- Biomedical Research (2015) Volume 26, Issue 2
Histomorphological analysis of peri-implant bone tissue of an implant in use for 10 years: A case report.
Ramón Fuentes1*, Iván Valdivia2, Eduardo Borie2, Víctor Beltran2, Cristina Bucchi21CIMOFIR Research Centre, Dental School, Universidad de La Frontera, Temuco, Chile.
2Department of Odontology and Biomedical Department, University of Antofagasta, Antofagasta, Chile
- *Corresponding Author:
- Ramón Fuentes Fernández
Dental School Universidad de La Frontera
Manuel Montt 112 Temuco
Chile PC: 4781176
Accepted date: February 17 2015
Abstract
In this case report, it was described the histomorphology through optical microscopy of the implant- bone interface in an implant in function during 10 years, which had to be removed due to problems associated with the connection of the abutment. A 73-year-old male patient came asking for prosthesis mobility. Due the impossibility to replace the abutment it was decided to remove the implant with a 5 mm internal diameter trephine and analyze this sample histomorphologically. The histological analysis showed a mature trabecular tissue between the implant threads with a regular cell distribution and lamellar organization of calcified matrix, observing that bone tissue follows the shape of the implant’s surface with which it was in contact. Also, was observed low osteoclast activity, collagen type I, without signs of inflammation or resorption. In conclusion, implants have been shown in this case report, as well as by studies in humans to have long duration and outstanding biocompatibility that permits the formation of mature and regular peri-implant bone tissue in certain conditions.
Keywords
osseointegration, peri-implant tissue, bone-implant interface; histomorphology.
Introduction
The use of dental implants to support prostheses is a common practice today [1], because of its high success rate, which varies between 90% and 95% in patients with no or few risk factors [2]. However, the success and longterm duration of this type of treatment is directly related to the formation of a bone?implant contact [3], which is also referred as osseointegration. The osseointegration process was originally defined as a direct structural and functional connection between the bone and the implant [4], which has been histologically demonstrated [5]. This phenomenon is a complex process with a multitemporal and multiscale nature [6].
Bone has the ability to regenerate being indistinguishable from the underlying bone tissue [7]. Histological studies of properly osseointegrated implants describe newly formed bone tissue in close contact with the implant and with a regular organization [5]. Other studies in animals show similar histological results [8] and have also made it possible to detail the process of osseointegration of implants in successive periods of time [9], a matter that is difficult to achieve in humans for bioethical reasons.
Despite a high success rate [10], implant failure may occur in some cases. Early failure may be due to factors such as inadequate surgical techniques [11], quality of and/or insufficient bone, patient's unhealthy habits, patient's systemic disease, and contamination during surgery [12,13], among others. Late implant failure, defined as a pathological process involving an osseointegrated implant, is less understood but is classified as overload or infection [11].
The aim of this study was to describe the histomorphology through optical microscopy of the tissue around a dental implant functioning for 10 years, which had to be removed due to problems associated with the connection of the implant with the abutment.
Case report
Patient and procedure
A 73-year-old male patient without systemic diseases or negative health habits came asking about prosthesis mobility. At clinical examination, damage was identified in the inner thread of the posterior–superior endosteal implant. A review of his medical record indicated that the implant (Ankylos, Dentsply, New York, NY, USA) of 3.5 mm in diameter and 11 mm in length, had been in place for 10 years and 3 months. At the time of implantation, the bone level was optimal, so no ridge augmentation techniques were used.
No pain, swelling, or implant mobility were observed. However, due the impossibility to replace the abutment it was decided to remove the implant with a 5 mm internal diameter trephine. The patient agreed with the suggested treatment and authorized through an informed consent the implant site extraction and histological examination of the sample. After removal of the implant, the resulting defect was filled with a biomaterial (DynaGraft-D putty, Keystone Dental, Burlington, MA, USA) for achieve bone tissue regeneration in order to schedule surgical placement of a new implant at a later date.
