- Biomedical Research (2012) Volume 23, Issue 1
High incidence of intracranial aneurysmal subarachnoid hemorrhage (SAH) in Kashmir, India
Abdul Rashid Bhat*, Mohammed Afzal Wani, Altaf R Kirmani, AU Ramzan, Shafiq Alam, Tariq Raina, Ashish Kumar Jain, Sajad Arif, MA Laharwal, Basharat MK
Department of Neurosurgery, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India
- *Corresponding Author:
- Abdul Rashid Bhat
Neurosurgeon, B-4 Faculty Quarters
Sher-i-Kashmir Institute of Medical Sciences
Srinagar, Kashmir 190011
India
E-mail: seven_rashid@rediffmail.com
Accepted date: July 07, 2011
Abstract
The Department of Neurosurgery, Sher-i-Kashmir Institute of Medical Sciences (SKIMS) Kashmir, sought to analyze all cases of subarachnoid hemorrhage (SAH) admitted from January 1983 to June, 2010 (27 years). The incidence of SAH in Kashmir at a given time is approximately 13/100000 population years. Of 2923 stroke patients admitted, SAH comprised of 31.02% (907/2923) with 92.06% adults and 7.93% children revealing a male: female ratio of 1.0: 1.78 (300 males: 535 females). The familial SAHs were 8.37% and familial aneurysms 8.51% common. The rupture of a saccular aneurysm was the most common (54.35%) cause of SAH in 191 males, 297 females and 5 children as detected by the carotid, digital subtraction and CT-angiography. The most common (36.10%) aneurysmal site was anterior communicating artery. About 30.83% aneurysmal patients had double/multiple aneurysms, thus more than 705 aneurysms were found in 493 patients. The arterio-venous malformation (AVM) in children was the commonest (65.51%) cause of SAH. The diagnostic tools of choice were lumbar puncture, carotid angiography and plain CT-scan brain. However CT angiography emerged as a better alternative to digital subtraction angiography, in revealing aneurysms and AVMs. While 35.61% cases were in Hunt-Hess poor grade (IV and V) with 63.15% deaths, an overall mortality of 36.60% in all SAH cases was observed. The initial bleeding episode led to 24.26% mortality while rebleeding occurred in 28.96% surviving cases and caused 12.35% mortality. A total of 32.04% aneurysms died. Good recovery was seen in 14.99% SAH cases and 19.6% aneurysmal ruptures. Noted finding was intra-operative enlargement of aneurysms that appeared smaller pre-operatively on CT-angiography. The special risk factors are ‘Jejeer’ Smoking and ‘Salt-Tea’ twice a day.
Keywords
Subarachnoid hemorrhage; aneurysms; Kashmir; outcome
Introduction
Spontaneous intracranial subarachnoid hemorrhage is the presence of blood in the subarachnoid space, bounded by the arachnoid and piamater, due to rupture of either an aneurysm or arterio-venous malformation or due to hypertension or an unknown cause. The literature reveals aneurysms as the most common cause of spontaneous SAH with 40% aneurysmal rupture on the Acom.A, 36% on the MCA, 34% on the ICA and Pcom.A and 6% on the ACA [1]. In 1924, London neurologist Sir Charles Symonds (1890–1978) gave a complete account of all major symptoms of subarachnoid hemorrhage, and he coined the term "spontaneous subarachnoid hemorrhage" [2]. SAH is a form of stroke and comprises 1-7% of all strokes [3]. Spontaneous SAH is sudden in 90% cases and is characterized by sudden onset severe headache of bursting nature in 60% cases usually in occipital region irrespective of aneurysmal rupture. This can be diagnosed by standard tools like lumbar puncture, CT-scan and 4-vessel cerebral angiography. The features of meningeal irritation, minimal neurologic findings of localizing value and presence of blood in CSF, along with headache, nausea, vomiting and transient loss of consciousness are described by twothirds of patients experiencing subarachnoid hemorrhage [4]. The incidence of SAH in United States of America (USA) on the average has remained constant at approximately 11 per 100,000 population annually while the deaths from SAH account for about 16 per 100,000 population [4,5]. The assessed natural history of SAH in a patient states that there is just one chance of good recovery out of five and one chance of getting crippled by the disease, while as there are three chances out of five to die sooner or later. Even 10-15% die before reaching hospital and those who survive often have neurological or cognitive impairment [6].
The early concept of aneurysmal SAH in India being uncommon, by B. Ramamurthi, has been proved wrong over time but the real incidence of SAH in India, due to lack of exact epidemiological data, remains questionable [7]. The purpose of this study, though hospital based, was to define and document the incidence of aneurysmal SAH in the Indian Kashmir, which was found to be lacking in the literature.
Material and Methods
This is a retrospective study of intracranial spontaneous subarachnoid hemorrhages (SAH) from January, 1983 to June, 2010 conducted by a single tertiary Neurosurgical Unit in J & K which has a uniform protocol to manage SAH patients and is backed by a methodical and advanced Medical Records Department to maintain the records. The patient case files were retrospectively studied from January 1983 to June, 2010. The patients were triaged according to the history and clinical status as assessed by the Glasgow Coma Scale (GCS), Hunt and Hess clinical grading, World Federation of Neurological Surgeons (WFNS) grading and imaging status as assessed by the Fischer grading system (amount of blood on CTscan), digital subtraction angiography (DSA), two/four vessel carotid angiography and CT-angiography. The protocol to manage all SAH patients in this study was early surgery, if able to sustain protocol requirements, and (since 1993) prophylactic hypertensive hypervolemic hemodilution “triple-H” therapy. After resuscitation, either a lumbar puncture (LP) or plain CT scan brain followed by bilateral carotid angiography (holds true from Jan., 1983 to Dec., 2002) or immediate NCCT-scan brain (if SAH was found, no lumbar punctures performed) followed by either digital subtraction angiography (DSA), bilateral carotid angiography or CT-angiography (from Jan., 2003 to June, 2010) . The SAH was recognized by the lumbar puncture and NCCT brain while as the cause of SAH like an aneurysm or an arterio-venous malformation, was identified on carotid angiography, CT-angiography and digital subtraction angiography. After angiography revealed an aneurysm(s) or AVM, surgical intervention was performed, while cases with negative angiography were subjected to the repeat angiography. All the SAHs with hypertension or of unknown cause were managed conservatively. The outcome was assessed by the most popular scale, Glasgow outcome Scoring (GOS) scale. The data was compiled and results analyzed statistically by SPSS 11.5 programme.
Observations
Incidence
Presuming the population of Kashmir, India, as 7 million (Census 2010-2011), observations revealed that the prevalence of subarachnoid hemorrhage (SAH) in Kashmir is about 13/100000 population years. Analysis showed that a total of 2923 strokes were admitted over a period of more than 27 years including 907 spontaneous subarachnoid hemorrhages (SAHs), comprising 31.02% = 907/2923 (Table 1). The most strokes i.e. 56.51% (1652 out of 2923) and the most SAHs i.e. 55.45% (503 out of 907) were found from the age-group of 41-60 years and most were aneurysmal (54.35% = 493/907) in origin. The 8.37% (76/907) patients with SAHs had history or evidence of blood-relations either being dead or treated of SAH and 55.26% (42/76) of these were aneurysmal.
