledge of reproduction, which makes it possible to guarantee the implementation of closed seasons, are not very well known; For example, the spawning season for this species has not been clearly defined, but studies [46] suggest periods in January, associated with coastal upwelling that guarantees larval development, and October, the period of greatest rainfall, subject to climatic variations which must be confirmed [55], but the presence of other important bivalve mollusks species has been observed in the natural environment, which is incorporated into the manual captures by artisanal fishermen, as is the case of Donax asper, D. californicus, D. carinatus, D. dentifer, and D. gracili, belonging to the Family Donacidae [56], but they are not as abundant as the white clam.
There is a need for studies related to the identification, evaluation, and rational use of the resource to have greater clarity about the information gaps that exist on the species that are being collected since clams continue to be extracted in the sector throughout the year.
The future is not very predictable for the extraction of L. asperrima, due to the lack of diverse studies in the sector. The characteristics and composition of the sediments in the area, directly related to the benthic community, define its structure, condition, and type of substrate, which these bivalves inhabit, characterizing these communities and making them different within the same region.
Factors such as environmental variations, the amplitude of the tide, and the impacts of the discharges generated in the adjacent areas, with high amounts of organic matter, by the fishing population that continues to grow, in addition to the construction of port facilities, transport operations of oil and especially the operations carried out in the surroundings of the Panama Canal, are activities that take place in the region near Playa Bique, however, the organisms have adjusted to these situations, and their abundance will depend on the number of larvae that can settle in the benthos and their ability to adapt to the stressful environment and the predators of the sector.
Even though the Panama Canal contributes between 3 to 6% of the transit of ships in world trade, it was estimated that after its expansion, the exchange towards the east coast of the United States would increase by 32% [57], this increase of transit in the Canal, increases the availability of Tributyltin (TBT) used in the antifouling paint of ships, a moderately toxic substance, however, it affects marine organisms, especially mollusks by affecting the endocrine system and producing imposex from concentrations of 0.06 to 2.3μg/l of TBT [58].
Imposex has been evaluated in various species of mollusks on the Pacific coast of Panama, near the main access to the Canal, including Acanthais brevidentata, Bostrycapulus calyptraeformis, Crepidula cf. nivea and Anachis fluctuata [59], especially in mollusk capture sites for commercial purposes, such as Bique beach, imposex was identified in Thais kiosquiformis [60], however, significant concentrations of other contaminants besides TBT have been reported, among these, DBT, MBT, irgarol, diuron, DCOIT which have not been evaluated in mollusk tissue [61], the effect of this physiological characteristic in species of commercial interest is unknown.
It is not ruled out that the activities mentioned above and the pollution problem, especially of a biological nature, as is the case of the red tides that have been detected in the area, can cause health problems by incorporating these bivalves as part of the diet of the residents and the community in general, by ingesting saxitoxins in concentrations greater than those established, and which has been regulated through the resolutions established in the Official Gazette from the country.
Although the death of corals has been determined in the Pacific areas of Costa Rica and Panama, where dinoflagellates that produce Harmful Algal Blooms (HAB) abound, such as Cochlodinium catenatum and Gonyaulax monilata, [62] and Gymnodinium catenatum in the entrance of the Channel [63]; HAB events produced by Noctiluca scintillans and Lepidodinium chloroform have been reported in the Gulf of Panama [64]. The direct relationship between these and mollusks of commercial interest has not been studied.
Until now, Playa de Bique continues to be important for the inhabitants of the area, who practically depend on and dedicate themselves exclusively to the extraction of the resource, where it is observed that in most of the collection points there is no predominance by any of the species that are captured and a good distribution of individuals by species continues to be maintained. However, it is necessary to incorporate sociocultural and economic programs in the communities related to the regulation and sustainable management of the resource to guarantee the production of clams.
Despite the permanent exploitation, the population of L. asperrima seems to remain stable, however, the strong extraction has caused a decrease in this resource, endangering not only the very existence of the fishery for this organism, but also the possibility of acquiring economic resources for the sustenance of the families, so this leads us to suggest the implementation of management techniques and models to improve the situation of collections of the populations of L. asperrima, and other groups that live associated. As it is an important species in the feeding of the fishing community, it is necessary to incorporate early warning systems (EWS) against the bloom of dinoflagellates that produce red tides whose neurotoxic toxins produce conditions associated with intoxication in humans, in addition to carrying out microbiological studies. Whose results serve to alert the population in terms of health?
Anadara tuberculosa (mangrove cockle)
One of the fundamental components of the marine environment is the mangroves, where the Phylum Mollusca is well represented [4], in particular the edible species, which are associated with the mangrove roots and can be good indicators due to their abundant presence or absence [65].
The mangrove cockle, A. tuberculosis, has been a food source for low-resource populations and is recognized as a cheap source of protein in tropical and subtropical areas [66], becoming one of the most exploited and important resources in the world the Pacific coast of Panama (Figure 10).
The species is distributed from the Gulf of California to Tumbes, Peru [4], characterized by living buried in muddy substrates, to depths of 15cm [67], in clayey or silt-clayey soils in the flooded area of mangroves [68]. and in the mangrove roots of the species Rhizophora mangle, R. harrisonni, and Pelliciera rizophora; permanently receive abundant organic matter from the tidal impact [69], increasing the availability of nutrients from which it feeds [70]. Its rapid growth makes the black shell one of the most important commercial renewable resources in mangrove forests, due to a large amount of biomass it produces in a short time, and it is considered the most palpable form of conversion and transfer of energy to the upper links of the food chain [71].
Historically A. tuberculosa has been a source of food subsistence as part of the culture of the communities that are settled in the nearby mangrove area in the Panamanian Pacific. Due to its economic importance, its traditional use as food has become an item for local marketing as an alternative income source for the livelihood of many families [72].
In Panama, no legislation regulates the commercial size of A. tuberculosa, which is a limiting factor for its sustainable use; while, in other countries, the catch size has been set from 47 mm onwards [73].
