Research Article - Biomedical Research (2017) Volume 28, Issue 7
High miRNA-221 expression is associated with poor overall survival in patients with non-small cell lung cancer
1Department of Respiratory Medicine, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
2Department of Respiratory Medicine, China Pingdingshan Coal Shen Ma Medical Group General Hospital, Pingdingshan, China
- *Corresponding Author:
- Jing Wang
Department of Respiratory Medicine
The First Affiliated Hospital of Zhengzhou University, China
Accepted date: November 30, 2016
Abstract
Objective: The objective of this study was to investigate miRNA-221 expression in non-small cell lung cancer (NSCLC) and to determine the correlation between miRNA-221 expression and overall survival.
Methods: Clinical data pertaining to 151 NSCLC patients who underwent complete tumor resection surgery between January 2012 and December 2015 were retrospectively analyzed, and miRNA-221 expression was detected via quantitative reverse transcription polymerase chain reaction (qRT-PCR). The relationship between miRNA-221 expression and patient clinical data, including overall survival (OS), was analyzed.
Results: The expression level of miRNA-221 was significantly higher in NSCLC tissues than in adjacent non-tumor tissues (P<0.05). Significantly higher miRNA-221 expression levels were observed in patients ≥ 50 years of age and with advanced disease (P<0.05). Univariate Cox regression analysis showed that clinical stage (OR=1.97, 95%CI: 1.14-2.67), pathological type (OR=2.55, 95% CI: 1.77-5.12), tumor size (OR=1.34, 95% CI: 1.01-2.14) and miRNA-221 expression (OR=3.08, 95% CI: 1.76-6.31) were risk factors for shorter OS. Multivariate Cox regression analysis showed that clinical stage (OR=3.35, 95%CI: 1.67-5.05), tumor size (OR=2.02, 95%CI: 1.03-3.24) and miRNA-221 expression (OR=2.58, 95%CI: 1.41-64.85) were independent risk factors for shorter OS. The median OS time in the low miRNA-221 expression group was 41 months (95% CI: 36.22-45.78), which was significantly higher than that in the high miRNA-221 expression group (25 months, 95%CI: 5.85-35.15, P=0.011).
Conclusion: Our data indicate that higher miRNA-221 expression levels are associated with shorter OS in patients with NSCLC and that miRNA-221 may be a molecular marker of patient prognosis in NSCLC.
Keywords
Non-small cell lung cancer, miRNA-221, Gene, Prognosis
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, and almost 85% of lung cancers are classified as non-small cell lung cancer (NSCLC). The majority of affected patients have a very poor prognosis, even during the early stages of the disease, and the 5-year survival rate is only 20-30% [1,2]. Several studies have focused on developing reliable biomarkers predicting therapy responses, which may enable clinicians to predict or improve the prognoses of patients with NSCLC [3-6].
MicroRNAs (miRNAs) are short (19-25 nucleotides in length), non-coding regulatory RNA molecules whose primary function involves silencing gene expression. It has been suggested that the effects of miRNAs are gene and cell specific and that these molecules may be considered biomarkers of patient prognosis in several human cancers [7-10].
miRNA-221 is an important miRNA molecule and is considered a type of carcinogenic miRNA, as it has been linked to gastrointestinal stromal tumors [11], glial tumors [2], and prostate cancer [12]. However, studies regarding the association between miRNA-221 expression and clinical outcomes in NSCLC, especially overall survival (OS), are scarce and have yielded controversial results [13,14]. Further investigations are necessary to explore the prognostic significance of miRNA-221 in patients with NSCLC. In this study, we evaluated miRNA-221 expression in tumor tissue samples from patients with NSCLC and analyzed the correlation among miRNA-221 expression, clinical and pathological factors and prognosis.