Age and Sex
The age group most struck by the subarachnoid hemorrhage (SAH) was 21 – 60 years, comprising 80.04% (726 patients) of a total of 907 SAHs, while 55.45% (503/907) of these were from the age group 41 – 60 years. The age group least effected was 0 – 20 years, where children (below 18 years) comprised of 7.93% (72/907) from a total of 9.26% (84/907) SAHs. Other than 7.93% (72/907) children with SAH, the males formed 33.07% (300/907) and females 58.98% (535/907) of all SAHs with a male/female ratio of 1.oo : 1.78. The aneurysmal SAHs, which contribute 54.35% (493/907) of all SAHs, mainly affect age group of 41 – 60 years with 71.39% (352/493) cases. The aneurysmal SAHs comprised of 38.74% (191/493) males, 60.24% (297/493) females and (5/493) children, with a M/F ratio of 1.00 : 1.55 (Table 1). While 65.51% (19/29) of all arterio venous malformations (AVMs), which form 3.19% (29/907) of all SAHs are found in the age group of 0 – 20 years (Table 1).
Neurological status on admission
Symptomatology
Among SAHs the most common symptoms were headache 90.51% (821/907), loss of consciousness 69.45% (630/907), weakness of a body part 40.79% (370/907), visual disturbances 29.98% (292/907), speech disturbances about 16% (145/907) and convulsions 7.82% (71/907). The patients of SAHs due to AVMs had most headaches and convulsions (86.20% each = 25/29 each). The aneurysmal SAHs had 73.22% (361/493) headaches and hypertensive SAHs had 80% (216/270) headaches. The meningeal irritation was the most common sign, 68.46% (621/907) found in all SAHs and 50.70% (250/493) in aneurysmal SAHs. The fundal abnormalities were 31.86% (289/907) common in all SAHs and 89.25% (241/270) in hypertensive SAHs, while 16.43% (81/493) aneurysmal SAHs showed fundal abnormalities. The 3rd nerve palsy was seen in 9.59% (87/907) of all SAHs and 15.61% (77/493) aneurysmal SAHs. The Electrocardiographic (ECG) changes in the form of QT prolongation, Q waves and cardiac dysrhythmias have been seen in 50.93% (462/907) of all SAHs and 55.78% (275/493) aneurysmal SAHs.
GCS (Glasgow coma Scale) Score and WFNS Grading System
Nearly half of the 907 SAH patients, 49.28% (447/907), were admitted with a GCS Score of 13-14 and half of these without a focal neurodeficit, thus fulfilling the WFNS grade criteria of 2 and 3. The worst GCS Score of 6 and less was found in 21.60% (196/907) cases, included in the WFNS grade 5. All the patients with good score (GCS 15) i.e. 15.10% (137/907) SAHs, on admission, were WFNS grade 1. None of the 907 SAHs presented symptomless or with unruptured and intact aneurysms, so no patients were in WFNS grade 0. The aneurysmal SAHs were admitted mostly, i.e. 49.49% (244/493), with a GCS Score of 13-14 and WFNS grade of 2 and3. The best admission GCS score of 15 and WFNS grade of 1 was found only in 19.26% (95/493) aneurysmal SAHs. While the worst GCS score of 3-6 and WFNS grade 5 was applicable to the 18.86% (93/493) SAHs caused by the aneurysms, comparatively small number (12.37% i.e. 93/493) of aneurysmal SAHs were in the GCS score of 7-12 and WFNS grade 4. Most of the hypertensive (56.66%=153/270), AVMs (75.86%=22/29) and idiopathic (60.86%=70/115) SAHs had admission GCS score of 13-15 and WFNS grade of 1, 2 and 3.
Hunt Hess Grading System
About 36.38% (330/907) of all SAHs were admitted in Hunt Hess good grade (I, II), resulting in a mortality of 19.69% (65/330). The SAHs with poor grade (IV, V), 35.61% (323/907), had worst mortality of 63.15% (204/323) and moderate grade (III) SAHs admitted, 28.00% (254/907), were having a death rate of 24.80% (63/254). By sex incidence, more females, i.e. 28.44% (258/907) were in poorer grade (IV, V) of SAH as compared to males with 4.18% (18/907). About only 16.20% (147/907) females were in good grade (I, II) of SAH as compared to the 18.19% (165/907) males in grade I and II (good grade). The overall female mortality was 59.33% (197/332) as compared to an overall male mortality of 30.42% (101/332). While most of the children, i.e. 5.94% (54/907) were admitted in the poor and moderate grade (I, II, III) of SAH, the total mortality of children was low at 10.24% (34/332), compared to males and females. The aneurysmal SAHs (493 cases) were equally found in poor grade (34.68%=171/493) and good grade (34.07%= 168/493) of Hunt Hess grading system with an overall mortality of 47.59% (158/332). Out of 29 SAHs due to AVMs 24.13% (7/29) were in the poor grade (IV, V) SAH and 62.06% (18/29) had grade I and II SAH. But a total death rate of 0.60% (2/332) for AVMs was negligible as compared to aneurysmal, hypertensive and idiopathic SAHs.
Risk Factors
Adults formed 92.06% (835/907) cases of all SAHs. Aneurysms were the leading cause, 54.35% (493/907), of all SAHs. The females outnumbered with 58.98% (535/907) SAHs, mostly postmenopausal, and depicted a male/- female ratio of 1.00/1.78. Familial SAHs were found to occur in 8.37% (76/907) and most, 55.26% (42/76), of these were aneurysmal in origin. The largest number of SAHs, 77.39% (702/907), got admitted in emergency, during chilly winter months of the year. Long history (more than 10 years) of tobacco smoking, in the form of cigarettes, huka (as “JEJEER” special to Kashmir) and pipes, was confirmed in 66.48% (603/907) patients. The use of sympathomimetic drugs like phenylpropanolamine, especially during chilly winters, was found in all cases. The history of intake of dietary salt in a high proportion, as Kashmiri “Salt-Tea twice a day” is found in all 100% (907) SAHs.
Causes and investigations
The analysis proved four major causes of SAH in the Kashmir valley. The aneurysmal rupture, as a cause of SAH, was found in about more than half the cases i.e. 54.35% (493/907) of all subarachnoid hemorrhages. A total of 705 aneurysms were found in 493 patients with a patient/aneurysm ratio of 1.00: 1.43. The aneurysmal SAHs comprised of 38.74% (191/493) males, 60.24% (297/493) females and (5/493) children, with a M/F ratio of 1.00: 1.55 (Table 1). The hypertension caused 29.76% (270/907) of all SAHs. The AVMs lead to 3.19% (29/907) subarachnoid hemorrhages and most i.e. 65.51% (19/29) of these were children.
Lumbar Puncture (LP)
The Lumbar puncture was performed in 69.45% (630/907) cases. Though initially every patient of SAH was diagnosed by lumbar puncture only, it was negative in 5.07% (32/630) cases. Thus LP was diagnostic (sensitive) in 94.9% (598/630) SAHs.