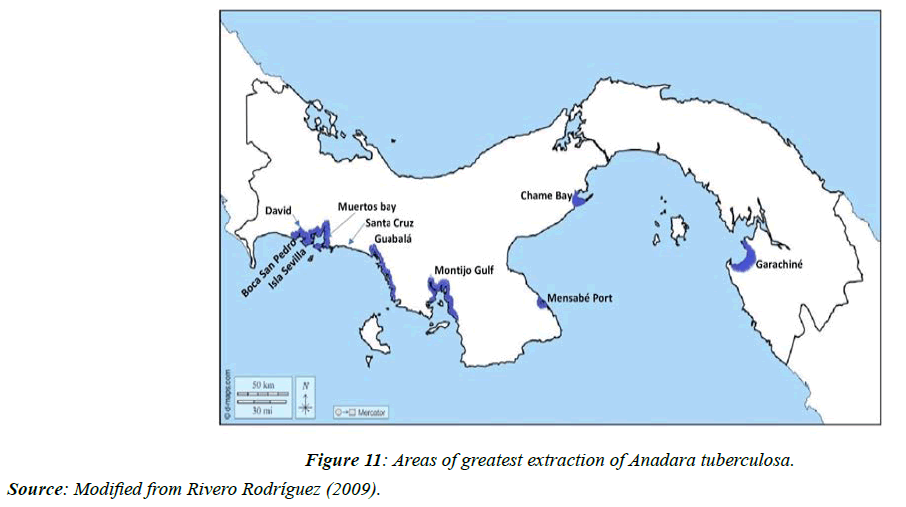
The predominant extraction zones for this species in the country are the provinces of Veraguas, Azuero, Panama, and Darién (Figure 11).
The lack of information that exists in the collection areas and the importance that this resource represents as a subsistence economic activity for the residents of the community has been a topic of discussion to guarantee sustainability.
It has been possible to determine that the extraction of the resource during the last years has been increasing due to its export and commercialization to the United States, Guatemala, and other nearby countries, which has motivated the communities of the extraction sectors to dedicate more effort to the activity.
The method of extraction has traditionally been manual and the sale of the species dates back more than four decades and has mainly served to support families in the Sajalices, Chame, and Montijo sectors. In its beginnings, the sale price was around 0.40 cents per dozen and due to the reduction caused by excessive extraction and deforestation of mangroves, prices have increased since the U.S. $1.00 to US $1.50. Distribution for purchase is through intermediary groups that distribute the shells.
Due to the importance that the species has had as a food source, studies related to fishing aspects and meat yield [74- 78], condition index [79], biometry [77-79], reproductive aspects [80,81], growth rate [82], population density [71,78], distribution [68,74], contamination [83] and biological evaluation [84].
Figure 12 shows one of the sales positions located along the country's Inter-American Highway.
The black shell as a source of protein and economic resource for the inhabitants of the Panamanian Pacific coast has become one of the most critical and exploited resources, particularly in Las Huacas area and other sectors of the Gulf of Montijo that the species is fast-growing and yield values of 33.88% with a condition index of 54.02 and sizes between 48.70 and 51.10mm that reflects an appropriate condition for the species to be marketed, in the case, particularly from the Gulf of Montijo, most specimens reach 45-65mm [84]. The reported size variation may be associated with the change of season, where contributions of continental origin generated by runoff bring the availability of nutrients [91].
The exploitation of the resource is quite rudimentary. Among the collection gear, bags are used to store the shells, and the primary tool is the hands of those who extract them (Figure 13).
The inputs required for the catches (fuel, boat, rowing boats, etc.) are used by the collecting groups to transport themselves, and they divide the effort only in transport. When the capture areas are close and accessible to the mangrove, they walk directly to the collection sites.
It has been detected that there are no formal organizations and collector groups are not associated to carry out extractive activities. The collections are made in family groups where women, men, and children or close friends are included and the yield benefits of the extraction are individual.
The collection activity is carried out during all the months of the year, during periods of low tide. Some collectors are selective in the extraction, the carvings that are not commercial are returned to the mangrove. In some cases, these carvings use for their consumption at the end of their work.
The maximum yield values (33.88%), the high sizes reported (48.7 and 50.5mm) and the average densities of 1.82org/m2 of A. tuberculosa, at the extraction site, are associated with the favorable conditions of the substratum of the sector, considered suitable for the survival and quality of the black shell, reasons that are sufficient to consider that the species is not overexploited and can continue to be marketed in the future [84].
Even though the species has not yet reached maximum extraction levels, its use as a food source requires great care, since, due to its ability to filter through its gill tissues and the body wall as a route for nutrient intake, it brings with it. trace metals from the sediments and the seawater column [85] or fluvial contributions, due to the effect of soil erosion and runoff waters that are considered during the rainy season that, since they are not assimilated, accumulate in visceral tissues [86,87].
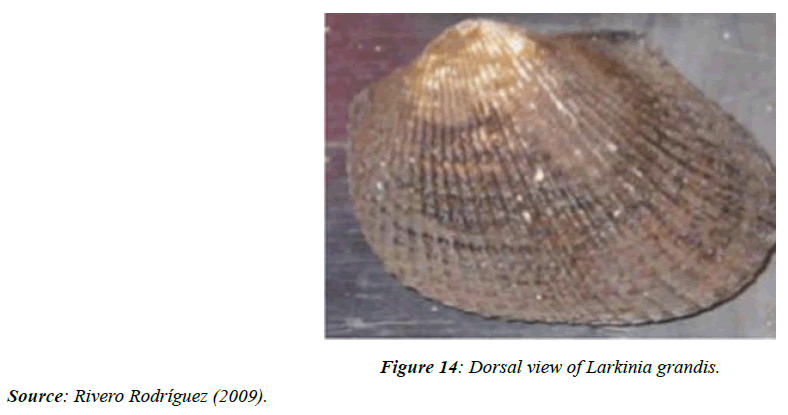
Larkinia grandis (mangrove cockle)
There are few studies on Larkinia grandis and the oysters of Panama. In the particular case of L. grandis, previously classified as Anadara grandis, it is found in mangrove areas in the Gulf of Panama (Figure 14). Before Europeans arrived in the Americas, the Indians used their shells as knives, as [88] describes after an archaeological study at Sitio Conte, Coclé.