Both the implant and the surrounding bone tissue were put in a bottle with 10% buffered formalin and stored at 4Cº for 3-4 weeks
Bone-implant separation and histological analysis
The sample was washed with PBS 1X 0.01M and then placed in a beaker with 300 mL of 10% ethylenediaminetetraacetic acid (EDTA) with 2% paraformaldehyde (pH 6.8) to partially decalcify and decrease the hardness of the bone tissue, generating a separation of the implant and bone tissue. Both solutions were changed every 6 hours for 15 days to obtain adequate decalcification.
Under a stereoscopic microscope with 10X magnification, it was analyzed at the bone–implant interface at the level of the bottom of the most apical thread, where a thin metal probe was carefully inserted to separate the tissue carefully with a micro-incision metal clamp serrated 20 Ga to hold its shape. Once completely separating the bone tissue from the metal, it was immersed in EDTA to complete its decalcification.
The tissue sample was cut 3 μm longitudinally with a microtome (Microm HM 325, Thermo Scientific, Florida, FL, USA) and subsequently stained using hematoxylineosin techniques, Picrosirius Red and Van Gieson’s stain. The samples were analyzed using an optical microscope (Olympus, Arquimed Innovation, Santiago, Chile) with magnification 4x, 10x and 50x. Histological analysis was performed on all sections obtained.
The histological analysis showed:
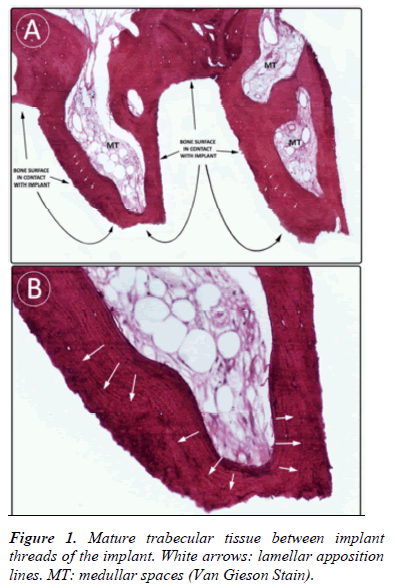
- A mature trabecular tissue between the implant threads with a regular cell distribution and lamellar organization of calcified matrix (Fig. 1).
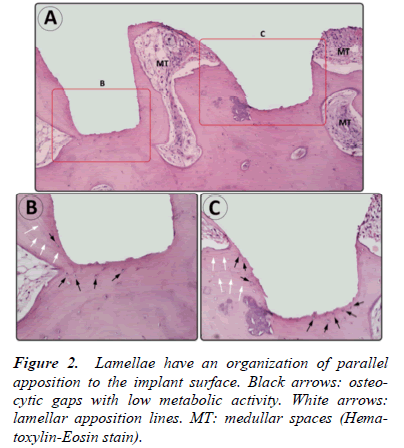
- Lamellae had a parallel organization to the surface of the implant, observing that bone tissue follows the shape of the implant’s surface with which it was in contact (Fig. 1 and 2).
- Sparse tissue fibroreticular regions and no presence of chondroid tissue; trabeculae described have morphology of bone tissue.
- The trabeculae showed moderate vascularity. Vessels of different sizes were observed both parallel and perpendicular to the surface of the implant
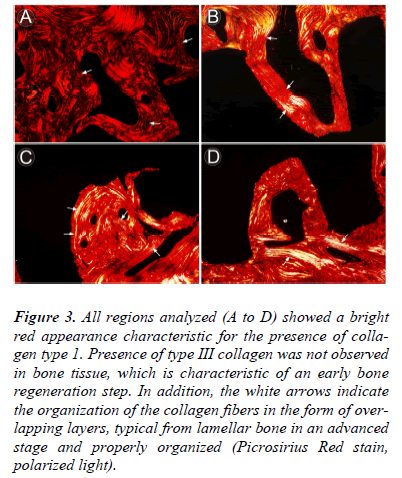
- Low osteoclast activity and collagen type I (Fig. 3).
Figure 3: All regions analyzed (A to D) showed a bright red appearance characteristic for the presence of collagen type 1. Presence of type III collagen was not observed in bone tissue, which is characteristic of an early bone regeneration step. In addition, the white arrows indicate the organization of the collagen fibers in the form of overlapping layers, typical from lamellar bone in an advanced stage and properly organized (Picrosirius Red stain, polarized light).