Non-Contrast CT-Scan Brain
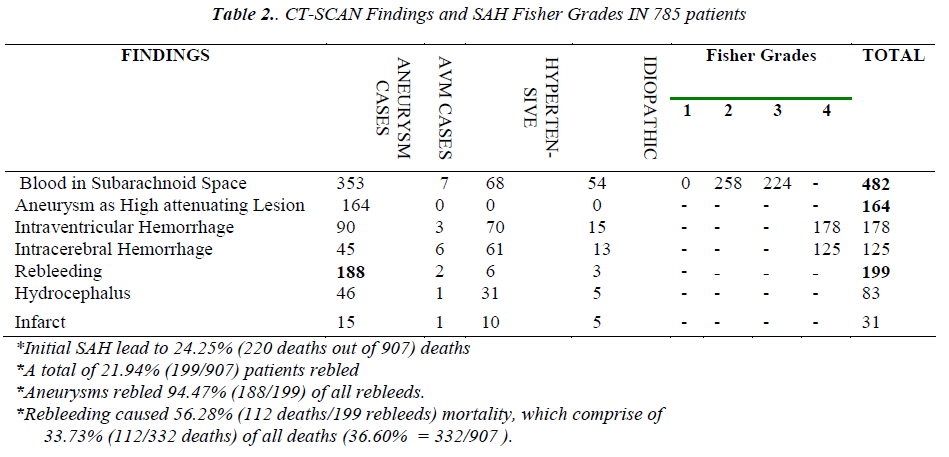
The non-contrast CT scan (NCCT) brain was performed in 86.54% (785/907) SAHs. The initial scan of 61.40% (482/785) SAH patients showed blood in subarachnoid space and 73.23% (353/482) of these were aneurysmal SAHs. About 20.89% (164/785) SAHs which underwent initial plain CT-scan brain, revealed aneurysms as high attenuating lesions (Figure 1). The NCCT brain showed that of all intraventricular hemorrhages (IVHs), 50.56% (90/178) IVHs were from aneurysmal SAHs. The intracerebral hemorrhages (ICH), lobar, thalamic etc; were found on initial CT-scan in 15.92% (125/785) of SAHs, mostly in hypertensive and aneurysmal SAHs. The rebleeding was confirmed in 25.35% (199/785) patients on CT-scan and 94.47% (188/199) rebleeds were aneurysmal SAHs. The hydrocephalus was found in 10.57% (83/785) scans, most of these i.e. 55.42% (46/83) belonged to aneurysmal SAHs. There were 3.94% (31/785) patients with unilateral and unilobar or bilobar infarcts, of which 48.38% (15/31) were aneurysmal SAHs [Table 2].
Fischer Grade Classification on NCCT Brain
The subarachnoid hemorrhage of Fisher grade 2 (subarachnoid hemorrhage less than 1mm thick on CT scan) and 3 (more than 1mm thick) appearance was found in 61.40% (482/785) scans. Out of all Fisher grade 2 and 3 SAHs, the aneurysms were the cause of 73.23% (353/482) patients. The Fisher grade 4 (Any thickness with intraventricular hemorrhage or parenchymal extension) appearance was found in 38.59% (303/785) of those scanned for SAH and of this 44.55% (135/303) patients were aneurysmal SAHs. The NCCT brain for SAH was 100% sensitive in the study [Table 2].
Angiography
The commonest diagnostic tool to reveal the cause of SAH was angiography which detected more than 705 aneurysms in 493 patients with a patient/aneurysmal ratio of 1.00: 1.43 and 29 AVMs in 29 SAHs, thus angiography was 62.36% (522/837) accurate. This procedure also revealed diffuse and local vasospasm in 41.93% (351/837) SAHs and had a mortality of 0.59% (5/837). Out of all SAHs (907), about 92.28% (837/907) patients were subjected to different types of angiographies available in different periods of time from 1983 to 2010 [Table 3]. However 7.71% (70/907) patients were either dead before angiography could be performed or were allergic to the contrast material used so that angiography was abandoned.
a. Carotid/vertebral angiography
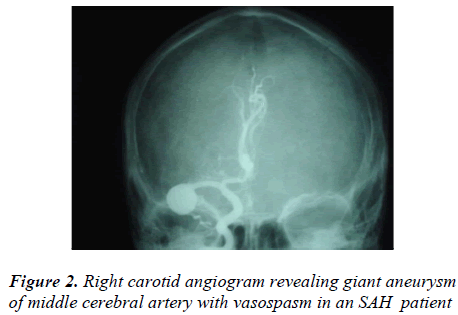
After lumbar puncture (LP) proved presence of SAH, 48.18% (437/907) patients were subjected to the unilateral or bilateral carotid and vertebral angiography to detect any aneurysms or/ and AVMs as a cause of SAH. The unilateral carotid angiography was performed in 19.90% (87/437), bilateral in 66.59% (291/437) and 4-vessel (+ vertebral) angiography was performed in 13.50% (59/437) SAH cases. The carotid/vertebral angiography proved positive in 49.19% (215/437) SAHs and negative in 50.80% (222/437) cases with a positive to negative ratio of 0.96 : 1.00. However vertebral angiography alone proved negative in 75.57% (44/59) cases i.e. less accurate than carotid angiography. The carotid angiography detected single aneurysms in 137 SAH cases and two or more than 2 aneurysms in 63 SAHs which amounted to more than 263 aneurysms in 200 aneurysmal SAHs (Figure 2). The carotid angiography detected 15 AVM cases. The local vasospasm was revealed in 34.09% (149/437) and diffuse vasospasm was found in 15.10% (66/437) SAH cases. The carotid/vertebral angiography led to 1.14% (5/437) deaths, mostly (3 out of 5) due to vertebral angiography [Table 3].
b. CT-Angiography
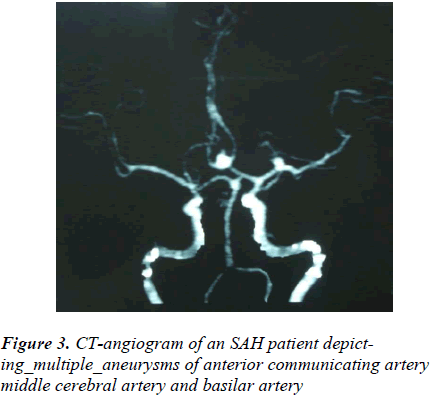
About 24.49% (205/837) patients were subjected to the CT-angiography. The CT-angiography was negative in 16.58% (34/205) and positive in 83.42% (171/205) patients with an accuracy of > 83%. More than half of the investigated patients, 50.24% (103/205) had single and 28.78% (59/205) had two or more than two (multiple) aneurysms, thereby detecting more than 221 aneurysms in 162 patients (Figure 3). CT-angiography also depicted 4.39% (9/205) AVMs. The vasospasm was found in 36.58% (75/205) patients. The procedure had negligible complications [Table 3].
c. Digital subtraction Angiography (DSA) or Catheter Angiography:
The DSA or catheter angiography was performed on 17.56% (147/837) with > 87% accuracy in determination of cause of SAH and was negative in 12.24% (18/147) patients. About 65.98% (97/147) patients had single aneurysm and 19.72% (29/147) SAHs revealed two or more than two aneurysms that made it 155 aneurysms in 126 patients of SAH. The 2.04% (3/147) patients who underwent DSA prove to be the AVMs. The DSA detected vasospasm in about 41.49% (61/147) patients and the procedure had no complications [Table 3].
d. MR-Angiography:
MR angiography was the least used tool to discern the cause of SAH. Only 5.73% (48/837) SAHs were subjected to this imaging procedure with only 14.58% (7/48) accuracy and 85.41% (41/48) negativity [Table 3]. Among SAHs caused by aneurysms and AVMs, 8.31% (4/48) showed single and 2.08% (1/48) depicted double aneurysms, while 4.16% (2/48) patients had AVMs.
