Research Article - Biomedical Research (2017) Volume 28, Issue 9
Histomorphology of chemoradiotherapy-induced oral mucositis in patients with gastrointestinal cancer
Ping-Gong Du1, Hua-Wei Liu2, Wen-Ting Bi3, Lin Feng2*1Department of Oral and Maxillofacial Surgery, Yantai Stomatological Hospital, Yantai, PR China
2Department of Stomatology, Chinese PLA General Hospital, Beijing, PR China
3Department of Stomatology, the First Cooperation of Chinese and Western Medicine Hospital in Beijing, Beijing, PR China
Accepted date: January 30, 2017
Abstract
Objective: To study the histomorphological changes of oral mucosa by observing the sections of lip buccal mucosae from patients with gastrointestinal cancer.
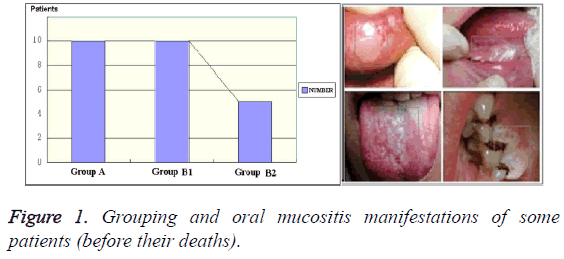
Methods: Oral mucosae from 15 patients aged 61-80 years old who died of gastrointestinal cancer were sampled. Group A (n=10) had received high-dose chemoradiotherapy. Group B (n=10) were divided two subgroups. Group B1 (n=5) had gastrointestinal cancer (before chemoradiotherapy), and Group B2 (n=5) consisted of healthy adult subjects. Oral mucosa (1 cm × 3 cm) was collected, prepared into 5-7 μm-thick paraffin sections, stained with haematoxylin-eosin, and observed under a light microscope. Meanwhile, indirect immunoperoxidase reaction was performed.
Results: No. 5 sample in Group A showed the atrophy rate of mucosal epithelial cells was 100%. No. 6 sample showed that the autologous mucosal layer was attenuated, and the hypodermal mucosa was loosened and arranged in deposited fibers. Besides, 75% of No. 3 and 9 samples showed moderate focal or diffusive inflammatory infiltrations of lymphocytes-macrophages in the basal layer of oral mucosa. The nerve fibers and muscles in 20% of No. 2 sample underwent dystrophy.
Conclusion: The oral mucosae of patients receiving chemoradiotherapy suffered from atrophy of epithelial cells and infiltration of numerous cells. In deeper layers, there was relaxation of the hypodermal mucosa as well as dystrophy of nerve fibers and muscles. The deep submucosa exhibited atrophic muscle fibers. In the meantime, the neuromodulation system was injured, especially in nonhyperplastic and hyperplastic muscle fiber bundles.
Keywords
Chemoradiotherapy, Oral mucosa, Morphology, Gastrointestinal cancer.
Introduction
Oral mucositis is the most important acute complication of chemoradiotherapy, especially in patients with bone marrow stem cell transplantation who receive myeloablative chemotherapy and those receiving radiotherapy for malignant tumors of oral and surrounding structures [1,2]. This disease evidently affects cancer treatment outcomes [3]. Generally, oral mucositis only invades the epithelium. Chemotherapy and radiotherapy directly injure the basal cells of mucosal epithelium and affect their regeneration, so there are no new cells in the basal layer and the existing ones migrate to the surface and shed. As a result, the epithelium is gradually attenuated, giving mucosal erythemas that finally develop into ulcers. Overall, chemoradiotherapy injures the oral mucosa on the DNA level [4], which has attracted global attention [5,6]. Under the same conditions, not all cancer patients are complicated with oral mucositis, the reasons for which remain elusive [7,8]. Chemoradiotherapy regimens induce significantly high levels of acute toxicity than radiotherapy alone. Chemotherapy introduces systemic toxicity and exacerbates local tissue reactions when combined with radiotherapy. In this case, mucositis is recognized as the principal factor limiting further effective treatment [9]. Radiotherapy and multiple-drug chemotherapy that are used concurrently have apparent interactions [10]. In this study, we studied the histomorphological changes of oral mucosa by observing the sections of lip buccal mucosae from patients with gastrointestinal cancer under a light microscope, also depending on the results of immunoperoxidase reaction.