Materials and Methods
Patients
A total of 151 patients who were diagnosed with NSCLC and underwent complete tumor resection surgery at our hospital from January 2012 to December 2015 were included in this study. The following patients were eligible for inclusion in this study: (1) patients diagnosed via pathology examinations, (2) patients who underwent complete tumor resection surgery, (3) patients who were not treated with radiotherapy, chemotherapy or immunotherapy before surgery and (4) patients with at least 3 months of comprehensive follow-up data. The following patients were excluded from this study: (1) patients with distant metastasis or malignant pleural effusions precluding complete tumor resection and (2) patients with additional tumors. All patients provided informed consent before beginning treatment, and the study was approved by the medical ethics committee of our hospital. All patients received regular examinations and underwent strict follow-up, during which their data were updated, until December 31, 2015.
Quantitative real-time PCR (qRT-PCR) for miRNA
All tissue samples were put in storage tubes within 30 min of collection for rapid freezing and then stored at -80. miRNA analysis was performed as follows: total RNA tissue samples were isolated using Trizol reagent (Invitrogen), and qRT-PCR was performed using Bulge-Loop miRNA qRT-PCR kits, according to the manufacturer’s instructions (RiboBio). Additional information regarding qRT-PCR has been presented in previous studies [15-17]. Briefly, miRNA-221 was synthesized using U6-specific cDNAs, a specific miRNA-221 stem loop primer and a U6 reverse primer and then amplified by real-time PCR with an miRNA-221-specific forward primer and a universal reverse primer. Each experiment was performed in triplicate. We used U6 small nuclear RNA as an endogenous control. The miRNA-221 expression level for each sample was calculated based on the ΔCT value. The relative miRNA-221 expression levels in paired tissues obtained from the same patient were determined via the comparative 2−ΔΔCT method [2,15].
Statistical analysis
T tests and chi-square tests were employed to analyze the correlations between miRNA-221 expression and patient clinical characteristics. Comparisons between tumor miRNA-221 expression levels and non-tumorous control miRNA-221 expression levels were analyzed using the 2-Ct method, 2-Ct=(CtmiRNA221-CtU6)-Avg. (CtmiRNA221-CtU6). The 2-Ct median was used as a threshold to divide patients into the following 2 groups: a low miRNA-221 expression group and a high miRNA-221 expression group.
OS curves were plotted via the Kaplan-Meier method, and differences between the groups were analyzed via the log-rank test. Univariate and multivariate analyses of OS were performed using the Cox proportional hazards regression model. SPSS 13 statistical software was used for all statistical analyses, and P<0.05 was considered statistically significant.
Results
A total of 151 NSCLC patients who underwent complete resection were included in the analysis. The median follow-up time was 45 months, the longest follow-up time was 60 months, and the shortest follow-up time was 3 months. Ninetysix patients were male, accounting for 63.6% of the sample, and 55 patients were female, accounting for 36.4% of the sample. The average age was 57.3 years, ranging from 39 to 79 years.
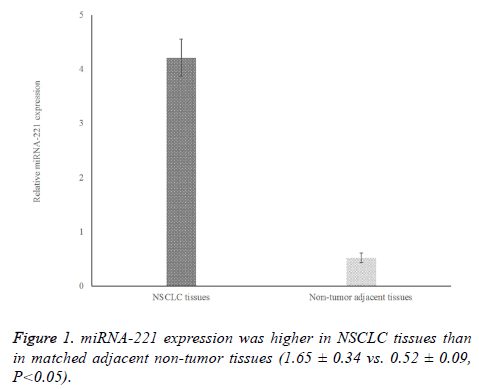
To determine the levels of miRNA-221 expression in NSCLC tissue samples, we performed qRT-PCR analyses on paired normal and tumor tissue samples from the same patient. As shown in Figure 1, the level of miRNA-221 expression in NSCLC tissues was significantly higher than that in adjacent non-tumor tissues (P<0.05). Based on the median miRNA-221 expression level, 79 cases of NSCLC were classified as having high miRNA-221 expression, and 72 cases were classified as having low expression.