Site and Number of Aneurysms (Multiple aneurysms)
The 493 aneurysmal SAH patients were harbouring 705 aneurysms, detectable angiographically. The aneurysms were the most common cause of SAH in 54.35% (493/907) patients. The 69.16% (341/493) aneurysmal SAHs, each, harbored single aneurysm and 30.83% (152/493) cases had 2 and more than 2 (multiple) aneurysms. Of the multiple aneurysms, the double aneurysms were found in 53.28% (81/152) and more than 2 (multiple) in 46.71% (71/152) cases. Out of the all aneurysmal SAHs, the double aneurysms comprised of 16.43% (81/493) cases and multiple (>2) aneurysms made 14.40% (71/493) of all aneurysmal SAHs. The patients with middle cerebral artery aneurysmal rupture had most multiple aneurysms i.e. 31.02% (41/132) [Table 4]. The most common arterial site for aneurysms was anterior communicating artery (A Com A), accounting for 36.10% (178/493) of all aneurysmal SAHs. The A. Com. Artery contributed 87.64% (156/178) cases of single-aneurysms and 0.56% (only one case i.e. 1/178) double-aneurysm case to the study. The rest of the 11.79% (21/178) A. Com. A. aneurysm cases had a single parent artery aneurysm in association with aneurysms of the other arteries, on the same side of the proximal control (7.30% = 13/178) and on the opposite side (4.49% = 8/178) of the proximal control [Table 4]. The 26.77% (132/493) aneurysmal SAHs were caused by the middle cerebral artery (MCA) aneurysms. Among MCA aneurysmal SAHs, the patients with single MCA aneurysms accounted to 68.93% (91/132) and double aneurysms on the same parent artery occurred in 9.09% (12/132) patients. The patients with multiple (> 2) aneurysms on the same parent (middle cerebral) artery accounted for 2.27% (3/132). The aneurysms on the other arteries in association with the parent artery (MCA) aneurysms, on the same side of the parent artery and on the opposite side to the parent artery, were found in 19.69% (26/132) patients. The aneurysms of internal carotid artery (ICA) were harbored by 20.89% (103/493) patients. The 63.10% (65/103) patients each, harbored a single aneurysm of ICA, while 7.76% (8/103) patients had double aneurysms on ICA and multiple (> 2) aneurysms were found on the parent artery (ICA) of 4.85% (5/103) patients. About 24.27% (25/103) patients with single ICA aneurysms each, had associated aneurysms on the other arteries of same and opposite sides to that of the parent artery. The anterior cerebral, posterior communicating, basilar and vertebral arteries had comparatively lower number of aneurysms [Table 4]. The commonest double aneurysm combination sites were anterior communicating and middle cerebral arteries, while commonest multiple (> 2) aneurysm combination sites were anterior communicating, middle cerebral and basilar arteries.
Aneurysmal Size and Familial Factor
The 493 aneurysmal SAHs with 705 aneurysms had variation in size.
(a) Small aneurysms (4 mm to 10 mm) = 68.22%
(481/705)
(b) Large aneurysms (11 mm to 25 mm) = 27.80%
(196/705)
(c) Giant aneurysms (> 25mm) = 3.97%
(28/705)
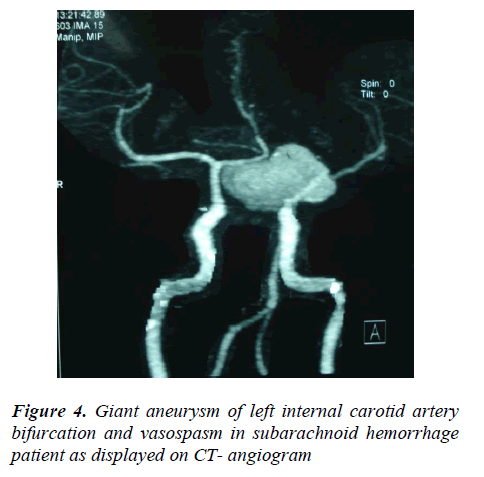
Half the giant aneurysms (50% = 14/28) were found on the MCA, another one-fourth (25% = 7/28) were detected internal carotid artery and the rest (25% = 7/28) occurred in the vertebra-basilar system (Figure 4). Though small, large or giant are absolute aneurysmal sizes, a parent artery- related type of aneurysmal size grading was found much accurate and comfortable intra-operatively. One finding is that an aneurysm of 5mm is much smaller for internal carotid artery than for M2 or M3 segments of middle cerebral artery or for pericallosal artery. The 8.37% (76/907) of all SAHs had evidence of blood relations to each other and about 55.26% (42/76) of these were aneurysmal, contributing 8.51% (42/493) patients to aneurysmal cause of SAHs. The rest of the 44.73% (34/76) familial SAHs were of hypertensive (34.21% = 26/76), idiopathic (9.21% = 7/76) and AVM (1.31% = 1/76) origin, amounting to 9.62% (26/270) hypertensive, 6.08% (7/115) idiopathic and 3.44% (1/29) AVMs.
Rebleed
The rebleeding was confirmed by CT scan in 25.35% (199/785) patients, leading to 56.28% (112/199) deaths. About 38.13% (188/493) aneurysmal SAHs rebled and contributed 94.47% (188/199) patients to all rebleeding [Table 2]. It was observed that after carotid angiography, increase in rebleedings was 17.58% (35/199) [Table 3].
Vasospasm
The vasospasm was found in 40.20% (351/873) patients who had undergone angiography [Table 3]. The local vasospasm (related to parent artery of aneurysm and its branches) was depicted in 74.92% (263/351) and the diffuse vasospasm (related to all vessels) in 25.07% (88/351). The carotid angiography showed vasospasm in 61.25% (215/351), CT angiography in 21.36% (75/351) and DSA detected vasospasm in 17.73% (61/351) patients. Of all cases (351), vasospasm due to the aneurysmal SAHs was found in 80.34% (282/351) patients.
Hydrocephalus
The hydrocephalus was found in about 10.57% (83/785) of those SAHs who underwent NCCT brain and more than half (55.42% = 46/83) of these were aneurysmal [Table 2].