The species can reach sizes up to 145mm [89], distributed in the sandy intertidal zone of mangrove estuaries [90].
Its extraction has been permanent for many years, like L. tuberculosa, there is no control over the sizes or the number of organisms, and its abundance has been significantly reduced, so both species are in danger of extinction Today, this clam is it is exported to other Central American countries, where it is consumed in "seviche" [91].
The information presented on these species makes it possible to establish important recommendations to be made, particularly oriented towards the problems of organic contamination and especially trace metals and their toxic effects, given the importance they have for the communities settled in the area and other sectors that include them in their diet, in addition to strategies that significantly guarantee the rational management of the populations of A. tuberculosa and L. grandis, due to the importance they have for the sustenance and improvement of the quality of life of the human populations in the environment. Similarly, the good practice of laboratory seed production and selection of suitable sites that meet the environmental conditions to repopulate natural banks is recommended. In this sense, the Aquatic Resources Authority of Panama has already been carrying out studies, since little information is available on these species [92].
Crassostrea corteziensis Cortez oyster, known as the native or pleasure oyster, is recognized for the flavour of its meat (Figure 14) and considered superior to that of other oysters, as is the case of C. gigas [93]. It is characterized by living in brackish water associated with mangrove roots [94] and is distributed from the Gulf of California to Panama [16] (Figure 15).
Oysters of the Crassostrea genus are considered one of the most important groups from the fisheries and aquaculture perspective [88]. In Panama, there are few studies on these groups and what is reported are capture figures at the level of artisanal fishing for food sustenance.
Some other species such as C. gigas or Japanese oysters (introduced species), Ostrea iridescens, and O. columbiensis; are edible and have shells that are rough on the outside and white with purple spots on the inside. They are harvested on rocky bottoms, especially in intertidal zones in mangrove areas, and are arranged gregariously (Figure 16).
Pinctada mazatlanica (pearl oyster)
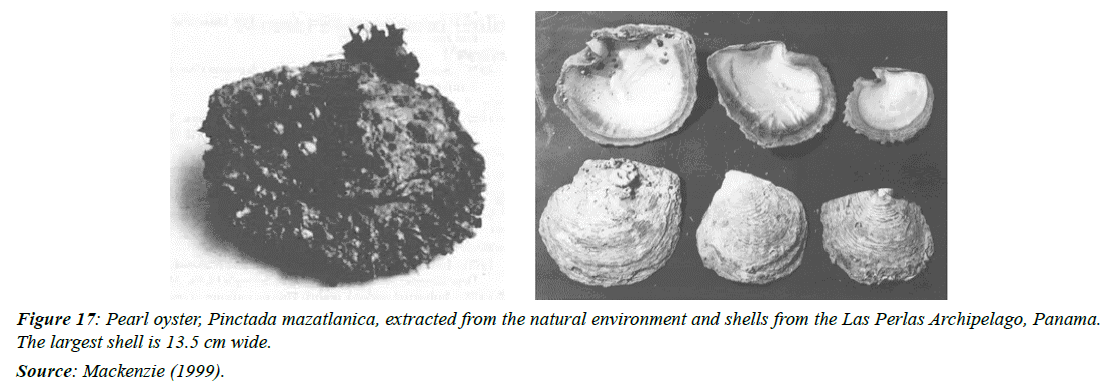
The pearl oyster, Pinctada mazatlanica, has a thick brown to gray shell and a maximum shell height of 10-12cm (4-4.75inches) (Figure 17). It extends along the Pacific coast from Baja California to Peru [16]. In Panama, this oyster is produced in the Gulf of Chiriquí and Panama, on rocky bottoms, where it is fixed by strong ligaments and characterized by not being gregarious [95].
Before the arrival of the Europeans, the Indians used to harvest oysters by diving without any type of gear to eat only the meat, but they did not use the pearls because they did not know their value [2]. The Spanish began oyster harvesting in small rowboats and sailboats called "brigantines". At the time, a small boat could be built from a single tree and could carry up to eight people [96].
The first records of pearl oyster fisheries date back to the 16th century, when the Spanish, including Vasco Núñez de Balboa in 1513, harvested pearls in the Gulf of Panama, particularly in the Pearl Archipelago, islands inhabited by indigenous people who extracted the Pacific pearl oyster. Historical records reveal that Balboa had extracted 96 ounces of pearls in just four days [97].
At first, the Spanish employed Indians to dive for pearl oysters, but disease and poor diet reduced their numbers. By the end of the 16th century, African divers had replaced the Indians, as they had more resistance to tropical diseases. The Spanish monarchy imposed various taxes on products brought from the New World, including pearls [96]. Pearls from Panama were sold in Santo Domingo (Dominican Republic) and European cities such as Seville, Venice, Antwerp, Nuremberg, Hamburg, and Lisbon.
Due to the quality of the type of pearl that was extracted in Panama and its abundance, the European market was flooded, which became a source of wealth for the wealthiest families, it is reported that the largest pearl known as "La peregrina" was sent to the Spanish crown and was extracted by a black slave, transited through France and Ireland and then returned to the new world, which was acquired by Elizabeth Taylor and auctioned for 11.8 million dollars after the death of the actress [97].
During the 17th century, pearl prices declined as some countries began producing imitation glass "pearls". During the 18th century, the Spanish continued to extract pearls from oysters, employing 400 divers and 230 small boats for extraction work in Panama. In 1812, an estimated 500 people harvested oysters, receiving a total income of 35,000 pesos. Around the same time, Panama declared its independence from Spain in 1821 and immediately joined Colombia.
Oyster fisheries continued, but in 1855, the industry declined when many divers gave up oysters and went to work building the Trans-Isthmus Railroad. When construction was finished, oyster harvesting resumed. Shortly after, overfishing in the Gulf of Panama caused a reduction and there is a change to continue harvesting in the Gulf of Chiriquí. In 1903, Panama seceded peacefully from Colombia and oyster harvests continued, and from 1900 to 1940, profits from pearl oysters were high, with exports subsequently declining during World War II.