The correlations between miRNA-221 expression and age, gender, clinical stage, pathological type, tumor size, and smoking history are shown in Table 1. Age, clinical stage, and pathological type were correlated with miRNA-221 gene expression (P<0.05). Moreover, as shown in Table 2, age and TNM stage were correlated with miRNA-221 expression (P<0.05), indicating that older patients (≥ 50 vs. <50) and patients with more advanced disease (II\III vs. I) were more likely to exhibit high levels of miRNA-221 expression.
| Characteristics | Samples size | miRNA-221 expression | T value | P value |
|---|---|---|---|---|
| Age (years) | 2.59 | 0.01 | ||
| <50 | 81 | 4.14 ± 0.48 | ||
| ≥ 50 | 70 | 4.33 ± 0.51 | ||
| Gender | 0.93 | 0.357 | ||
| Male | 96 | 4.32 ± 0.54 | ||
| Female | 55 | 4.24 ± 0.46 | ||
| Clinical stage | 10.09 | <0.001 | ||
| I | 54 | 3.80 ± 0.55 | ||
| II,III | 97 | 4.66 ± 0.47 | ||
| Pathological type | 2.11 | 0.037 | ||
| SCC | 62 | 4.31 ± 0.59 | ||
| ADC | 89 | 4.12 ± 0.52 | ||
| Tumor size(cm) | 1.89 | 0.061 | ||
| ≥ 3 | 66 | 4.34 ± 0.45 | ||
| <3 | 85 | 4.21 ± 0.39 | ||
| Smoking history | 1.86 | 0.064 | ||
| Yes | 71 | 4.01 ± 0.47 | ||
| No | 80 | 4.16 ± 0.52 |
Table 1. The correlation between miRNA-221 expression levels and the clinical characteristics of patients with non-small cell lung cancer.
| Characteristics | Sample size | miRNA-221 expression | χ2 | P value | |
|---|---|---|---|---|---|
| high | low | ||||
| Age(years) | 9.38 | 0.002 | |||
| <50 | 81 | 33 (40.74) | 48 (59.26) | ||
| ≥ 50 | 70 | 46 (65.71) | 24 (34.29) | ||
| Gender | 0.01 | 0.939 | |||
| Male | 96 | 50 (52.08) | 46 (47.92) | ||
| Female | 55 | 29 (52.73) | 26 (47.27) | ||
| Clinical stage | 4.51 | 0.031 | |||
| I | 54 | 22 (40.74) | 32 (59.26) | ||
| II,III | 97 | 57 (58.76) | 40 (41.24) | ||
| Pathological type | 0.03 | 0.993 | |||
| SCC | 62 | 32 (51.61) | 30 (48.39) | ||
| ADC | 89 | 47 (52.81) | 42 (47.19) | ||
| Tumor size(cm) | 1.23 | 0.254 | |||
| ≥ 3 | 66 | 38 (57.58) | 28 (42.42) | ||
| <3 | 85 | 41 (48.24) | 44 (51.76) | ||
| Smoking history | 0.35 | 0.545 | |||
| Yes | 71 | 39 (54.93) | 32 (45.07) | ||
| No | 80 | 40 (50.00) | 40 (50.00) | ||
Table 2. The correlation between miRNA-221 expression and the clinical characteristics of patients with non-small cell lung cancer.
The correlations between clinical characteristics, including miRNA-221 expression levels, and OS are shown in Table 3. Univariate Cox regression analysis showed that advanced clinical stage (II/III), ADC, tumor size ≥ 3 cm and high miRNA-221 expression levels were risk factors for shorter OS. Multivariate Cox regression analysis showed that clinical stage (OR=3.35, 95% CI: 1.67-5.05), tumor size (OR=2.02, 95% CI: 1.03-3.24) and miRNA-221 expression levels (OR=2.58, 95% CI: 1.41-64.85) were independent risk factors for shorter OS, as shown in Table 4.
