Research Article - Biomedical Research (2017) Volume 28, Issue 11
High-performance liquid chromatography-tandem mass spectrometry for the determination of bile acid in mice serum
Tiantian Gao1, Chao Sun2, Hui Tang1, Ye Bi3, Yongfeng Song3 and Jian Zhang1*
1Department of Pharmacy, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China
2Department of Pharmacy, the Second Hospital of Shandong University, Jinan, PR China
3Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China
- *Corresponding Author:
- Jian Zhang
Department of Pharmacy
Shandong Provincial Hospital Affiliated to Shandong University, PR China
Accepted on March 21, 2017
Abstract
In this study, we developed and validated a High-Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS) method to determine bile acid in mouse serum. The serum samples were analysed after solid-phase extraction. The analytes were separated on a Diamonsil C18 column with a mobile phase of methanol and water containing 10 mmol/L ammonium acetate and 0.005% formic acid (70:30) at a flow rate of 0.5 mL/min. Analytes were detected by tandem mass spectrometry in negative ion mode. The results demonstrated that the calibration curve was linear for all bile acids over a range of 10-10000 ng/L. The specificity, matrix effect, recovery, linearity, accuracy, and precision were validated for bile acid in mouse serum. The HPLC/MS/MS method was selective, sensitive, and simple, and was applied successfully to determine the bile acid in more than 200 mouse serum samples. In conclusion, this method is suitable for the quantitative detection of bile acid.
Keywords
Liquid chromatography-tandem mass spectrometry, HPLC-MS/MS, Mice serum, Bile acid
Introduction
Bile acid is the main lipid component of bile, which is derived from cholesterol in liver microsomes and transported actively into biliary by a secretory pathway [1].
Approximately, 95-98% of the bile acid is reabsorbed at the terminal ileum after entry into the small intestine and is transported back to the liver by portal vein, which forms the bile acid enterohepatic circulation [2]. Bile acid is associated with hepatobiliary diseases, gastrointestinal diseases, and other diseases that cause changes to bile acid metabolism [3-10]. Therefore, it is necessary to establish a simple, rapid, and effective analysis method to detect bile acids in an organism. However, this is difficult because of their complex nature and low concentration in biological fluids.
Currently, the classification and detection methods for bile acid mainly focus on High-Performance Liquid Chromatography (HPLC), Gas Chromatography/Mass Spectrometry (GC/MS), and High-Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS) [11]. For GC/MS, the bile acids to be treated via hydrolysis and derivation according to their category [12,13], and HPLC requires fluorescence derivation [14]. Meanwhile, while taurine-conjugated bile acid needs to derivatized after hydrolysis. However, HPLC-MS/MS does not need hydrolysis and derivation, and represents a relatively simple operating method to detect various types of bile acid in serum simultaneously, with advantages of speed, reproducibility, high separation efficiency, and sensitivity. In the present study, we aimed to develop an HPLC-MS/MS method to determine bile acids. The developed method has the advantage of a quick and easy-to-use sample preparation and clean-up without further complex derivatization techniques, and was applied successfully to determine the bile acids in more than 200 mouse serum samples.
Material and Methods
Chemicals and reagents
Cholic Acid (CA), Taurocholic Acid (TCA), Deoxycholic Acid (DCA), Ursodeoxycholic Acid (UDCA), and Lithocholic Acid (LCA) were purchased from Sigma-Aldrich (USA). β- Muricholic acid (β-MCA) was purchased from Steraloids. Co. The internal standard cholic-2, 2, 4, 4-d4 acid (CA-d4) was purchased from CDN Isotopes (Quebec, Canada). Activated carbon was purchased from Sigma-Aldrich.
Methanol and acetonitrile were purchased from J. T. Baker Solusorb Co. (USA), and ethanol was purchased from Tianjin Kemiou Chemical Reagents Ltd. Co.; all were of chromatographic purity. Ammonium acetate was purchased from Chemical Technology Academy of Shandong Province, and formic acid was purchased from New Jerser Co. (USA); both were analytical grade. Wahaha purified water was obtained from the market.
Preparation of standard solutions
CA, TCA, DCA, UDCA, LCA, and β-MCA standards were weighed accurately (0.010 g) and dissolved separately in 10 ml of anhydrous methanol to prepare standard solutions at 1 mg/ml. The standard solutions were then used to prepare mixed solutions of the six analytes at 100 ng/ml, 1 μg/ml, and 10 μg/ml in anhydrous methanol. All the solutions were stored at 4°C.
The CA-d4 standard was weighed accurately and dissolved in anhydrous methanol at 1 μg/ml as an internal standard solution.