Management
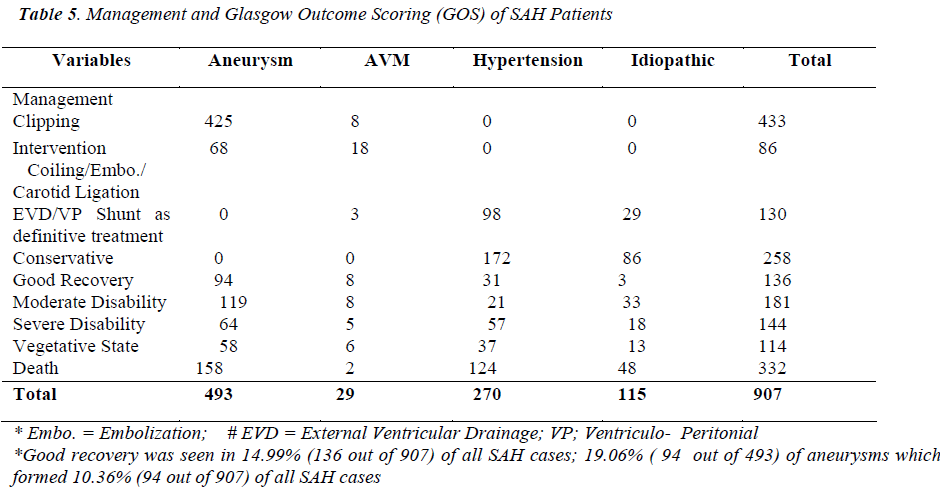
The 71.50% (649/907) SAHs underwent surgical and endovascular type of treatment and 28.40% (258/907), all of these hypertensive and idiopathic SAHs, were treated conservatively. The surgical treatment included clipping, Ventriculo peritoneal (VP) shunts, external ventricular drainages (EVD) and carotid ligation (a few giant aneurysms). The 14.53% (130/907) SAH patients, with ventriculomegaly, were subjected to either external ventricular drainage (EVD) or/and Ventriculo peritoneal (VP) shunts, of mostly hypertensive and idiopathic origin. Endovascular treatment in the form of coiling / embolization and carotid ligation was required in 9.48% (86/907) aneurysms and AVMs [Table 5].
Clipping
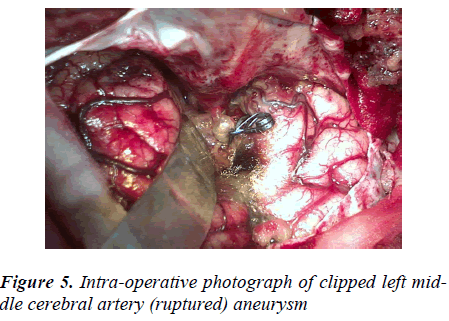
The clipping was possible in 47.70% (433/907) of all SAHs, 0.88% (8/907) of these being AVMs and the rest, 46.82% (425/907), aneurysms. Of all the aneurysmal SAHs (493), only 86.20% (425/493) aneurysms were clipped (Figure 5). The single aneurysms were clipped in 96.23% (409/425) and the double aneurysms in 3.76% (16/425) patients. About 85.96% (153/178) Acom.A aneurysms, 98.48% (130/132) MCA and 85.44% (88/103) ICA aneurysms were clipped. The 27.58% (8/29) AVMs were trapped by clipping. The intraoperative aneurysmal size in a patient varied from the size to that of same aneurysm and patient on preoperative CT angiography by 0.5 –1.5 mm (being larger) especially in case of distal anterior cerebral artery (DACA) and middle cerebral artery (MCA) aneurysms [Table 5].
Mortality
The initial episode of subarachnoid hemorrhage led to 24.26% (220/907) deaths and rebleeding caused 12.35% (112/907) deaths. About 30.32% (275/907) died in first two weeks and the remainder 6.29% (57/907) within following four weeks. A total of 36.60% (332/907) patients of SAH died. Almost 1/3, 32.04% (158/493) of all aneurysms died, contributing 47.59% (158/332) to total mortality. The rebleeding contributed 33.73% (112/332) to the overall mortality. The SAH patients in the Hunt Hess grade V had maximum mortality of 72.95% (143/196), contributing 43.07% (143/332) to overall mortality. The least mortality of 15.17% (17/112) was observed in the Hunt Hess grade I, contributing 5.12% (17/332) to total mortality. Of all deaths, there were 59.33% (197/332) female deaths, 30.42% (101/332) male deaths and 10.24% (34/332) child deaths in SAH patients. The carotid angiography resulted in 1.14% (5/437) deaths, increasing the whole mortality by 1.50% (5/332) deaths [Table 3, 5].
Outcome
Of all (907) SAH patients, 14.99% (136/907) SAHs showed good recovery, most of these were aneurysmal SAHs (69.11%; 94/136), although good recovery among aneurysmal SAHs was 19.06% (94/493). Similar results, for good recovery, were seen in SAH patients due to AVM, hypertension and idiopathic causes. About 19.95% (181/907) SAHs of all causes ended up in moderate disability, 65.74% (119/181) of these were due to aneurysmal SAHs. And 24.13% (119/493) SAHs due to aneurysmal rupture were moderately disabled. The severe disability and vegetative state occurred in 15.87% (144/907) and 12.56% (114/907) SAHs respectively. However, more than 50% (58/114) patients in vegetative state were due to aneurysmal rupture [Table 5]. The hypertensive SAHs had a mortality of 45.92% (124/270), idiopathic SAHs 41.73% (48/115), aneurysmal SAHs 32.04% (158/493) and SAHs due to AVMs had 6.89% (2/29) mortality, a total SAH mortality of 36.60% (332/709).