Materials and Methods
Oral mucosae from 15 patients aged 61-80 years old who died of gastrointestinal cancer were sampled. We have obtained the "Ethical Committee Permission" for patient tissue sampling before the research (No. 2013223). Group A (n=10) had received high-dose chemoradiotherapy. Group B (n=10) was divided into two subgroups. Group B1 (n=5) had gastrointestinal cancer (before chemoradiotherapy), and Group B2 (n=5) consisted of healthy adult subjects (Figure 1). Samples of Group A were collected from of the morgue of our forensic unit. Oral mucosae (1 cm × 3 cm) were collected from lip buccal mucosae. The samples were fixed in 10% neutral formalin, paraffin-embedded, prepared into 5-7 μm-thick paraffin sections, stained with Haematoxylin-Eosin (HE), and observed under a light microscope (lens: X40 and × 90 magnifications, ocular lens: X7 magnification). Meanwhile, indirect immunoperoxidase reaction was conducted to detect leukocytes and the reaction with Leukocyte Common Antigen (LCA). Besides, CD-3, CD-20, CD-4, CD-8 and CD-56 leukocytes were also detected. All experiments were carried out by Leica Microsystems Inc. and Morphology Master video test system.
The injuries of cancer to oral mucosa were first examined before determining the negative effects of chemoradiotherapy. All patients with gastrointestinal cancer received oral examinations to clarify the states of dental hard tissues, periodontal tissues as well as mouth and lip mucosae before sampling. Oral mucosal smears were prepared by scraping and smearing cells onto slides that were then oven-dried for 1 h, fixed in a 1:1 mixture of 96% ethanol and medical diethyl ether for 10 min, stained with the Romanowski-Giemsa method, and observed under the light microscope (lens: X40 and X90 magnifications, ocular lens: X7 magnification).
Results
Morphological changes
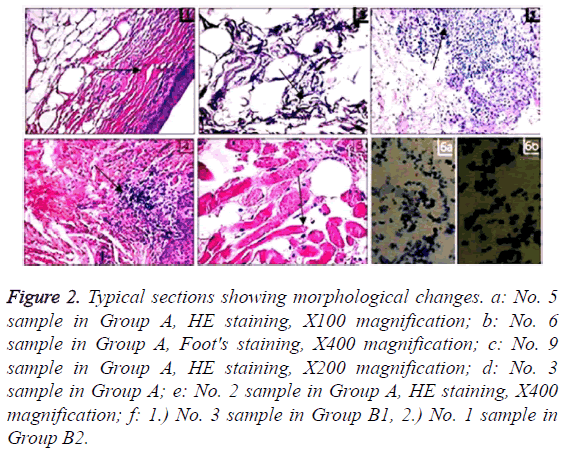
For No. 5 sample in Group A, mucosal epithelial cells had the atrophy rate of 100% (Figure 2a). As to No. 6 sample, the autologous mucosal layer was attenuated, and the hypodermal mucosa was loosely arranged in deposited fibers (Figure 2b). Moreover, 75% of No. 3 (Figure 2c) and No. 9 (Figure 2d) samples showed that lymphocytes-macrophages underwent moderate focal or diffusive inflammatory infiltrations in the basal layers of oral mucosae. Additionally, 20% of No. 2 sample was subjected to dystrophy of nerve fibers and muscles (Figure 2e). Figure 2f shows that samples from Groups B1 and B2 have similar initial states in intact oral and lip mucosae as well as injured mucosae.