Blank serum pre-treatment
Normal mouse serum was extracted and mixed with 100 mg/ml of activated carbon by gentle rocking. The mixture was shocked mildly and kept at room temperature overnight (around 17 h), before being centrifuged at 19,500 rpm for 1 h. The supernatant was filtered to a clean tube through a 0.22 μm membrane (MCM, Agela technologies Co.). The blank serum sample was then tested immediately, or stored at -70°C [12].
HPLC-MS/MS analysis
The chromatographic system comprised an Agilent 1200/6410 series HPLC system (Agilent Technologies, Waldbronn, Germany) with an on-line degasser. The chromatographic separation was carried out on a Diamonsil C18 chromatographic column (150 mm × 4.6 mm, 5 μm). The mobile phase consisted of methanol:water (containing 10 mmol/L ammonium acetate and 0.005% formic acid) (70:30, v/v). The injection volume was 10 μL and the mobile phase flow rate was 0.5 ml/min. The column oven temperature was set to 25°C.
The Atmospheric Pressure Electronic Spray Ionization (APESI) ion source was operated in the negative ion mode using the following settings: dry gas engine (nitrogen) flow velocity 9.0 L/min, dry gas pressure 45 psi, dry gas temperature 350°C, capillary voltage 4000 V. Analytes were monitored in the Multiple Reaction Monitoring (MRM) mode, and selected monitoring of ion pairs 514.3 → 514.3 (TCA, β-MCA), 407.4 → 407.4 (CA), 391.4 → 391.4 (UDCA, DCA), 375.4 → 375.4 (LCA), 411.4 → 411.4 (CA-d4) was performed with debris voltages of 130 V, 120 V, 140 V, 130 V, 120 V, respectively; the collision energy was 0 eV.
Sample preparation
Mouse serum samples (200 μL) were mixed with 50 μL of 1 μg/ml internal standard solution and 1 ml of 0.05% formic acid. After mixing thoroughly by vortexing for 2 min, the mixture was loaded onto an HLB Cartridge extraction column (Waters Co.), which had been pre-conditioned with 1 ml of methanol and 1 ml of 0.05% formic acid. The cartridge was subsequently washed with 1 mL of water and 1 ml of 5% methanol. The bile acids were eluted with 1 ml of methanol and 2 ml of acetonitrile. The eluent was dried at 60°C under a nitrogen stream, and the residue was dissolved in 100 μL of 90% methanol. After vortexing for 1 min to mix well, followed by 1 min of ultrasonication, 10 μL of the sample was injected into the HPLC-MS/MS system.
Specificity experiments
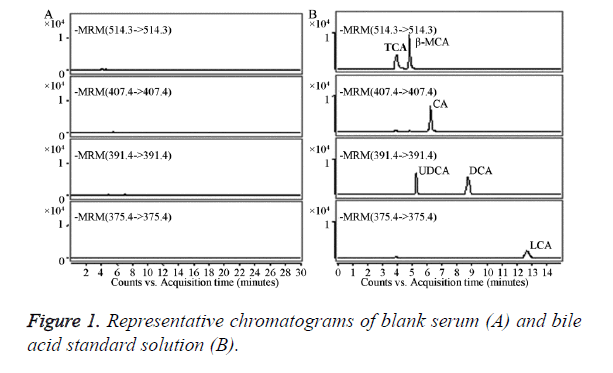
The blank serum (a) and bile acid standard solution (b) were injected separately into the HPLC-MS/MS system for analysis.
Preparation of the standard curve
To prepare different concentrations of 10, 25, 50, 100, 200, 500, 1000, 10000 ng/ml bile acid serum samples, the appropriate amount of standard solution was dried with nitrogen and dissolved in 200 μL of mouse blank serum. Following the “sample processing” operation, five samples were analysed for each concentration. The bile acid concentration (x) was set as the abscissa, and the bile acid and internal standard peak area ratio (y) was set as the ordinate, and were used in a computing weighted (W=1/x2) least squares method to establish the standard curve.
Matrix effects and recovery rate
The low, medium, and high concentrations (10, 100, 10000 ng/ml) of bile acid standard solutions were analysed, and the corresponding peak area was designated as A1. The blank serum was then used to perform sample processing (without the internal standards), using 100 μL of 5, 50, and 5000 ng/ml of the bile acid standard solution; the corresponding peak area was designated as A2/An appropriate amount of blank serum was prepared with low, medium, and high concentrations (10, 100, 10000 ng/ml) of bile acid serum samples, following the “sample processing” operation (without internal standards); the corresponding peak area was designated as A3. The A2/A1 ratio was used to calculate the influence of the matrix on bile acids at low, middle, and high concentrations. The A3/A2 ratio was used to calculate the absolute recovery of bile acids at low, middle, and high concentrations.