| Clinical characteristics | β | Standard error | P value | OR (95% CI) |
|---|---|---|---|---|
| Sex (1=female, 2=male) | 0.46 | 0.23 | 0.342 | 1.59 (0.57-2.46) |
| Age (1 ≤ 50 years, 2 ≥ 50 years) | 0.16 | 0.12 | 0.480 | 1.17 (0.68-1.82) |
| Clinical stage (I=1, II, III=2) | 0.68 | 0.33 | 0.036 | 1.97 (1.14-2.67) |
| Pathological type (1=SCC, 2=ADC) | 0.94 | 1.45 | 0.022 | 2.55 (1.77-5.12) |
| Tumor size (1 ≤ 3 cm, 2 ≥ 3 cm) | 0.29 | 0.28 | 0.046 | 1.34 (1.01-2.14) |
| Smoking (1=No, 2=Yes) | 0.21 | 0.18 | 0.858 | 1.23 (0.47-1.73) |
| miRNA-221 expression (1=low?2=high) | 1.12 | 3.54 | 0.015 | 3.08 (1.76-6.31) |
Table 3. Univariate analysis of overall survival in patients with non-small cell lung cancer
| Clinical characteristics | β | standard error | P value | OR (95% CI) |
|---|---|---|---|---|
| Sex (1=female?2=male) | 0.15 | 0.44 | 0.841 | 1.16 (0.40-2.69) |
| Age (1 ≤ 50 years, 2 ≥ 50 years) | 0.76 | 0.41 | 0.613 | 2.14 (0.59-3.78) |
| Clinical stage (I=1, II, III=2) | 1.21 | 3.39 | 0.013 | 3.35 (1.67-5.05) |
| Pathological type (1=SCC, 2=ADC) | 0.29 | 1.46 | 0.589 | 2.49 (0.34-4.57) |
| Tumor size (1 ≤ 3 cm, 2 ≥ 3 cm) | 0.70 | 0.58 | 0.039 | 2.02 (1.03-3.24) |
| Smoking (1=No, 2=Yes) | 0.75 | 2.61 | 0.211 | 2.12 (0.39-5.72) |
| miRNA-221 expression (1=low, 2=high) | 0.95 | 0.42 | 0.001 | 2.58 (1.41-4.85) |
Table 4. Multivariate analysis of overall survival in patients with non-small cell lung cancer.
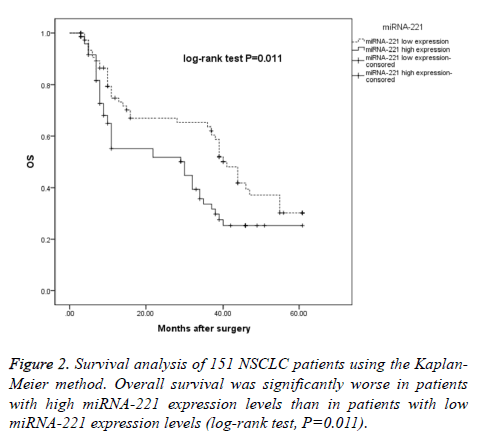
Patients were stratified into high miRNA-221 expression and low miRNA-221 expression groups to determine the effects of miRNA-221 expression on OS, and we found that the median OS time in the low miRNA-221 expression group was 41 months (95% CI: 36.22-45.78), while the median OS time in the high miRNA-221 expression group was 25 months (95% CI: 5.85-35.15), a difference that was statistically significant (log-rank χ2=6.520, P=0.011), as shown in Figure 2.
Discussion
In recent years, significant progress has been made regarding NSCLC clinical diagnosis and laboratory testing, but patient prognoses are still poor, and the 5-year survival rate ranges from approximately 20-30% [2]. Exploring the pathogenesis of lung cancer may facilitate development of targeted therapies, resulting in improved patient outcomes [18]. miRNAs can modulate key signaling pathways or genes related to tumorigenesis, and abnormal miRNA expression may induce tumorigenesis [2,3]. Although the processes associated with miRNA-mediated regulation of tumor behavior are very complex and diverse, miRNAs are tissue specific and may be used as tools for the early diagnosis or prediction of lung cancer [19,20].