Figure 2: Typical sections showing morphological changes. a: No. 5 sample in Group A, HE staining, X100 magnification; b: No. 6 sample in Group A, Foot's staining, X400 magnification; c: No. 9 sample in Group A, HE staining, X200 magnification; d: No. 3 sample in Group A; e: No. 2 sample in Group A, HE staining, X400 magnification; f: 1.) No. 3 sample in Group B1, 2.) No. 1 sample in Group B2.
Results of immunoperoxidase reaction
The results of immunoperoxidase reaction are summarized in Table 1, being consistent with the morphological changes under the light microscope. Clearly, chemoradiotherapy induced the immune response of hosts and release of leukocytes.
| A5 | A9 | A6 | A3 | A2 | B13 | B21 | |
|---|---|---|---|---|---|---|---|
| LCA | - | + | + | - | + | + | + |
| CD-3 CD-20 | - | - | - | + | + | + | + |
| CD-4 | + | - | - | - | - | - | + |
| CD-8 | - | - | + | - | - | + | + |
| CD-56 | + | + | + | - | + | + | - |
Table 1: Results of immunoperoxidase reaction.
Discussion
The pathogenesis of chemoradiotherapy-induced oral mucositis remains unclear, which has now mainly been attributed to direct or indirect injuries. Direct injury means the mucosa is directly injured by anticancer agents or radiation on the 5th~14th day on average, further inducing the apoptosis of oral mucosal epithelial cells. Indirect injury means oral mucosa is injured by the loss of saliva due to release of inflammatory mediators, radiation or anticancer agents [11-13]. In addition, methotrexate contributes to oral mucositis because of enhanced adaptability to the immune system function, manifested as increase in oral lymphocytes [14].
The pathophysiological process of chemoradiotherapy-induced oral mucositis is divided into start-up phase, inflammatory/ vascular phase, epithelial phase, ulcer/bacteria phase and recovery phase [15]. In the inflammatory phase, epithelial, endothelial and connective tissues are injured, releasing free radicals, modified proteins and primitive inflammatory cytokines such as interleukin (IL)-1β, prostaglandin and tumor necrosis factor α. Such inflammatory mediators directly or indirectly aggravate vascular permeability, increase the accumulation of cytotoxic factors, and finally exacerbate tissue injury. In contrast, release of anti-inflammatory factors such as IL-11 offsets early inflammatory response [16]. The epithelial phase starts 4-5 days after radiotherapy, during which the injury degree is directly correlated with the proliferation rate of oral epithelial tissues. Young patients are prone to radiationinduced oral mucositis and recover rapidly, whereas oral basal cells of the elderly tend to undergo mitosis [17]. Meanwhile, the patients have high levels of transforming growth factor B1 before chemoradiotherapy, which plummet after treatment while oral mucositis occurs accordingly [18].
After one week of treatment with anticancer agents, the epithelium of oral mucosa collapses, leading to oral mucositis in the ulcer phase, loss of epithelial cells and fibrin exudation. As a result, pseudomembrane and ulcer form. In this phase, gram-negative bacteria and fungi grow in to the injured mucosal surface, accompanied by decrease in leukocytes to further aggravate the disease. Moreover, release of bacterial metabolites including endotoxin results in excessive oxidation of monocytes, further enhancing the release of inflammatory mediators such as IL-1, NO and tumor necrosis factor A [5]. For patients receiving chemotherapy, the recovery phase usually lasts for 12-16 days, which is predominantly controlled by proliferation of epithelial cells, recovery of hematopoietic function, rebuilding of local microflora, and lack of factors that impede recovery [15,19].