1913 was the time when four major companies and many small groups were harvesting oysters. The largest company owned two large 100-ton ships. Each of these large vessels had an auxiliary fleet of 10 small boats approximately 10.5m (35ft) long, with crews of 10, including crew, divers, and inspectors. Divers were paid U.S. $1.25 for each quintal (100 pounds) of oysters harvested, with some harvesting up to 7 quintals per day.
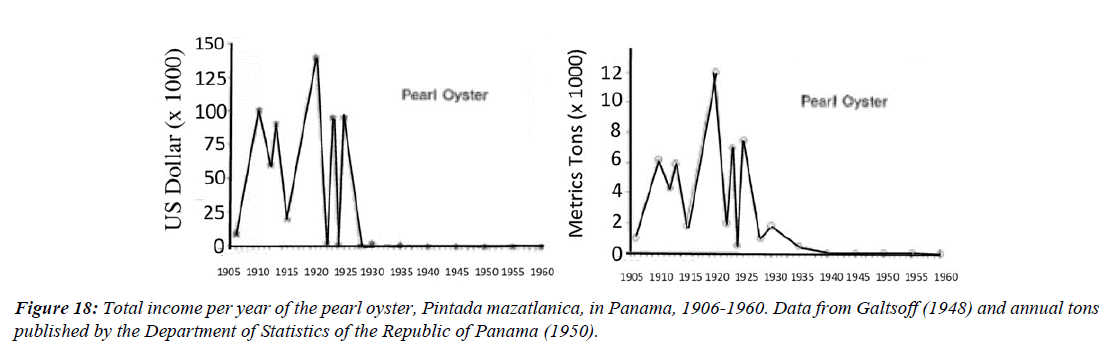
Figure 16 shows the records of income and weight in tons of pearl oyster catches from 1906 to 1960. The information was reported by [14,95] who describe the income and tons of extraction reported from 1905 to 1930, From then on, little was possible to record until 1960. The sales transactions were private and there were no official records, data were only available from 1933 to 1938, but they were still very useful to see the trend toward the reduction of the pearl fishery from 1925 to 1938.
When the Panamanian government showed data indicating that annual exports of pearl oysters had reached 700 tons represented by 2,000,000 pearl oysters, extracted since 1925, it was concluded that overfishing was the main reason why the fishing industry of this species failed (Figure 18).
Fisheries were active during those periods, and pearl oysters were harvested around several islands in the Gulf of Chiriquí and Panama, including Cébaco, Coiba, Taboga, Otoque, Pacheca, Saboga, Chapera, Pedro González, and San Miguel (Rey). During the 1940s, pearl exports began to decline. Although the causes were never documented, some people claimed that the Japanese poisoned the beds, while others blamed overfishing.
From 1944 to 1945 the fishery was resumed and pearl oysters were still found, although, in very small quantities, the data showed that there were no landings between 1948 and 1960, but a reduction in the species [14].
Schools of P. mazatlanica studied in 1950, did not believe that poisoning was the cause of the decline because it would have affected many other species, not just pearl oysters [95]. He also ruled out diseases and parasites, although he found that Nematopsis and Bucephalus were present in the pearl oyster; and he ruled out the deterioration of the bottom because the divers who collected the oysters did not damage or alter the bottom.
The first oyster fishing regulations were issued by the president of Panama, Belisario Porras, who, to restore the fishery, recommended that fishing for this species be prohibited and an investigation into its fishery be initiated. Oyster overfishing was reported in the area in the 16th century, similar to what happened in Panama, which forced the cessation of oyster fishing in the country for many years, reason enough to create regulations.
In this sense, the Fiscal Code was implemented through Law 8 of January 27, 1956 [98]: that in Chapter V on hunting and fishing includes in articles 290 to 294 regulations on the fishing of the mother-of-pearl shell and establishes the acquisition of the fishing license, the number of people and equipment to be used, the amount of the right that corresponds to the National Treasury and the areas within which the holder of the license can fish, aid to the municipalities destined in favour of them twenty percent (20%) of the product, the fishing of the motherof- pearl shell by a system of divers, either head or mechanical apparatus in waters less than eight (8) fathoms deep, at the lowest tides and the regulation for extraction that prohibits the use of dredges, trawlers or any other similar device. Likewise, it is forbidden to throw substances into the hatcheries that can destroy them, to fish for shells whose size is less than thirtyfive millimeters (35mm) in diameter.
Due to strong fishing pressure and the deterioration of ecosystems in many places, oyster populations have been declining, and in some cases have disappeared, causing various ecological and economic repercussions.
History in the Panamanian Caribbean
In the Panamanian Caribbean, the tidal range is 30 cm, and the fishing settlements are very close to the coast. The most exploited area, concerning mollusks, corresponds to the province of Bocas del Toro (Figure 19) and the San Blas region, particularly in Bocas del Toro, with a West Indian population that is characterized by incorporating mollusks into their frequent diet seafood.
References
- Rodriguez FA. Snails and claims: Uses, conservation and economic aspects in Panama. In Natural History of the Isthmus of Panama. 2015;242.
- Cook R, Sanchez LA. Archeology in Panama (1880-2003). In University Commission of the centenary of the Republic (ed.) Panama one hundred years of the Republic. 2004;3-65.
- Isaza Aizpurua II. The ancestors of Parita: Pre-Columbian settlement patterns in the lower La Villa River valley, Azuero Peninsula, Panama. Unpublished PhD. dissertation, Department of Anthropology, Boston University, Boston. 2017;717.
- Mayo C, Cook R. The pre-Hispanic industry of marine shells in Gran Cocle, Panama. Technological analysis of shell artifacts from the midden-workshop of the Cerro Juan Diaz Site, Los Santos, Panama. Archaeofauna. 2005;14:285-98.
- Cook R, Sanchez LA. Contemporary metallurgy, shell crafts and painted ceramics on Juan Diaz Hill, Gran Cocle, Panama. Gold Museum Newsletter. 1997;42:56-85.