In this study, we retrospectively collected clinical data pertaining to 151 NSCLC patients who underwent complete tumor resection surgery and detected miRNA-221 expression in preserved tissue samples using qRT-PCR. We noted higher miRNA-221 expression in NSCLC tissues than in paired adjacent normal lung tissues, indicating that miRNA-221 may be used to distinguish cancerous tissues from normal tissues and to determine prognosis in NSCLC patients [10,13].
We then analyzed miRNA-221 expression levels in lung cancer tissues and divided patients into the following 2 groups based on these levels: a high expression group and a low expression group. All these results showed that miRNA-221 expression was correlated with age and clinical stage. As shown in Tables 1 and 2, significantly higher miRNA-221 expression was noted among patients ≥ 50 years and with advanced disease (II/III) than among patients <50 years and with early disease (I). Yoruker EE showed that miRNA-221 was overexpressed in papillary thyroid carcinoma and that miRNA expression was correlated with tumor aggressiveness and adverse clinical characteristics. This group also found that miRNA-221 levels were significantly lower after surgery, indicating that miRNA-221 expression levels may be correlated with tumor burden [9].
Several studies have shown that high levels of miRNA-221 expression were correlated with poor prognoses in some tumors, such as hepatocellular carcinoma [21], thyroid cancer [9], and prostate cancer [12], indicating that miRNA-221 may play a significant role in tumorigenesis or tumor progression. One study showed that the level of miRNA-221 expression in lung cancer was significantly increased compared to that in benign lung lesions in 40 patients [15]. Overexpression of miRNA-221 may lead to targeting of some important tumor suppressor genes (for example, P57, FOXO3, and DDIT4) and induce tumor proliferation and invasion. Zhang et al. found that miRNA-221 targeted the PUMA gene and regulated cell apoptosis by directly acting on the 3' UTR of PUMA in glioma [22]. Garofalo et al. found that miRNA-221 induced TRAIL resistance and enhanced cellular migration by targeting the tumor suppressors PTEN and TIMP3 via activation of the AKT pathway and metallopeptidases in lung and liver cancer [23-25], confirming the role of miRNA-221 in NSCLC development and prognosis. Moreover, Xu et al. showed that miRNA-221 overexpression can result in increased H460 cell proliferation and viability. Down-regulation of miRNA-221 attenuates these effects, which indicates that inhibition of miRNA-221 may represent a novel treatment approach for human NSCLC [26].
However, studies regarding the correlation between miRNA-221 expression and NSCLC prognosis in terms of OS are still scarce. In this study, univariate Cox regression analysis showed that advanced clinical stage, adenocarcinoma, tumor size ≥ 3 cm and high miRNA-221 expression are risk factors for shorter OS. The median OS time (25 months) in the high miRNA-221 expression group was significantly shorter than that (41 months) in the low miRNA-221 expression group. Multivariate Cox regression analysis showed that clinical stage and miRNA-221 expression were independent risk factors for shorter OS, suggesting that miRNA-221 may be a prognostic factor for OS in NSCLC patients. Lv et al. retrospectively analyzed clinical data, including data regarding miRNA-221 expression, pertaining to 117 NSCLC patients and found that miRNA-221 expression was an independent risk factor for shorter OS in patients with NSCLC and that the median OS time (35.34 months) in the high miRNA-221 expression group was significantly shorter than that in the low miRNA-221 expression group (48.72 months) [13].
Similarly, studies have explored the correlation between miRNA-221 expression and OS in other tumors. For example, in patients with NK/T-cell lymphoma, higher plasma miRNA-221 levels were correlated with shorter OS, and plasma miRNA-221 levels were found to be an independent prognostic factor for OS; therefore, the authors concluded that plasma miRNA-221 levels may serve as diagnostic and prognostic markers in patients with NK/T-cell lymphoma [27]. Smid et al. noted significantly high miRNA-221 levels in gastric cancer patients who underwent palliative chemotherapy and that high miRNA-221 expression levels were correlated with shorter times to disease progression [28]. Moreover, Yuan et al. showed that low miRNA-221 expression levels were inversely associated with OS and DFS in patients with colorectal cancer (CRC) via combined analyses of patient samples, cell lines, and mouse models [3].