- Osteocytic lacunae had a consistent morphology with low metabolic activity (Fig. 2).
- No signs of inflammation or resorption activity were observed.
In general, the peri-implant tissue in this case report was a mature, regular, and vital bone, which was remodeled actively, presumably depending on the forces applied to the implant.
Discussion
Several studies have reported histomorphology of tissue surrounding implants with early loss [14,15] removed because of infection or mobility of the implant. In general, this is described and characterized histologically for the presence of stratified connective tissue, proliferation of epithelial tissue, and inflammatory cells, as well as a lack of osseointegration [14]. Also, there have been reports of late implant failure, because of peri-implantitis or excessive occlusal load [16,17]. In both cases, osseointegration loss is observed. The main histological features of peri-implantitis consist of the presence of bone sequestra near the implant, bacteria on the surface of the implant, and inflammatory infiltrates (macrophages, lymphocytes, and plasma cells) in the adjacent area [18].
The traditional classification of failure in endosseous implant is defined as early or late, the first being a failure to achieve osseointegration and the second the inability to maintain it. However, as observed in this study and others [5,19], implant failure can also occur as a result of factors not directly related to osseointegration as such, so this is important to consider. Therefore, histological descriptions of the implants removed for different failures from those classically described, are important evidence of bone– implant interaction.
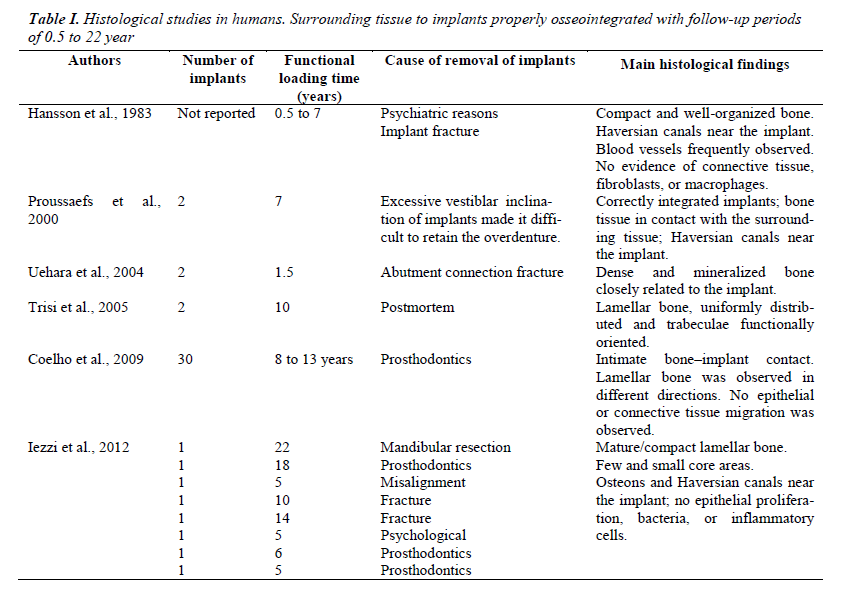
Several studies in humans and with follow-up periods between 0.5 and 22 years have confirmed histological findings described in this study (Table I). The studies reported to date indicate that the reasons for the removal of properly osseointegrated implants are of the following types: psychiatric [5,20], prosthetic [19-21], fracture of implant or the abutment [5,20,22] and/or mandibular resection [20]. Postmortem cases have also been reported, in which osseointegrated implants are removed for histological analysis [23]. Histology described in these articles is independent of patient gender, the position of the implant and time of functional loading of the implant. In general, it describes bone tissue in intimate contact with the implant [21,23] and regular and mature organization [5,20], as here described. In addition, osteocytes and Halversian systems are described near the bone–implant interface [5,19,20]. Finally, similar to that observed in this study, there is no evidence of connective tissue formation, presence of fibroblasts, macrophages, or inflammatory cells [5,20,21].
Conclusion
Endosseous implants have been shown in this case report, as well as by studies in humans, to have long duration and outstanding biocompatibility that permits the formation of mature and regular peri-implant bone tissue.