Discussion
Subarachnoid hemorrhage (SAH) from a ruptured aneurysm accounts for approximately 5% of all strokes and incidence of SAH had remained stable at around 8 per 100000 personıyears over 35 years. In a small subset of studies, gender specific incidences were given, which indicated a higher incidence in women [8]. The study at sher–i-Kashmir Institute of Medical Sciences (SKIMS), Kashmir observed that SAH formed 31.02% of all strokes and the overall incidence is about 13/100000 population (Table 1). The high proportion of SAHs compared to overall strokes in Kashmir may be due to racial or ethnic (population group of mountain locked Kashmir) factors. Since community-based studies reported an incidence that ranged from 8.1 per 100 000 in Australia and New Zealand to 23 per 100 000 in Japan [9]. The lower incidence in some regions may be explained by racial differences, although in some studies the incidence of SAH in black populations was higher in comparison with white popultions [10, 11]. Incidences per 100000 personıyears were 22.7 (95% CI 21.9 to 23.5) in Japan, 19.7 (18.1 to 21.3) in Finland, 4.2 (3.1 to 5.7) in South and Central America, and 9.1 (8.8 to 9.5) in the other regions. There was wide variation in SAH incidence, ranging from 2 to 25 per 100000 personıyears, with most regional incidences between 7 and 13 per 100000 personıyears.The overall incidence of SAH is approximately 9 per 100000 personıyears [12]. A large multinational World Health Orga nization study found that the age-adjusted annual incidence of SAH varied 10-fold between different countries, from 2.0 cases per 100 000 population in China to 22.5 per 100 000 in Finland [13].SAH is reported to be 62% common in 5th and 6th decades of life. This is 54.3% common in females and 45.7% in males, while aneurysms are 59% common in females and 41% in males [14]. The incidence in women was 1.24 (1.09 to 1.42) times higher than in men; this gender difference started at age 55 years and increased thereafter. In the age group 25–45 years, incidence was significantly higher in men than in women, but in the age group 55–85 years, incidence was significantly higher in women than in men [12]. The Studies have suggested that the gender difference is related to hormonal status, with incidence of SAH increases with age, occurring most commonly between 40 and 60 years of age (mean age 50 years), but SAH can occur from childhood to old age and is 1.6 times higher in women than in men [15, 16]. The incidence of aneurysmal rupture is only 2-20/100,000 individuals/year [13]. Hemorrhage is more frequent in women than men (3:2 ratio) over the age of 40, but the reverse is true in those younger than 40. Peak rupture rates occur between the ages of 50 and 60 years [17]. A series of 167 SAH patients is reported to have mean age ± SD 52.6 ± 14.1 and predominance (71%) of women [18]. Similar findings were revealed in the Kashmir study. The present study at SKIMS revealed that the most common symptoms were headache 90.51%, loss of consciousness 69.45%, weakness of a body part 40.79%, visual disturbances 29.98%, speech disturbances 16% and convulsions 7.82%. The meningeal irritation was the most common sign, 68.46% found in all SAHs and 50.70% in aneurysmal SAHs. The fundal abnormalities were 31.86% common in all SAHs and 89.25% in hypertensive SAHs, while 16.43% aneurysmal SAHs showed fundal abnormalities. The 3rd nerve palsy was seen in 9.59% of all SAHs and 15.61% aneurysmal SAHs. Headache was reported in 10% to 48% of SAHs [14]. About 67.8% SAHs present with paralysis of a side, 75% loss of consciousness while 78% patients had headache [19]. Fontanarosa retrospectively studied 109 patients with proven SAH and found headache in 74%, nausea or vomiting in 77%, loss of consciousness in 53%, and nuchal rigidity in 35% [20]. The speech disturbances ranged from 2% to 21.3% . Reporedly visual disturbances occurred in 4% to 43% of patients [14, 19]. A study reported meningeal irritation in 75%, 3rd nerve palsy in 14% and fundal abnormalities in 35% of SAH patients [19]. Seizures may occur in up to 20% of patients after SAH, most commonly in the first 24 hours and more commonly in SAH associated with intracerebral hemorrhage, hypertension, and middle cerebral and anterior communicating artery aneurysms [21]. Other study showed 4% convulsions occurring in SAHs [14]. Delayed seizures occurred in 7% of patients in another series [22]. The symptoms and signs of SAH patients in the Kashmir study are quite comparable to the above studies. The patient's clinical status is assessed using the World Federation of Neurological Surgeons Scales and Hunt and Hess Scale [23, 24, 25]. A study showed poor clinical condition on admission in 22% SAHs and amount of cisternal blood >median in 43% [18]. In the present study 36.38% of all SAHs were admitted in Hunt Hess good grade (I, II), resulting in a mortality of 19.69%. The SAHs with poor grade (IV, V), 35.61%, had worst mortality of 63.15% and moderate grade (III) SAHs admitted, 28.00%, were having a death rate of 24.80%. The aneurysmal SAHs (493 cases) were equally found in poor grade (34.68%) and good grade (34.07%) of Hunt Hess grading system with an overall mortality of 47.59%. The first scale of severity was described by Hunt and Hess in 1968, which showed 80% and 90% mortality in Hunt-Hess grade 4 and 5 respectively [25]. The present study has shown parallel results in Kashmiri patients. The study observed that being an adult is a risk factor for SAH. Adults comprised of 92.06% of all SAHs. Aneurysms are the major cause of SAH with 54.35% of all SAHs. Familial SAHs occurred in 8.37% and mostly (55.26%) were aneurysmal in origin. However it is reported that genetic factors explain only 10% of SAH. Group Smoking in the form of a special Kashmiri clay/copper water container with two wooden barrels, utilizing wet/dry tobacco called “JEJEER” is popular in Kashmir. This study revealed 66.48% patients were smokers. Racial differences have been reported in risk of SAH. Black Americans are at higher risk than white Americans [26]. Multivariate models have found hypertension, smoking, and heavy alcohol use to be independent risk factors for SAH in the United States, Japan, the Netherlands, Finland and Portugal [27]. Sympathomimetic drugs, including cocaine and phenylpropanolamine are known risk factors for SAH [28]. This study noted that phenylpropanolamine, as a decongestant and cold remedy, was much used during winters by all patients. The risk factors like smoking, female gender, hypertension, family history of cerebrovascular disease, and postmenopausal state are thought to be common for SAH and multiple aneurysms [29]. The SKIMS study found females form more than half, 58.98%, of Kashmir study, mostly postmenopausal, showing male: female ratio of 1.00 : 1.78.The Kashmir study revealed that most SAHs, 77.39%, got admitted during winters. Moreover the use of high amount of dietary salt as “Salt-Tea Twice A Day” by all patients has been a risk factor for hypertension and SAH. Symonds described the use of lumbar puncture and xanthochromia in diagnosis of SAH and lumbar puncture showed evidence of hemorrhage in 3% of people in whom CT was found normal [2, 6]. The detection rate of SAH by lumbar puncture is reported to be 96.5% [19]. The study at SKIMS confirmed the diagnostic sensitivity of lumbar puncture as 94.9%. The sensitivity of CT in the first 12 hours after SAH is 98% to 100%, declining to 93% at 24 hours and to 57% to 85% 6 days after SAH [30, 31]. Computed tomography (CT scan) of the brain has a high sensitivity and will correctly identify over 95% of cases [6, 32]. The ruptured aneurysms in 21.1% SAH patients were detected as high attenuating lesions on plain CT-scan [33]. The SKIMS study revealed 100% sensitivity of plain CT scan brain and 20.89% SAHs, which had high attenuating lesions on CT-scan, were confirmed aneurysms by angiography. In study at Kashmir, 48.18% patients were subjected to carotid angiography. The unilateral carotid angiography was performed in 19.90% and bilateral in 66.59% patients and detected single aneurysms in 137 SAH cases and multiple aneurysms in 63 SAHs which amounted to more than 263 aneurysms in 200 aneurysmal SAHs. A study reports that adequate carotid angiography detects berry aneurysms in 80% of those SAHs which are CT-negative. The carotid angiography has high positive results with a positive to negative ratio of 3.50 : 1.00 [1]. In a series of SAH patients carotid angiography was performed unilaterally in 38.2% and bilaterally in 17% patients with 7.8% complications [33]. The SKIMS study shows carotid angiography positive in 49.19% SAHs with a positive/negative ratio of 0.96/1.00 with a mortality rate of 1.14%. The Kashmir study proves that CT-angiography (CTA) was positive in 83.42% patients with an accuracy of > 83% and negative in 16.58%. About 50.24% had single and 28.78% had multiple aneurysms, thereby detecting more than 221 aneurysms in 162 patients. Authors of a study have reported sensitivity of CTA for aneurysms between 77% and 100% and a specificity between 79% and 100%.For aneurysms of 5 mm in size, CTA has a sensitivity between 95% and 100% compared with between 64% and 83% when aneurysms are <5 mm [34]. Among aneurysms detected on CTA and then undergoing surgery, 100% correlation was observed between CTA and catheter angiography. Velthuis and colleagues found that CTA is equal to catheter angiography in 80% to 83% of cases [35]. In 74% of patients, catheter angiography performed after CTA did not reveal any additional information [36]. At SKIMS DS-angiography detected single aneurysms in 65.98% patients and multiple aneurysms in 19.72% SAHs which disclosed 155 aneurysms in 126 patients. DSA proved 87.76% sensitive and detected vasospasm in about 41.49% patients. The sensitivity of 3-dimensional time-of-flight MRA for cerebral aneurysms is between 55% and 93%.With aneurysms 5 mm, the sensitivity is 85%to 100%, whereas the sensitivity of MRA for detecting aneurysms <5 mm drops to 56% [37]. The present study disclosed 14.58% accuracy for detecting aneurysms in SAH patients. Among SAHs caused by aneurysms and AVMs, 8.31% showed single and 2.08% depicted double aneurysms.