The limitations of this study were its relatively small sample size and retrospective design. Additional studies, especially prospective cohort studies, are needed to evaluate the prognostic value of miRNA-221 in NSCLC.
In conclusion, we demonstrated that miRNA-221 may play an important role in NSCLC and that increased miRNA-221 expression may facilitate tumor progression. Therefore, miRNA-221 may be a prognostic marker or therapeutic target in patients with NSCLC.
References
- Rambow F, Job B, Petit V, Gesbert F, Delmas V, Seberg H, Meurice G, Van Otterloo E, Dessen P, Robert C, Gautheret D, Cornell RA, Sarasin A, Larue L. New Functional Signatures for Understanding Melanoma Biology from Tumor Cell Lineage-Specific Analysis. Cell Rep 2015; 13: 840-853.
- Tong BC, Harpole DH. Molecular markers for incidence, prognosis, and response to therapy. Surg Oncol Clin N Am 2012; 21: 161-175.
- Yuan K, Xie K, Fox J, Zeng H, Gao H, Huang C, Wu M. Decreased levels of miR-224 and the passenger strand of miR-221 increase MBD2, suppressing maspin and promoting colorectal tumor growth and metastasis in mice. Gastroenterol 2013; 145: 853-64.e9.
- Navarro A, Pairet S, Alvarez-Larran A, Pons A, Ferrer G, Longaron R, Fernandez-Rodriguez C, Camacho L, Monzo M, Besses C, Bellosillo B. miR-203 and miR-221 regulate SOCS1 and SOCS3 in essential thrombocythemia. Blood Can J 2016; 6: e406.
- Lin Q, Mao W, Shu Y, Lin F, Liu S, Shen H, Gao W, Li S, Shen D. A cluster of specified microRNAs in peripheral blood as biomarkers for metastatic non-small-cell lung cancer by stem-loop RT-PCR. J Cancer Research and Clin Oncol 2012; 138: 85-93.
- Gu YF, Zhang H, Su D, Mo ML, Song P, Zhang F, Zhang SC. miR-30b and miR-30c expression predicted response to tyrosine kinase inhibitors as first line treatment in non-small cell lung cancer. Chin Med J 2013; 126: 4435-4439.
- Waters PS, McDermott AM, Wall D, Heneghan HM, Miller N, Newell J, Kerin MJ, Dwyer RM. Relationship between circulating and tissue microRNAs in a murine model of breast cancer. PloS One 2012; 7: e50459.
- Chen JC, Su YH, Chiu CF, Chang YW, Yu YH, Tseng CF, Chen HA, Su JL. Suppression of Dicer increases sensitivity to gefitinib in human lung cancer cells. Ann Surg Oncol 2014; 4: S555-63.
- Yoruker EE, Terzioglu D, Teksoz S, Uslu FE, Gezer U, Dalay N. MicroRNA Expression Profiles in Papillary Thyroid Carcinoma, Benign Thyroid Nodules and Healthy Controls. J Cancer 2016; 7: 803-809.
- Li Y, Di C, Li W, Cai W, Tan X, Xu L, Yang L, Lou G, Yan Y. Oncomirs miRNA-221/222 and Tumor Suppressors miRNA-199a/195 Are Crucial miRNAs in Liver Cancer: A Systematic Analysis. Dig Dis Sci 2016.
- Ihle MA, Trautmann M, Kuenstlinger H, Huss S, Heydt C, Fassunke J, Wardelmann E, Bauer S, Schildhaus HU, Buettner R, Merkelbach-Bruse S. miRNA-221 and miRNA-222 induce apoptosis via the KIT/AKT signalling pathway in gastrointestinal stromal tumours. Mol Oncol 2015; 9: 1421-1433.