References
- Winnett W, Tenenbaum HC, Ganss B, Jokstad A. Perioperative use of non-steroidal anti-inflammatory drugs might impair dental implant osseointegration. Clin Oral Implants Res. 2014. doi: 10.1111/clr.12493.
- Pjetursson BE, Thoma D, Jung R, Zwahlen M, Zembic A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin Oral Implants Res 2012; 23: 22-38.
- Javed F, Vohra F, Zafar S, Almas K. Significance of Osteogenic Surface Coatings on Implants to Enhance Osseointegration Under Osteoporotic-like Conditions. Implant Dent 2014; 23: 679-686.
- Brånemark P-I, Hansson BO, Adell R, Breine U, Lindström J,Hallán O, Öhman A. Osseointegrated implants in the treatment of the edentulous jaw. Stockholm: Almqvist and Wiksell; 1977.
- Hansson HA, Albrektsson T, Brånemark PI. Structural aspects of the interface between tissue and titanium implants. J Prosthet Dent 1983; 50: 108-113.
- Mathieu V, Vayron R, Richard G, Lambert G, Naili S, Meningaud JP, Haiat G. Biomechanical determinants of the stability of dental implants: influence of the boneimplant interface properties. J Biomech 2014; 47:3-13.
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 1998; 355: S7-21.
- Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium andzirconium bone implants. Biomaterials 1985; 6: 97-101.
- Johansson C, Albrektsson T. Integration of screw implants in the rabbit: a 1-year follow-up of removal torque of titanium implants. Int J Oral Maxillofac Implants 1987; 2: 69-75.
- Curto, AA. Evaluación de las Tasas de Supervivencia Clínica de los Implantes Cortos: Revisión de la Literatura. Int J Odontostomat 2012; 6: 201-203.
- Tonetti MS, Schmid J. Pathogenesis of implant failures. Periodontol 2000 1994; 4: 127-138.
- Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Impl Res 2002; 13: 1-19.
- Martin W, Lewis E, Nicol A. Local risk factors for implant therapy. Int J Oral Maxillofac Implants 2009; 24: S28-38.
- Esposito M, Thomsen P, Ericson LE, Lekholm U.Histopathologic observations on early oral implant failures. Int J Oral Maxillofac Implants 1999; 14: 798-810.
- Piattelli A, Scarano A, Piattelli M. Microscopical aspects of failure in osseointegrated dental implants: a report of five cases. Biomaterials 1996; 17: 1235-1241.
- Sanz M, Alandez J, Lazaro P, Calvo JL, Quirynen M, van Steenberghe D. Histo-pathologic characteristics of peri-implant soft tissues in Brånemark implants with 2 distinct clinical and radiological patterns. Clin Oral Implants Res 1991; 2: 128-134.
- Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin Oral Implants Res 1996; 7: 143-152.
- Piattelli A, Scarano A, Piattelli M. Histologic observations on 230 retrieved dental implants: 8 years' experience (1989-1996). J Periodontol 1998; 69: 178-184.
- Proussaefs PT, Tatakis DN, Lozada J, Caplanis N, Rohrer MD. Histologic evaluation of hydroxyapatitecoated root-form implant retrieved after 7 years in function: a case report. Int J Oral Maxillofac Implants 2000; 15: 438-443.
- Iezzi G, Vantaggiato G, Shibli JA, Fiera E, Falco A, Piattelli A, Perrotti V. Machined and sandblasted human dental implants retrieved after 5 years: a histologic and histomorphometric analysis of three cases. Quintessence Int 2012; 43: 287-292.
- Coelho PG, Marin C, Granato R, Suzuki M. Histomorphologic analysis of 30 plateau root form implants retrieved after 8 to 13 years in function. A human retrieval study. J Biomed Mater Res B Appl Biomater 2009; 91: 975-979.
- Uehara T, Takaoka K, Ito K. Histological evidence of osseointegration in human retrieved fractured hydroxyapatite-coated screw-type implants: a case report. Clin Oral Implants Res 2004; 15: 540-545.
- Trisi P, Keith DJ, Rocco S. Human histologic and histomorphometric analyses of hydroxyapatite-coated implants after 10 years of function: a case report. Int J Oral Maxillofac Implants 2005; 20: 124-130.