- Linne S. Darien in the Past: The Archeology of eastern Panama and north-western Colombia. Elanders Boktryckeri Aktiebolag, Gothenburg. 1929;318.
- Mayo C. The pre-Hispanic industry of marine shells in "Gran Cocle", Panama. PhD Thesis, Complutense University of Madrid. 2004;367.
- Carvajal Contreras DR, Hansell P. Mollusks in Central Panama: A review. Archaeofauna. 2008;17:157-74.
- Martin-Rincon JM, Rodriguez F. Marine molluscs from Panama Viejo. Resource selectivity from a long-term perspective. Canto Rodado. 2006; 1: 85-100.
- Villalaz JR, Gomez JA. Scheme of bivalve culture in Panama. Aquaculture Research in Latin America, Pudoc Wageningen. 1987;310-19.
- Prieto A, Montes A, Ruiz LJ. Biomass production potential in a natural population of the oyster Crassostrea rhizophorae, in the Grande de Obispo lagoon, Gulf of Cariaco, Venezuela. Interscience. 2008;33(10):747-53.
- Morales VV, Muniz JL. Diagnosis of bivalve mollusc aquaculture in Panama. International Co-operation of the Spanish Agency with Panama. 1992;150.
- D'Croz L, Arosemena G. The Biological Inventory of the Panama Canal: The Marine Study. I. University of Panama, Vice President for Research and Postgraduate; 1994;1:1-598.
- Mackenzie CL, Burrel VG, Rosenfield A, et al. The history, present condition, and future of the molluscan fisheries of North and Central America and Europe. U.S. In NOAA Technical Report NMFS. 1997;3:33-40.
- Glynn PW. Observations on the ecology of the Caribbean and Pacific coasts of Panama: 13-30. The Panamic biota: Some observations prior to a sea level canal. Bull Biol Soc Wash. 1972;2:13-30.
- Smayda TJ. A quantitative analysis of the phytoplankton of the Gulf of Panama. III. General ecological conditions, and the phytoplankton dynamics at. Inter-Amer Trop Tuna Comm Bull. 1966;11(5):355-612.
- Forsbergh ED. On the climatology, oceanography and fisheries of the Panama bight. Bull Inter-Amer Trop Tuna Comm. 1969;14:49-259.
- D'Croz L, Del Rosario JB, Gomez JA. Upwelling and phytoplankton in the Bay of Panama. Rev Biol Trop. 1991;39(2):237-45.
- D'Croz L, Robertson DR. Coastal oceanographic conditions affecting coral reefs on both sides of the Isthmus of Panama. Proc 8th Int Coral Reef Symp. 1997;2:2053-8.
- Bayne BL. Marine mussels: Their ecology and physiology. Cambridge University Press. 1985;506.
- Fay C, Neves R, Pardue G. Bay scallop. Fish and Wildlife Service. 1983;23.
- ARAP. Regional exchange, experiences in the extraction and culture of Central American mollusks. Extraordinary Meeting Report. 2011;25.
- Keen AM. Seashells of tropical west America (2nd Ed). Stanford Univ Press Stanford Calif. 1971:1064.
- Vergara-Lopez P, Mazon-Suastegui JM, Guerra-Lima ZI, et al. Advances in the marine culture of conchuela Argopecten ventricosus in the Republic of Panama. Ministry of Agri Devt (MIDA). 2002:4.
- Villalaz J, Gomez JA. Larval development of Argopecten circularis. Science. 1990;5(1):7-11.
- Villalaz J Gomez JA. Experimental study of the gametogenesis of the tropical conchuela Argopecten circularis in the Gulf of Panama. Science. 1989;4(1):7-23.
- Arosemena D, Martinez A. Contribution to the fishing biological knowledge of the conchuela (Argopecten circularis), exploited in the Gulf of Panama. Minist Comer Ind Direction General de Recursos Mar. 1986:22.
- USDOC. Industrial outlook report: Fishing industry (Panama). U.S Dep Commer Nat Tech Inf Sen Springfield Va. 1979:53.
- Villalaz J. Reproductive biology of Argopecten circularis. Univ Delaware Newark, PhD Thesis. 1992:100.
- Anonymous. The Latin American scallop fisheries 1980-86. Mar Fish Rev. 1987;49(1):72-6.
- Financial Gazette. The conchuela industry: Much manoco.1986;4(11).
- Gaceta Oficial. Decree 6. Ministry of Commerce and Industries. Issues transitory measures on conchuela fishing Official Gazette 20740. 1987.
- Caceres C, Alarcon FJ. Histological and histochemical studies of the digestive system of the clam Argopecten ventricosus (Sowerby, 1842). Bol Inst Spec Oceanogr. 2002;18(1-4):289-92.
- Romalde JL. Heroes and villains: Bacteria associated with shellfish farming. AquaTIC Magazine. 2016;37: 45-59.
- Cordero H, Esteban MA, Cuesta A. Use of probiotic bacteria against bacterial and viral infections in shellfish and fish aquaculture. Sustainable Aquaculture Tech. InTech. 2014; 239-65.
- Sanchez-Ortiz AC, Luna-Gonzalez A, Campa-Cordova AI, et al. Isolation and characterization of potential probiotic bacteria from pustulose ark (Anadara tuberculosa) suitable for shrimp farming. Latin American Journal of Aquatic Research. 2015;43(1):123-36.
- Abasolo‐Pacheco F, Saucedo PE, Mazon‐Suastegui JM, et al. Isolation and use of beneficial microbiota from the digestive tract of lions‐paw scallop Nodipecten subnodosus and winged pearl oyster Pteria sterna in oyster aquaculture. Aquaculture Res. 2016;47(10):3042-51.
- Iversen ES. Report on the analysis of Panamanian conchuela samples. In D Arosemena A Martinez (eds.). Contribution to the fishing biological knowledge of the conchuela (Argopecten circularis), exploited in the Gulf of Panama. Ministry Eat. Ind. General Directorate Sea Resources. 1978:22.
- Hoverman J, Auld J, Relyea R. Putting prey back together again: integrating predator-induced behavior, morphology, and life history. Ecology. 2005;144:481-91.