Chemotherapy-induced oral mucositis is related with chemotherapeutic agents, route of administration, treatment duration, dosage, supplementary drugs and previous mucosal toxicity treatment [20]. The risk of oral mucositis is increased due to continuous or repeated treatment with low-dose cytotoxic drugs, increase in chemotherapy cycle and history of this disease. The degree of radiotherapy-induced oral mucositis is associated with treatment duration, radioactive source, cumulative radiation dose, dosage, volume of radiated mucosa, history of smoking or drinking, and other factors such as dry mouth and oral infections [21,22]. In immunocompetent hosts, radiotherapy-induced oral mucositis often recovers within three weeks after the treatment terminates. Commonly occurring 7-10 days after treatment, oral mucositis induced by implantation radiation becomes most severe within two weeks. Such injuries, except for large areas of mucosal ones, usually recover within several weeks. This disease can also be triggered by disrupted mechanisms of metabolic enzymes and DNA modification, deficiency of folic acid and vitamin B12, anticancer agent-induced damage of liver and kidney functions, exudative pleural and peritoneal effusions, and delayed elimination of drugs owing to the use of special drugs (e.g. leucovorin) [23,24]. Potential decrease in haemocytes [15] and previous oral lesions such as dry mouth also promote the onset of oral mucositis. This symptom limits the production of saliva, weakens its buffering effect, as well as elevates its stickiness and acidity and oral IgA level, finally affecting the onset of dental caries [25,26].
In this study, changes in the mucosal epithelial cells are typified by atrophy of epithelial cells and invasion of numerous lymphocytes and macrophages. In deeper layers, there was relaxation of the hypodermal mucosa as well as dystrophy of nerve fibers and muscles. Deeper part of the submucosal layer showed atrophy of muscle fibers, manifested as injury of the neuromodulation system. Particularly, the proliferative and nonproliferative muscle fiber bundles were more prone to neural Injury. Similarly, it has previously been reported that complications of chemoradiotherapy were induced by intramucosal matrix components but not surface structures.
After high-dose chemoradiotherapy, the percentage of viable oral epithelial cells increases. Also, cells in the buccal epithelium shift from mature to immature, possibly due to desquamation of the upper oral epithelial layer. Newer treatment modalities that can circumvent the side effects of chemotherapeutic agents should be considered. Clinical trials with larger sample sizes will give more accurate results in this regard. Decreased loco-regional control, poorer quality of life and shortened overall survival have recently been associated with unplanned treatment breaks and reduction in dose intensity [27]. Therefore, such assessment aids in mucositis may be valuable in the future. The in vitro assay may also be useful as an adjunct in studies focusing on oral mucositis prevention.
Conclusion
The findings herein provide pathological and physiological evidence for the prevention and treatment of radiotherapy- and chemotherapy-induced oral mucositis. However, the mucosa was collected near the cheeks or lips with the area of 1 × 3 cm2, so we observed limitations of the study. We are looking forward to in-depth studies.
References
- Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Nigudgi S. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy-a randomized controlled trial. Support Care Cancer 2013; 21: 1421-1428.
- Gouvêa de Lima A, Villar RC, de Castro G, Antequera R, Gil E, Rosalmeida MC, Federico MH, Snitcovsky IM. Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys 2012; 82: 270-275.
- Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers. The patients perspective. Caner Nurs 2002; 25: 461-467.
- Fleckenstein J, Kuhne M, Seegmüller K, Derschang S, Melchior P, Gräber S, Fricke A, Rübe CE, Rube C. The impact of individual in vivo repair of DNA double strand breaks on oral mucositis in adjuvant radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011; 81: 1465-1472.
- Minhas S, Kashif M, Nagi AH. Evaluation of various nuclear cytological changes in normal buccal mucosa and peritumoural area in patients with oral squamous cell carcinoma receiving concomitant chemoradiotherapy. Patholog Res Int 2016; 2016: 6293795.
- Campos MI, Campos CN, Aarestrup FM, Aarestrup BJ. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol Clin Oncol 2014; 2: 337-340.
- Dreizen S. Description and incidence of oral complication. NCI Monogr 1990; 9: 11-15.