Precision and accuracy
The appropriate amount of blank serum was added to prepare serum samples of low, medium, and high (10, 100, 10000 ng/ml) concentrations following the “sample processing” operation. The intra-day precision and accuracy were evaluated by analysing five repeats of low, medium, and high concentrations in 1 day. The inter-day precision and accuracy were obtained by continuous detection of the low, medium, and high concentrations on three consecutive days.
Results
Specificity
The results showed that each of the analytes had no other interfering peak impurities, and the reservation times were TCA, 4.18 min; β-MCA, 4.82 min; CA, 6.09 min; UDCA, 5.36 min; DCA, 8.76 min; and LCA, 12.96 min (Figure 1). The blank serum processed by activated carbon had no significant endogenous impurities that interfered at the peak position of six bile acid standards; therefore, the method had good specificity.
Standard curve
The bile acid standard curves and correlation coefficients are shown in in Table 1. The results showed the detection of all the bile acids was linear within the range of 10-10000 ng/ml.
| Bile acids | Linear regression equation y=ax+b | Correlation coefficient γ |
|---|---|---|
| CA | y=0.0049x+0.0054 | 0.9909 |
| TCA | y=0.0052x-0.0063 | 0.9903 |
| DCA | y=0.0052x-0.0056 | 0.9913 |
| UDCA | y=0.0036x-0.0086 | 0.9927 |
| LCA | y=0.0028x-0.0045 | 0.9919 |
| β-MCA | y=0.0036x+0.0313 | 0.9908 |
Table 1. The calibration curve of all the bile acids.
Matrix effect
The results in Table 2 showed that the blank serum processed by activated carbon could be used as a standard for the blank matrix, and produced no significant matrix effect.
| CA | TCA | DCA | UDCA | LCA | β-MCA | |
|---|---|---|---|---|---|---|
| Low concentration (10 ηg/ml) | 99.23 ± 15.18 | 111.38 ± 17.40 | 102.93 ± 14.49 | 109.51 ± 16.29 | 90.17 ± 15.99 | 97.34 ± 11.47 |
| Moderate concentration (100 ηg/ml) | 99.75 ± 5.20 | 99.44 ± 4.03 | 100.09 ± 7.96 | 89.88 ± 3.09 | 100.40 ± 7.67 | 101.11 ± 2.36 |
| High concentration (10,000 ηg/ml) | 98.54 ± 4.09 | 101.70 ± 3.48 | 98.97 ± 2.96 | 108.36 ± 5.53 | 96.12 ± 7.42 | 100.53 ± 5.15 |
Table 2. The matrix effects of blank serum.
Method precision and recovery
The results in Table 3 showed that all the ingredients’ Relative Standard Deviation (RSD) were below 12%, with good precision. The average extraction recovery of all the ingredients was 50-90%, and the average relative recovery was 90-115%.
| Bile acids | Precision (RSD%) | Recovery (%) | |||
|---|---|---|---|---|---|
| Inter-day | Intra-day | Extraction recovery | Relative recovery | ||
| CA | Low concentration (10 ηg/ml) | 4.3 | 3 | 78.25 | 108.52 |
| Moderate concentration (100 ηg/ml) | 3.1 | 4.4 | 89.16 | 95.8 | |
| High concentration (10,000 ηg/mL) | 1.4 | 1.4 | 82.65 | 104.86 | |
| TCA | Low concentration (10 ηg/ml) | 3.6 | 11.3 | 80.73 | 105.22 |
| Moderate concentration (100 ηg/ml) | 2.2 | 7.2 | 72.36 | 96.16 | |
| High concentration (10,000 ηg/ml) | 7.9 | 1.1 | 72.44 | 93.44 | |
| DCA | Low concentration (10 ηg/ml) | 9 | 6.9 | 79.15 | 107.56 |
| Moderate concentration (100 ηg/ml) | 5.3 | 6.4 | 68.92 | 93.2 | |
| High concentration (10,000 ηg/ml) | 0.7 | 4.4 | 65.03 | 112.7 | |
| UDCA | Low concentration (10 ηg/ml) | 5.1 | 6.6 | 87.52 | 100.3 |
| Moderate concentration (100 ηg/ml) | 1.7 | 4.8 | 87.17 | 90.82 | |
| High concentration (10,000 ηg/ml) | 2.8 | 2.8 | 76.91 | 109.34 | |
| LCA | Low concentration (10 ηg/ml) | 10.6 | 7.5 | 59.52 | 102.14 |
| Moderate concentration (100 ηg/ml) | 4.4 | 5.7 | 56.76 | 93.36 | |
| High concentration (10,000 ηg/ml) | 2.6 | 1 | 64.34 | 111.3 | |
| β-MCA | Low concentration (10 ηg/ml) | 5.2 | 7 | 89.75 | 96.8 |
| Moderate concentration (100 ηg/ml) | 2.3 | 5 | 83.05 | 111.28 | |
| High concentration (10,000 ηg/ml) | 3.5 | 0.7 | 82.95 | 91.5 | |
Table 3. The precision and recovery of all the bile acids.