The SKIMS study disclosed the causes of SAH as 54.35% aneurysms, 29.76% hypertensive bleeds, 12.67% unknown- cause and 3.19% AVMs. Locksley reports the causes of spontaneous SAH in a study as 51% aneurysms, 6% AVMs, 15% hypertension and 22% idiopathic [14]. Another author reported 36.7% SAHs due to aneurysms, 7.1% AVMs, 9.4% hypertension and 46.8% as unknown causes [19]. In one study, 85% cases of spontaneous SAH are the caused by rupture of a cerebral aneurysm [6]. Rinkel et al report that 15–20% spontaneous SAH has no detectable cause on the first angiogram [38]. The study at Kashmir observed that angiography detected 705 aneurysms in 493 aneurysmal SAH patients. The anterior communicating artery (Acom.A) accounted for 36.10% aneurysms, middle cerebral artery (MCA) 26.77%, internal carotid artery (ICA) 20.89%, anterior cerebral artery (ACA) 7.70% and vertebra-basilar (VB) arteries accounted for 6.49% aneurysms. A study, by Pakarinen 1967, revealed 40% aneurysms on the Acom.A, 36% on the MCA, 34% on the ICA and Pcom.A and 6% aneurysms on the ACA [1]. However Locksley 1966 in a study reported 38.1% aneurysms on ICA and Pcom.A, 30.3% on Acom.A, 20.9% MCA, 5.8% ACA and 5.5% aneurysms on the VB arteries [14]. Tandon, India 1988, detected 45.9% aneurysms on the ICA and Pcom.A, 24.8% Acom.A, 17.3% MCA, 10.2% ACA and 1.08% aneurysms on the VB arteries [19]. Another series showed 42.9% aneurysms on the ICA-Pcom.A complex, 25.7% aneurysms on the Acom.A, 18.7% aneurysms on the MCA, 8.5% on ACA and 4% aneurysms on the VBsystem [33]. The SKIMS study also found the present classification of aneurysmal-size like small, large and giant as an absolute to all arteries of various sizes. The study found a new parent-artery related aneurysmal size grading accurate and intra-operatively adjusting. For example two aneurysms of 5 mm size (small) each, located separately on the larger internal carotid artery and smaller M2 segment of MCA or distal anterior cerebral artery (DACA), is found intra-operatively small for the internal carotid artery and larger for the DACA or M2 of MCA. A series of studies reported multiple aneurysms in patients of spontaneous SAH, at the rate of 9%, 18.5% and to 20% [14, 33, 39]. The study in Kashmir found that 30.83% aneurysmal SAHs had multiple aneurysms accounting for > 364 aneurysms and 69.16% aneurysmal SAHs harbored single aneurysms i.e. 341 aneurysms. Thus 493 aneurysmal SAHs carried > 705 aneurysms in them. True familial intracranial aneurysm syndrome occurs when 2 firstthrough third-degree relatives have intracranial aneurysms [40]. In family members with the familial intracranial aneurysm syndrome, the risk of harboring an unruptured aneurysm was 8% [41].
A study of 23 families with familial SAH found that having 3 affected relatives tripled the risk of SAH. When magnetic resonance angiography (MRA) was used to screen 8680 asymptomatic individuals for intracranial aneurysms, the overall incidence of aneurysms was 7.0% but rose to 10.5% in those with a family history of SAH [42]. However, another magnetic resonance imaging (MRI) study reported that 4% of relatives of sporadic SAH patients had aneurysms [43]. In a large case-control study family history was found to be an independent risk factor for SAH [44]. The study at SKIMS found that 8.37% of all SAHs had evidence of first through third degree blood relations to each other and about 55.26% of these were aneurysmal, contributing 8.51% patients to aneurysmal SAHs. The rest of the 44.73% familial SAHs were of hypertensive (34.21%), idiopathic (9.21%) and AVM (1.31%) origin.
Pakarinen found rebleeding in 51.7% of SAH patients with 77.5% mortality [1]. Rebleeding was reported in 14% in a study of 167 patients [18]. At SKIMS rebleeding occurred in 25.35% SAH patients, leading to 56.28% deaths of rebled cases. The Kashmir study observed vasospasm in 40.20% of SAHs. The local vasospasm was more (74.92%) prevalent than the diffuse entity. The vasospasm was found in 30% patients of SAH in a study from India [19]. After aneurysmal SAH, angiographic vasospasm is seen in 30% to 70% of patients, with a typical onset 3 to 5 days after the hemorrhage, maximal narrowing at 5 to 14 days, and a gradual resolution over 2 to 4 weeks [45]. The secondary cerebral ischemia occurred in 27% of a series [18]. The 1980s saw the introduction of triple H therapy (prophylactic hypertensive hypervolemic hemodilution “triple-H” therapy) as a treatment for delayed ischemia due to vasospasm, and trials with nimodipine in an attempt to prevent this complication [46, 47]. The study at Kashmir found 10.57% SAH cases with hydrocephalus. Acute hydrocephalus (ventricular enlargement within 72 hours) is reported to occur in 20% to 30% of patients and is more frequent in patients with poor clinical grade and higher Fischer Scale scores [48]. Hydrocephalus was reported in 14.1% of SAH patients in a study [33]. Some SAH patients with acute hydrocephalus may benefit from early placement of a ventricular drain at the initial hospital [49]. Before 1970, carotid ligation was commonly used to treat recently ruptured intracranial aneurysms. Now it is used for aneurysms that cannot be treated by direct surgical clipping or coil embolization. The Italian neurosurgeon Dr Guido Guiglielmi introduced his endovascular coil treatment in 1991 [50]. The International Subarachnoid Aneurysm Trial (ISAT) showed that in the aneurysms of anterior circulation (Acom.A/ACA) the likelihood of death or being dependent on others for activities of daily living was reduced (7.4% absolute risk reduction, 23.5% relative risk reduction) if endovascular coiling was used as opposed to surgery [51]. Aneurysms of the VB complex and posterior cerebral artery (PCA) are hard to reach surgically and are more accessible for endovascular management [51, 52]. Aneurysms in the cavernous segment of the internal carotid artery are also difficult to treat with surgery but may be treated relatively easily with coil embolization [53]. The SKIMS series required endovascular treatment in the form of coil embolization and carotid ligation in 9.48% aneurysms and AVMs [Table 5]. Because of their morphology, MCA aneurysms can be difficult to treat by coil embolization, and surgical results for these aneurysms are often reported as more favorable [52, 54]. The study at Kashmir revealed that out of all aneurysmal SAHs (493), only 86.20% patients were clipped. The single aneurysms were clipped in 96.23% and double aneurysms in 3.76% patients [Table 5]. About 85.96% Acom.A aneurysms, 98.48% MCA and 85.44% ICA aneurysms were clipped. This study noted that a specific size of