- Epstein JB, Schubert MM. Oropharyngeal mucositis in cancer therapy. Review of pathogenesis, diagnosis and management. Oncology 2003; 17: 1767-1779.
- Bensadoun RJ, Magné N, Marcy PY, Demard F. Chemotherapy- and radiotherapy-induced mucositis in head and neck cancer patients: new trends in pathophysiology, prevention and treatment. Eur Arch Otorhinolaryngol 2001; 258: 481-487.
- Phillips TL, Fu KK. Quantification of combined radiation therapy and chemotherapy effects on critical normal tissues. Cancer 1976; 37: 1186-1200.
- Silva GB, Sacono NT, Othon-Leite AF, Mendonca EF, Arantes AM, Bariani C, Duarte LG, Abreu MH, Queiroz-Junior CM, Silva TA, Batista AC. Effect of low-level laser therapy on inflammatory mediator release during chemotherapy-induced oral mucositis: a randomized preliminary study. Lasers Med Sci 2015; 30: 117-126.
- Ferreira B, da Motta Silveira FM, de Orange FA. Low-level laser therapy prevents severe oral mucositis in patients submitted to hematopoietic stem cell transplantation: a randomized clinical trial. Support Care Cancer 2016; 24: 1035-1042.
- Ibrahim EM, Mulhim FA. Effect of granulocyte-macrophage colony stimulating factor on chemotherapy-induced oral mucositis in non-neutropenic cancer patients. Med Oncol 1997; 14: 47-51.
- de Koning BA, van Dieren JM, Lindenbergh-Kortleve DJ, van der Sluis M, Matsumoto T, Yamaguchi K, Einerhand AW, Samsom JN, Pieters R, Nieuwenhuis EE. Contributions of mucosal immune cells to methotrexate induced mucositis. Int Immunol 2006; 18: 941-949.
- Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 1998; 34: 39-43.
- Sonis ST, Lindquist L, VanVugt A, Stewart AA, Stam K, Qu GY, Iwata KK, Haley JD. Prevention of chemotherapy induced ulcerative mucositis by transforming growth factor beta 3. Cancer Res 1994; 54: 1135-1138.
- Sari J, Nasiloski KS, Gomes APN. Oral complications in patients receiving head and neck radiation therapy: a literature review. Rev Gauch Odontol 2014; 62: 395-400.
- Chen HW. Change of plasma transforming growth factor-b1 levels In nasopharyngeal carcinoma patients treated with concurrent chemo-radiotherapy. Jpn J Clin Oncol 2005; 35: 427-432.
- Plevova P. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 1999; 35: 225-226.
- Riley P, Glenny AM, Worthington HV, Littlewood A, Clarkson JE, McCabe MG. Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy. Cochrane Database Syst Rev 2015; 12: 11552.
- Franzen L, Funegard U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer 1992; 28: 457-462.
- Rugg T, Saunders MI, Dische S. Smoking and mucosal reactions to radiotherapy. Br J Radiol 1990; 63: 554-556.
- Peterson DE, Ohrn K, Bowen J, Fliedner M, Lees J, Loprinzi C, Mori T, Osaguona A, Weikel DS, Elad S, Lalla RV. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer 2013; 21: 327-332.
- Lee JS, Murphy WK, Shirinian MH, Pang A, Hong WK. Alleviation by leucovorin of the dose-limiting toxicity of edatrexate: potential for improved therapeutic efficacy. Cancer Chemother Pharmacol 1991; 28: 199-204.
- Greenspan D. Oral complications of cancer therapies. Management of salivary dysfunction. NCI Monogr 1990; 159-161.
- Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, Quinn B, Epstein JB, Blijlevens NM, Waltimo T, Passweg JR, Correa ME, Dahllof G, Garming-Legert KU, Logan RM, Potting CM, Shapira MY, Soga Y, Stringer J, Stokman MA, Vokurka S, Wallhult E, Yarom N, Jensen SB. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer 2015; 23: 223-236.
- Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol 2007; 5: 23-31.