- Guerra C, Maeda-Martinez AN, Hernandez-Llamas A, et al. The influence of temperature and presence of predators on growth, survival, and energy allocation for reproduction in the Catarina scallop Argopecten ventricosus. Aquacult Res. 2011; 43: 756-66.
- Delgado GA, Glazer RA, Stewart NJ. Predator-induced behavioral and morphological plasticity in the tropical marine gastropod Strombus gigas. Biol Bull. 2002;203:112-20.
- Lafrance M, Cliche G, Haugum GA, et al. Comparison of cultured and wild sea scallop Placopecten magellanicus, using behavioral responses and morphometric and biochemical indices. Mar Ecol Prog Ser. 2003;250:183-95.
- Palacios JA, Rodriguez JA, Cruz RA, et al. Study of the biology of Protothaca asperrima (Pelecypoda: Veneridae), reproductive cycle. Brenesia. 1986;25:23-32.
- Chamizo AN. Study of growth and reproduction in Protothaca asperrima (Sowerby, 1835) during the rainy and dry season in Puerto Caimito (October 1994 to March 1995). Bachelor Thesis, School of Biology. University of Panama. 1997:145.
- Lopez I, Gutierrez A. Study of the biomass and reproduction of the white clam Protothaca asperrima (Pelecypoda: Veneridae) in Playa Bique, Arraijan. Graduate Thesis, University of Panama. 1998:87.
- Olsson AA. Mollusks of the tropical Eastern Pacific Particularly from the Southern of the Panamic Pacific faunal province (Panama to Peru). Paleontological Research Inst Ithaca New York. 1961;574.
- Lopez I, Luna IG, Gutierrez A, et al. Relationship of the gonadal development of the white clam (Protothaca asperrima) (Pelecypoda: Veneridae) with the rate of oxygen consumption. Technoscience. 2003;5(2):87-96.
- Aguila Y, Luna IG, Villalaz JR. Zonacion de una playa arenosa fangosa. Tesis de Licenciatura, Universidad de Panama. 1978:135.
- Munoz EA, Diaz CA. Some aspects of the sexual maturation and production of Protothaca asperrima in the Panama Bay. Degree Thesis. University of Panama. 1984:63.
- Telesca AT, Visuetti IA. Study of the growth and horizontal migration of Protothaca asperrima (Sowerby, 1835) (Lamellibranchia: Veneridae) in Bique Bay, Panama. Bachelor's Thesis University of Panama. 1985:120.
- Marciaga BB, Mencomo J. Seasonal influence on the growth and reproduction of Protothaca asperrima, (Sowerby 1835); and their variations in the energy substrates. Bachelor's Thesis, School of Biology. University of Panama. 1993:87.
- Barcenas S, Guzman N. Effect of phytoplankton concentration on the growth of Protothaca asperrima under laboratory conditions. Bachelor's Thesis, University of Panama. 1996:125.
- Lopez I, Luna IG, Gutierrez A, et al. Reproductive cycle of the white clam Protothaca asperrima (Pelecypoda: Veneridae) at Playa Bique, Arraijan. Technoscience. 2005;7(2):43-53.
- Posada JM, Piedra A, Ross E, et al. Commercially important marine invertebrates on the Pacific coast of Panama. AquaDoc. 2014;121.
- Liu Q, Wilson WW, Luo M. The impact of Panama Canal expansion on the container-shipping market: a cooperative game theory approach. Maritime Policy & Management. 2016;43(2):209-21.
- UNEP. Regionally based assessment of persistent toxic substances: Central America and the caribbean regional report/united nations environment programme, chemicals; global environment facility. Policy Commons. 2002:133.
- Li C, Collin R. Imposex in one of the world’s busiest shipping zones. Smithsonian Contributions to the Marine Sci. 2009;38:189-96.
- Rodriguez F, Alvarado NE. Biomonitoring for TBT contamination; Incidence of imposex towards the western sector of the Panamanian Pacific. Analytical Magazine. 2014;2:18-21.
- Batista Andrade JA, Caldas SS, Batista RM, et al. From TBT to booster biocides: levels and impacts of antifouling along coastal areas of Panama. Environmental Pollution. 2018;234:243-52.
- Guzman HM, Cortes J, Glynn PW, et al. Coral mortality associated with dinoflagellate blooms in the eastern Pacific (Costa Rica and Panama). Mar Ecol Prog Ser. 1990;60:209-303.
- Gomez Herrera JA. Soler A. Red tide in the Pacific of Panama. scientia, 1991;6(1):81-93.
- Seixas C. Two reports of red tides on the coasts of the Gulf of Parita, Pacific of Panama. Vision Antataura. 2017; 1(2): 97-98.
- Lee S. Mangrove macrobenthos: Assemblages, services and linkages. J Sea Research. 2008;59:16-29.
- Broom MJ. The biology and culture of marine bivalve mollusks of the genus Anadara. ICLARM Stud Rev. 1985;12:37.
- Mackenzie C Jr. A history of the pearl oyster fishery in the Archipielago de las Perlas, Panama. Marine Fisheries Review. 1999;61(2):58-65.
- Cruz RA, Jimenez JA. Molluscs associated with mangrove areas of the Pacific coast of Central America. Heredia, Costa Rica: UNA Foundation Editorial. 1994:182.
- Quizhpe PF, Yanez DM, Jimbo JI. Growth and fattening of black shell (Anadara tuberculosa) in pens in the mangroves of Payana Island. Technical University of machala. Conference Proceedings. 2017;1(1):33-6.
- Canas FA, Sierra SO. Proximal bromatological analysis in Anadara tuberculosa (hairy shell) from the Bay of Jiquilisco Department of Usulutan El Salvador. [Bachelor Thesis, University of El Salvador]. 2019.
- Campos JA, Fournier ML, Soto R. Estimation of the population of Anadara tuberculosa (Bivalvia: Arcidae) in Sierpe Terraba, Costa Rica. Tropical Biology J. 1990;32(2b):477-80.