Determination of samples
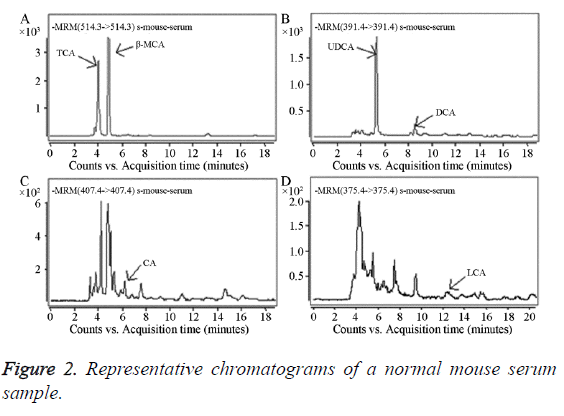
Forty mouse serum samples were analysed using the established method. The bile acids that needed to be determined were completely separated within 20 min; the performance of the method was adequate and stable. Representative results from a normal mouse are shown in Figure 2: TCA 96.25 ng/ml, β-MCA 115.95 ng/ml, UDCA 88.12 ng/ml, DCA 9.02 ng/ml, CA 3.31 ng/ml, and LCA 3.21 ng/ml.
Discussion
According to reference [15], most HPLC/MS/MS determination methods for bile acid use the gradient elution method. In this experiment, the mobile phase was methanol-water containing 10 mmol/L ammonium acetate and 0.005% formic acid (70:30), which achieved good separation. The MS analysis used an AP-ESI ion source, negative ions, and Multiple Reaction Monitoring (MRM) modes. However, there was no obviously enhanced response using different collision energies to detect the ion pairs; therefore, 0 eV was chosen as the collision energy. Agilent and Waters column extractors were used in this experiment. The Agilent column showed lower recovery than the Waters HLB Cartridge column did; therefore, the latter was chosen. After the extract was dried by a nitrogen stream, it was dissolved in the mobile phase; however, the peak from β-MCA was not good. This was resolved using a 90% methanol solution, which produced a good peak for β-MCA.
Conclusion
The developed method had good specificity, and the bile acids were detected in a linear manner within a range of 10-10000 ng/L. The inter-day and intra-day RSDs were all below 12%, the average extraction recovery of each ingredient was 50-90%, and the average relative recovery was 90-115%. Therefore, this method is sensitive, specific, and simple, and showed good reproducibility, with a short analysis time and wide linear range. Therefore, we believe that this HPLC-MS/ MS is suitable for the quantitative detection of mouse serum bile acids in scientific research.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Steiner C, von Eckardstein A, Rentsch KM. Quantification of the 15 major human bile acids and their precursor 7a-hydroxy-4-cholesten-3-one in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2870-2880.
- Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet 2002; 41: 751-790.
- Tazuma S, Kanno K, Sugiyama A, Kishikawa N. Nutritional factors (nutritional aspects) in biliary disorders: Bile acid and lipid metabolism in gallstone diseases and pancreaticobiliary maljunction. J Gastroenterol Hepatol 2013; 28: 103-107.
- Hofmann AF. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology 2009; 49: 1403-1418.
- Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther 2014; 39: 923-939.
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009; 50: 1955-1966.
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009; 89: 147-191.
- Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G. Bile acids as regulatory molecules. J Lipid Res 2009; 50: 1509-1520.
- Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141: 1773-1781.
- Tsuei J, Chau T, Mills D, Wan YJ. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med (Maywood) 2014; 239: 1489-1504.
- Burkard I, von Eckardstein A, Rentsch KM. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2005; 826: 147-159.
- Birk JJ, Dippold M, Wiesenberg GL, Glaser B. Combined quantification of faecal sterols, stanols, stanones and bile acids in soils and terrestrial sediments by gas chromatography-mass spectrometry. J Chromatogr A 2012; 1242: 1-10.
- Matysik S, Schmitz G. Application of gas chromatography-triple quadrupole mass spectrometry to the determination of sterol components in biological samples in consideration of the ionization mode. Biochimie 2013; 95: 489-495.
- You J, Fu Y, Sun Z, Suo Y. 2-(5-Benzoacridine)ethyl-p-toluenesulfonate as sensitive reagent for the determination of bile acids by HPLC with fluorescence detection and online atmospheric chemical ionization-mass spectrometric identification. Anal Bioanal Chem 2010; 396: 2657-2666.
- Xiang X, Han Y, Neuvonen M, Laitila J, Neuvonen PJ. High performance liquid chromatography-tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 51-60.