an aneurysm, on the CT angiography, grew larger intra-operatively by 0.5 –1.5 mm, especially in distal anterior cerebral artery and MCA aneurysms. This may be the result of dividing arachnoid membrane that formed cicatrized and tight adhesions around the aneurysm to shrink it after its rupture. This was more a finding noted in delayed surgery. The Kashmir study found 14.53% SAH patients having IVH, ventriculomegaly and hydrocephalus were subjected to either external ventricular drainage (EVD) or/and Ventriculo peritoneal (VP) shunts. The SKIMS study recorded 36.60% mortality of SAH patients. About one-third of all aneurysmal patients i.e. 32.04% died, thus aneurysmal deaths contributed 47.59% to total mortality. About 33.73% of all deaths were those SAHs who died of rebleeding. In a population-based study by Broderick et al the 30-day mortality rate among all patients who suffered SAH was 45%, with the majority of deaths occurring in the first days after SAH [55]. Recurrent hemorrhage remains a serious consequence of aneurysmal SAH with a case fatality rate of 70% for persons who rebled [14]. Death rate of 17% in a total of 167 patients is observed [18]. Up to half of all cases of SAH are fatal and 10–15% die before reaching a hospital [6]. The mortality for SAH is between 40 and 50% [48]. It is reported that a mortality of 77.5% occurred in patients of SAH who had rebled [1]. The initial hemorrhage can be devastating and up to a quarter of patients die before reaching medical attention [56]. A SKIMS the initial episode of subarachnoid hemorrhage led to 24.26% deaths and rebleeding caused 12.35% deaths. About 30.32% SAHs died in first two weeks and the remaining 6.29% died within following four weeks. The outcome for patients with SAH remains poor, with population-based mortality rates as high as 45% and significant morbidity among survivors [57, 15, 58]. Perhaps the most meaningful and simplest measure of the effect of these deficits is whether the patient is able to return to his or her previous occupation [59]. The study at Kashmir found good recovery in 14.99% SAH patients, most of these were aneurysmal SAHs 10.36%. The outcome of 167 SAHs in a series is reported as 17% deaths; vegetative or severe disability 15%; moderate disability 25% and good recovery 43% [18]. At SKIMS about 19.95% SAHs of all causes ended up in moderate disability. The severe disability occurred in 15.87% and vegetative state was found in 12.56% SAHs. A total of 36.60% of all SAHs died.
Conclusion
The incidence of spontaneous aneurysmal SAH in Kashmir is (13/100000 population) as common as in other geographical locations found in the literature. Familial and multiple aneurysmal SAHs are common. The risk factors specific to Kashmir are group-smoking from Jejeer and salt tea twice a day, although other common risk factors like adult females, cold remedies in chilly winters, racial and genetic are also seen. The best diagnostic tool is CTangiography. A relative (parent artery related) aneurysmal classification, than an absolute one, was found much adjustable intra-operatively. Clipping is the best treatment in multiple and anterior circulation aneurysms and coiling in posterior ones. The variation in the size of a single specific aneurysm on pre-operative angiography and intraoperatively was noteworthy. Good recovery was found in only 14.99% patients. However, high morbidity, mortality, complications and worsening outcome associated with the SAH still remain the challenges of future for a neurosurgeon.
References
- Pakarinen S. Incidence, aetiology and prognosis of primary subarachnoid hemorrhage; a study based on 589 cases diagnosed in a defined Urban Population during a defined period. Acta Neurol Scand 1967; 29: 1128.
- Symonds CP. "Spontaneous subarachnoid hemorrhage". Quarterly Journal of Medicine 1924; 18: 93- 122.
- Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, Anderson CS. Risk factors for subarachnoid hemorrhage: an update systemic review of epidemiological studies. J Stroke 2005; 36(12): 2773- 2780.
- Sahs A, Perret GE, Locksley HB, Nishioka H. Intracranial aneurysms and subarachnoid hemorrhage. Philadelphia, J.B. Lippincott, 1969.
- Smith RR, Up-Church JJ. Monitoring anti-fibrinolytic therapy in subarachnoid hemorrhage. J Neurosurgery 1973; 38: 339-344.
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007; 369 (9558): 306-318.
- Ramamurthi B. Are subarachnoid hemorrhages uncommon in India. Neurology India 1965; 13: 42-43.
- Feigin VL, Lawes CM, Bennett DA, Anderson CA. Stroke epidemiology: a review of population‐based studies of incidence, prevalence, and case‐fatality in the late 20th century. Lancet Neurol 2003; 2: 43-53.
- Inagawa T, Takechi A, Yahara K, Saito J, Moritake K, Kobayashi S, Fujii Y, Sugimura C. Primary intracerebral and aneurysmal subarachnoid hemorrhage in Izumo City, Japan, part I: incidence and seasonal and diurnal variations. J Neurosurg. 2000; 93: 958-966.
- Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, Araya F, Feuerhake W, Galvez M, Salinas R. Incidence, 30‐day case‐fatality rate, and prognosis of stroke in Iquique, Chile: a 2‐year community‐based prospective study (PISCIS project). Lancet 2005; 365: 2206-2215.
- Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004; 35: 426-431.
- De Rooij NK, Linn FHH, Van der Plas JA, Algra A, and Rinkel GJE. Incidence of subarachnoid haemor rhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007 December; 78(12): 1365-1372.
- Ingall TJ, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000; 31: 1054-1061.
- Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations: based on 6368 cases in the cooperative study. J Neurosurg. 1966; 25: 219-239.
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001; 124 (pt 2): 249–278.
- Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998; 29: 251-256.
- Ohkuma H, Fujita S, Suzuki S. Incidence of aneurysmal subarachnoid hemorrhage in Shimokita, Japan, from 1989 to 1998. Stroke 2002; 33 (1): 195-199.
- Ynte MR, SlooterAJC, Bardoel A, Frijns CJM, Rinkel GJE, and Wijmenga C. Genes and outcome after aneurysmal subarachnoid hemorrhage J Neurol 2005; 252: 417-422
- Tandon PN. Subarachnoid hemorrhage in India: An Epidemiological study. ICMR Bulletin 1988; 18: 33- 38.
- Fontanarosa PB. Recognition of subarachnoid hemorrhage. Ann Emerg Med. 1989; 18: 1199-1205.
- Ohman J. Hypertension as a risk factor for epilepsy after aneurysmal subarachnoid hemorrhage and surgery. Neurosurgery 1990; 27: 578-581.
- Claassen J, Peery S, Kreiter KT, Hirsch LJ, Du EY, Connolly ES, Mayer SA. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 2003; 60: 208-21