- INRENARE/OIMT. Descriptive study of the terrestrial fauna associated with the mangrove ecosystem in the areas of Azuero, Chame and Chiriqui. Mangrove Management, Conservation and Development Project. 1995a:50.
- FAO. Integral management of a mangrove area. Terraba-Sierpe Forest Reserve, Costa Rica, Basic information. Prepared for the Government of Costa Rica by the Food and Agriculture Organization of the United Nations (FAO). 1988:140.
- Squires HJ, Esteves M, Barona O, et al. Mangrove cockles, Anadara spp. of the Pacific Coast of Colombia. Veliger. 1975;18:57-68.
- Betancourt J, Cantera J. Ecological and economic study of the piangua. Seminar on the South American Pacific Ocean, Cali, 1978 Set. 1976:6.
- Borda AC, Cruz R. Artisanal fishery for Anadara tuberculosa and Anadara similis bivalves and their relationship with environmental events. J Mar Res. 2004;25(3):197-208.
- Cruz RA, Palacios JA. Biometry of the Anadara tuberculosa mollusk (Pelecypoda: Arcidae) in Punta Morales, Puntarenas, Costa Rica. Tropical Biology J. 1983;31:175-9.
- Silva A, Bonilla R. Abundance and morphometry of Anadara tuberculosa and A. similis (Mollusca: Bivalvia) in the Purruja mangrove swamp, Golfo Dulce, Costa Rica. Tropical Biology J. 2001;49(2):315-20.
- Cruz RA. Monthly variation of the condition index of the Anadara tuberculosa mollusk (Pelecypoda: Arcidae) in Punta Morales. Puntarenas Costa Rica. Tropical Biology J. 1982;30:1-4.
- Sibaja WG. Sexual maturity in the chora mussel Mytella guyanensis Lamarck, 1819 (Bivalvia: Mytilidae) from the mangrove swamp in Jicaral, Puntarenas, Costa Rica. Tropical Biology J. 1986; 34(1):151-5.
- Ampie CL, Cruz RA. Size and sexual maturity of Anadara tuberculosa (Bivalvia: Arcidae) in Costa Rica. Brenesia. 1989;31:21-4.
- Villalobos CR, Baez AL. Tasa de crecimiento y mortalidad en Anadara tuberculosa (Bivalvia: Arcidae) bajo dos sistemas de cultivo en el Pacifico de Costa Rica. Rev Lat Acuic. 1983;1-54.
- Duran IL. Determination of heavy metals in Anadara tuberculosa in the Mangrove swamp of Taborcillo Island, Punta Chame. Bachelor's Thesis, University of Panama. 1999:101.
- Jordan LY, Gomez JA. Biological evaluation of Anadara tuberculosa, Gulf of Montijo, Republic of Panama. Technoscience. 2006;8(2):191-205.
- Del Cid A, Carneiro D, Tunon O, et al. Biometry and gonadal development of the black shell Anadara tuberculosa in Estero Cate, Gulf of Montijo. Technoscience. 2001;23(1):276-92.
- Fuentes MV, Gomez JA. Trace metals in Ascidia nigra (Savigny, 1816) collected in coastal areas of Sucre State, Venezuela. Scientia. 2001;15(2): 45-5.
- Duran IL, Gomez JA. Concentration of Fe, Cu and Zn in the tissues of A. tuberculosa (Pelecipoda. Arcidae) during the rainy and dry season (October 1998-March 1999) in Punta Chame, Panama, Rep. Panama. Scientia. 2001;26(1):43-51.
- Lathrop SK. Cocle: An archaeological study of central Panama. Peabody Museum of Archaeology and Ethnology, Harvard University. 1937;8.
- Fischer W, Krupp F, Shneide W, et al. Guide for the identification of species for fishing purposes. Central-Eastern Pacific. Volume I Plants and Invertebrates. FAO. 1995:646.
- Duran IL, Fuentes MV, Gomez JA. Concentration of cadmium, lead and copper in Anadara tuberculosa in the mangrove swamp of Taborcillo Island, Punta Chame, Republic of Panama. Technoscience. 2004;6(2): 91-104.
- Rivero Rodriguez S. Diagnosis of the cultivation and extraction of molluscs in Central America. Towards a regional strategy. OSPESCA/CETMAR Foundation of the Xunta de Galicia. 2009:87.
- ARAP. Basic guide for the culture of bivalve molluscs from the Panamanian Pacific: Conchuela, oysters and black shell. Internal Report. 2012:36.
- Stuardo J, Martinez A. Relationals between some ecological factors and the biology of populations of Crassostrea corteziensis (Hertlein, 1951) from San Blas, Nayarit. Center for Marine Science and Limnology. Mexico. Univ National Auton Mexico. 1975;2(1):89-130.
- Mazon-Suastegui JM. Culture of Japanese oyster Crassostrea gigas (Thunberg). In: Casas-Valdez M. and G. Ponce-Diaz (Eds.), Study of the Fishing and Aquaculture Potential of the State of Baja California Sur, Mexico. CIBNOR S.C., La Paz, B.C.S. Mexico. 1996;2:625-50.
- Galtsoff P. The pearl-oyster resources of Panama. U.S Dep Inter Fish Wildl Serv Spec Sci Rep Fish.1950;28:53.
- Camargo M. Pearl and mother-of-pearl shell fisheries in Panama. Rev Lottery. 1983;326-327:32-76
- Rodriguez FA. Snails and clams: Uses, conservation and economic aspects in Panama. In Rodriguez, FA., O'Dea, A (edn). Natural History of the Isthmus of Panama. 2005;139-53.
- Gaceta Oficial. Ley 8. Por el cual se aprueba el codigo fiscal. Gaceta Oficial. 12995.1956.
- Viloria E, Acero A, Blanco J. The collapse of the striped mojarra Eugerres plumieri (Pisces:Gerreidae) fishery in the Cienaga Grande de Santa Marta: Fishing, environmental or biological causes?. Bol Invest Mar Cost. 2012;41(2):399-428.
- Cramer K. History of the human impact on the coastal ecosystems of the Panamanian Caribbean. In Natural History of the Isthmus of Panama. Smithsonian Tropical Institute. 2015:242.