- Sen CK, Gordillo GM, Khanna S, Roy S. Micromanaging vascular biology: tiny microRNAs play big band. J Vasc Res 2009; 46: 527-540.
- Lv J, Xu L, Xu Y, Qiu M, Yang X. Expression of MiRNA-221 in non-small cell lung cancer tissues and correlation with prognosis. Zhongguo Fei Ai Za Zhi 2014; 17: 221-225.
- Yamashita R, Sato M, Kakumu T, Hase T, Yogo N. Growth inhibitory effects of miR-221 and miR-222 in non-small cell lung cancer cells. Cancer Med 2015; 4: 551-564.
- Han HS, Jo YN, Lee JY, Choi SY, Jeong Y, Yun J, Lee OJ. Identification of suitable reference genes for the relative quantification of microRNAs in pleural effusion. Oncol Lett 2014; 8: 1889-1895.
- Mirzadeh Azad F, Naeli P, Malakootian M, Baradaran A, Tavallaei M. Two lung development-related microRNAs, miR-134 and miR-187, are differentially expressed in lung tumors. Gene 2016; 577: 221-226.
- Wang C, Ding M, Xia M, Chen S, Van Le A, Soto-Gil R, Shen Y, Wang N, Wang J, Gu W, Wang X, Zhang Y, Zen K, Chen X, Zhang C, Zhang CY. A Five-miRNA Panel Identified From a Multicentric Case-control Study Serves as a Novel Diagnostic Tool for Ethnically Diverse Non-small-cell Lung Cancer Patients. EBioMedicine 2015; 2: 1377-1385.
- Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, Engelman JA, Ono M, Rho JK, Cascione L, Volinia S, Nephew KP, Croce CM. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nature Med 2012; 18: 74-82.
- Molina-Pinelo S, Suarez R, Pastor MD, Nogal A, Marquez-Martin E, Martin-Juan J, Carnero A, Paz-Ares L. Association between the miRNA signatures in plasma and bronchoalveolar fluid in respiratory pathologies. Dis Mar 2012; 32: 221-230.
- Geng Q, Fan T, Zhang B, Wang W, Xu Y. Five microRNAs in plasma as novel biomarkers for screening of early-stage non-small cell lung cancer. Respir Res 2014; 15: 149.
- Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, Wong N. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterol 2008; 135: 257-269.
- Zhang C, Zhang J, Zhang A, Wang Y, Han L. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int J Oncol 2010; 37: 1621-1626.
- Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Can Cell 2009; 16: 498-509.
- Garofalo M, Quintavalle C, Di Leva G, Zanca C, Romano G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene 2008; 27: 3845-3855.
- Yang W, Yang Y, Xia L, Yang Y, Wang F, Song M, Chen X, Liu J, Song Y, Zhao Y, Yang C. MiR-221 Promotes Capan-2 Pancreatic Ductal Adenocarcinoma Cells Proliferation by Targeting PTEN-Akt. Cell Physiol Biochem 2016; 38: 2366-2374.
- Xu Y, Zhong C, Ding S, Huang H, Shen Z. MicroRNA-221 promotes human non-small cell lung cancer cell H460 growth. Int J Clin Exp Med 2015; 8: 2024-2030.
- Guo HQ, Huang GL, Guo CC, Pu XX, Lin TY. Diagnostic and prognostic value of circulating miR-221 for extranodal natural killer/T-cell lymphoma. Dis Mark 2010; 29: 251-258.
- Smid D, Kulda V, Srbecka K, Kubackova D, Dolezal J, Daum O, Kucera R, Topolcan O, Treska V, Skalicky T, Pesta M. Tissue microRNAs as predictive markers for gastric cancer patients undergoing palliative chemotherapy. Int J Oncol 2016; 48: 2693-2703.