- Cordoba DE, Aviles MC, Valdes I, et al. Diversity of mollusks (Bivalves and Gastropods), which serve as a source of food on Isla Colón, Bocas del Toro province, Panama. Technoscience. 2010;12(1):23-33.
- FAO. The state of the world's food and agriculture. Global analysis, regional analysis, agricultural development financing. 1986;191.
- ARAP. National report on the fishery for Strombus gigas. Presentation. 2016;10.
- Official Gazette. Executive Decree 159. By which a closed period of the sea snail Strombus gigas is established in the Republic of Panama. official Gazette 24963. 2004.
- Gaceta Oficial. Executive Decree 98. By which the national plan of the Republic of Panama is approved to prevent, discourage and eliminate illegal, unreported and unregulated fishing. official Gazette. 26413. 2009.
- Uribe E. Production of larvae and juveniles of marine species. Cent Int Invest Dev. 1988;184.
- Escobar J. River pollution and its effects on coastal areas and the sea. CEPAL; 2002.
- Miloslavich P, Klein E, Diaz JM, et al. Marine biodiversity in the atlantic and pacific coasts of south America: knowledge and gaps. PloS one. 2011;6(1):e14631.
- Villalaz J, Gomez JA. Outline of Panama. In Proceedings of the Workshop on Aquaculture in Latin America. Int Fund Sci culture of bivalves. 1986; 310-319.
- Gomez JA, Villalaz J. Annual cycle of sexual maturation of the conchuela argopecten circularis. Science. 1988;3.
- Villalaz J, Gomez JA. Larval development of Argopecten circularis. Science. 1990; 5(1):7-11.
- Villaláz, J. Laboratory study of food concentration and temperature effect on the reproductive cycle of Argopecten ventricosus. J Shell Res. 1994; 12(2):516-519.
- Villalaz JR. Morphometric and biochemical-changes in 2 age classes of the tropical scallop, argopecten-ventricosus, under laboratory conditions. Am Malacol Bull. 1994;11(1):67-72.
- Mexican Commission for Cooperation with Central America and the Caribbean. Mexican cooperation with Central America and the Caribbean 2003. Technical Secretariat. 2004; 76.
- Vergara Lopez P, Mazon Suastegui JM, Guerra Lima ZI, et al. Advances in the marine culture of conchuela Argopecten ventricosus in the Republic of Panama.
- MIDA. Report 2004. Ministry of Agricultural Development. 2004; 96.
- Gómez JA, Villalaz JR. Induction of spawning by serotonin in the tropical bivalve Lyropecten nodosus. In Memories II National Aquaculture Congress. 1991:13-15.
- Medina B, Guzman HM, Mair JM. Failed recovery of a collapsed scallop Argopecten ventricosus fishery in Las Perlas Archipelago, Panama J Shellfish Res. 2007;26(1):9-15.
- Financial Capital. Low production of shellfish in Panama. 2012.
- Jansen H, van den Bogaart L. Blue carbon by marine bivalves: Perspective of carbon sequestration by cultured and wild bivalve stocks in the Dutch coastal areas. Wageningen Marine Research; 2020.
- Yao Z, Xia M, Li H, et al. Bivalve shell: Not an abundant useless waste but a functional and versatile biomaterial. Crit Rev Environ Sci Technol. 2014;44(22):2502-30.
- Baker P, Baker SM. Carbon mineralization associated with aquaculture of the Northern Quahog Mercenaria mercenaria. J Shellfish Res. 2019;38(3):519-27.
- Bevelander G, Nakahara H. An electron microscope study of the formation of the nacreous layer in the shell of certain bivalve molluscs. Calcif Tissue Int. 1969;3(1):84-92.
- Schäffer TE, Ionescu-Zanetti C, Proksch R, et al. Does abalone nacre form by heteroepitaxial nucleation or by growth through mineral bridges?. Chemistry of Materials. 1997;9(8):1731-40.
- Moore D, Heilweck M, Fears MD, et al. An assessment of the potential value for climate remediation of ocean calcifiers in sequestration of atmospheric carbon. Science Open Preprints. 2022.
- Alonso AA, Alvarez-Salgado XA, Antelo LT. Assessing the impact of bivalve aquaculture on the carbon circular economy. J Clean Prod. 2021;279:123873.
- Bertolini C, Bernardini I, Brigolin D, et al. A bioenergetic model to address carbon sequestration potential of shellfish farming: Example from Ruditapes philippinarum in the Venice lagoon. ICES Mar Sci Symp 2021;78(6):2082-91.
- Ray NE, Maguire TJ, Al-Haj AN, et al. Low greenhouse gas emissions from oyster aquaculture. Environ Sci Technol. 2019;53(15):9118-27.
- Filgueira R, Byron CJ, Comeau LA, et al. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar Ecol Prog Ser. 2015;518:281-7.
- The state of world fisheries and aquaculture 2022. FAO. 2022; 236.
- Tamburini E, Turolla E, Lanzoni M, et al. Manila clam and mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci Total Environ. 2022;848:157508.
- Aquaculture Advisory Council. Recommendation on carbon sequestration by molluscs. 2022; CCA2022-16, 9.
- Álvarez-Salgado XA, Fernández-Reiriz MJ, Fuentes-Santos I, et al. CO2 budget of cultured mussels metabolism in the highly productive Northwest Iberian upwelling system. Sci Total Environ. 2022;849:157867.
- Munari C, Rossetti E, Mistri M. Shell formation in cultivated bivalves cannot be part of carbon trading systems: a study case with Mytilus galloprovincialis. Mar Environ Res. 2013;92:264-7.
- Wang H, Ge C, Mao Y, et al. Effect of hybrid abalone, haliotis discus hannai×haliotis discus discus, cultivation on the carbon cycle: Carbon source/sink. J World Aquac Soc. 2016;47(5):720-8.
- Fodrie FJ, Rodriguez AB, Gittman RK, et al. Oyster reefs as carbon sources and sinks. Proc Royal Soc B. 2017;284(1859):20170891.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref