Research Article - Biomedical Research (2017) Volume 28, Issue 18
High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer
Hu Song1, Qi-yu Liu2 and Zhi-wei Huang3*
1Department of Gastrointestinal Surgery, the Affiliated Hospital of Xuzhou Medical University, Xu Zhou City, Jiangsu Province, PR China
2Ganmei Affiliated Hospital of Kunming Medical University, the First People's hospital of Kunming, PR China
3Laiyang Central Hospital of Yantai, Changshan Road 111#, Laiyang City, Shandong province, PR China
- *Corresponding Author:
- Zhi-wei Huang
Department of Gastrointestinal Surgery
The Affiliated Hospital of Xuzhou Medical University PR China
Accepted date: August 03, 2017
Abstract
Background: Human Fibroblast Activation Protein (FAP) is a surface glycoprotein expressed on cancer associated fibroblasts in the majority of epithelial cancers. Tumor promoting effects of FAP expression was reported in several cancers. However, in gastric cancer, its clinical significance is unclear.
Methods: Sections of primary human gastric cancer (adenocarcinoma) and adjacent normal gastric tissue specimens were collected from 112 patients. Immunohistochemistry method was used to evaluate FAP expression on sections. The overall percentage of FAP staining was assessed semi quantitatively (score=1, 2, 3). Prognostic value of FAP expression in gastric cancer was evaluated.
Results: The gastric cancer tissues showed a higher amount of FAP positive cells than adjacent normal gastric tissues. High expression of FAP was correlated with primary tumor invasion (P value=0.042) and high TNM stage (P value=0.036). In survival analysis, patients with high expression of FAP showed shorter overall survival and progression free survival compared with patients showed low expression of FAP (P value=0.026 and 0.015, respectively). High expression of FAP was identified as an independent prognosticator of gastric cancer as well (P value=0.017).
Conclusion: Our data suggested that FAP positive cells were accumulated in gastric cancer tissues. Meanwhile, patients whose gastric tumors have high level of FAP expression were more likely to have aggressive disease development and poor survival. Studies that elucidate the mechanism of how the FAP positive cells promote gastric cancer development are needed.
Keywords
Fibroblast activation protein (FAP), Gastric cancer, Survival, Prognosis, Cancer associated fibroblasts.
Introduction
Stromal fibroblasts play an important role in supporting cancer progression via inducing chronic cancer-related inflammation, secreting growth and nutrient factors, as well as reconstructing the extracellular compartment [1,2]. Human Fibroblast Activation Protein (FAP) is a 97-kDa cell surface glycoprotein with gelatinase and dipeptidyl peptidase activity and belongs to the serine protease family [3,4]. It was known that FAP is selectively expressed in activated fibroblasts of epithelial cancers and healing wounds, and malignant cells of sarcomas [3,4]. In cancer animal models, up-regulation of FAP resulted in enhanced tumor growth, invasion, and immunosuppression [5-7]. In colorectal cancer and pancreatic cancer patients, high expression of FAP predicted poor survival, suggesting the rationale of investigating FAP expression in more cancer types [8,9].
Gastric cancer is one of the most common malignancies worldwide and causes more than 720,000 deaths each year [10]. In China, 679,000 new gastric cancer cases are diagnosed each year, and more than 70% patients finally died due to the disease [11]. Accumulating evidence has suggested that the tumor stroma has crucial role in the development and progression of gastric cancer [12]. It was known that loss of stromal caveolin-1 expression promoted gastric cancer development and predicted a poor prognosis, indicating the significance of studying tumor stroma related proteins in gastric cancer [12]. Given that upregulation of FAP expression has shown tumor promoting roles, in the present study, we aimed to investigate the clinical values of FAP expression in gastric cancer patient.
Material and Method
Patient samples
We collected 112 archived Formalin-Fixed, Paraffin-Embedded (FFPE) gastric cancer tumor tissues of the patients diagnosed at our hospital during December 2010 to 2014. Meanwhile, tumor adjacent normal gastric tissues were collected as well. The study was approved by the local Ethical Committee. All the included patients had signed the informed consent of this study. The clinicopathological features of each patient were obtained from the archived medical record. The TNM classification was performed according to the 7th Edition AJCC Cancer Staging Manual.
Patient follow-up
Follow-up of patients was performed by searching the archived medical records and making phone calls. The start point of follow-up was the date of surgical treatment. The endpoint was the date when the patient died of gastric cancer or December 1st, 2016 depending on which came first. The follow-up period ranged from 2 months to 59 months with the average of 42 months. Overall Survival (OS) was defined as the interval between the date of surgery and the date of death due to gastric cancer. Progression-Free Survival (PFS) time was defined as the elapsed time between the date of surgery and the date of observed clinical progression.
Immunohistochemistry
We measured the expression of FAP on the FFPE slides of the gastric cancer patients by Immunohistochemistry (IHC). Sample sections were de-paraffinized in xylene and then rehydrated with decreased concentrations of ethanol. Slides were then washed with 1x PBS for 5 min. To block the endogenous peroxides, slides were submerged in 3% H2O2 methanol for 10 min. Antigen retrieve was performed by heating the sections with Reveal Decloaker (Biocare Medical, CA, USA) in the microwave for 15 min. Slides were then permeabilized by 0.1% Triton X-100 PBS-T Buffer for 20 min. Primary anti-FAP antibody (1:100 dilution, Abcam, USA) was added to incubate at 4°C overnight. On the second day, HRP conjugated secondary antibody (1:1000 dilution, Abcam, USA) was added to incubate for 1 h at room temperature. DAB was used as the chromogenic reagent. Each slide was evaluated by two researchers independently and blindly to the clinical information. The FAP positive area of each slide was estimated.
Statistical analysis
The statistical analysis was performed with SPSS 17.0 (IBM, USA) and GraphPad Prism 7 software. The association between FAP expression and clinicopathological features was analyzed via Chi-square analysis. To analyze the prognostic value of FAP expression, we conducted Cox’s proportional hazard model analyses. Survival curves were plotted by Kaplan Meier method.
Patients who survived through the endpoint of follow-up or got lost from the follow-up were considered as censored cases in the survival curves. ROC curve analysis was performed to determine the cut-off point of FAP expression. The point with highest sum of sensitivity and specificity regarding to the OS of patients was chosen as the cut-off point.
Difference of means was tested by t-test. Two-tailed P values of all the statistical analysis less than 0.05 was considered as statistically significant.
Results
FAP expression in the tumor tissue and adjacent normal tissue of gastric cancer patients
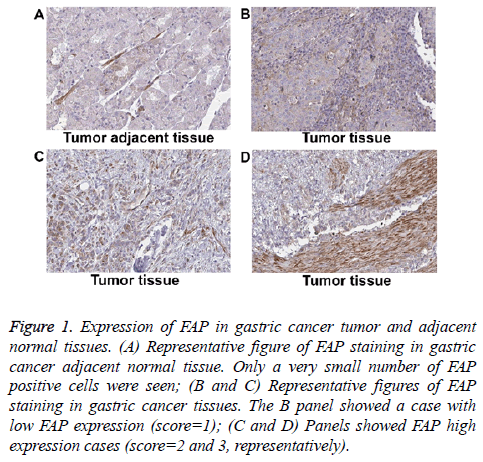
We collected a total number of 112 gastric cancer patient’s tumor tissue and the corresponding adjacent normal tissue to evaluate FAP expression. The basic information of included patients was shown in Table 1. Briefly, the average age of these patients was sixty-four, with 31.25% females and 68.75% males. All of the collected tumors were adenocarcinoma. Around half of these patients were at advanced TNM stage (III or IV stage) at diagnosis. FAP expression was evaluated via IHC method on FFPE tissue samples. The FAP positive signal was predominantly detected in the fibroblastic cells, but not in the gland cells. As shown in Figure 1, tumor adjacent normal gastric tissues had minimal amount of FAP positive cells (Figure 1A). Whereas, in cancer samples, a large amount of FAP positive cells were detected (Figures 2A-2C). Based on positive area, we assigned an expression score for each case: 1 (less than 25% positive), 2 (25%-50% positive), and 3 (more than 50% positive).
Figure 1: Expression of FAP in gastric cancer tumor and adjacent normal tissues. (A) Representative figure of FAP staining in gastric cancer adjacent normal tissue. Only a very small number of FAP positive cells were seen; (B and C) Representative figures of FAP staining in gastric cancer tissues. The B panel showed a case with low FAP expression (score=1); (C and D) Panels showed FAP high expression cases (score=2 and 3, representatively).
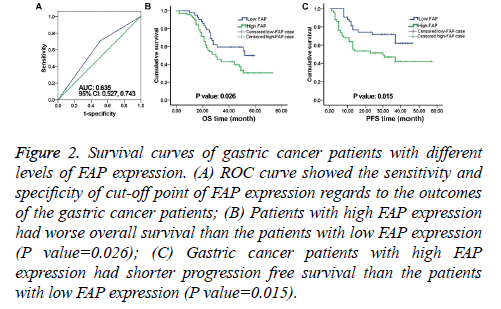
Figure 2: Survival curves of gastric cancer patients with different levels of FAP expression. (A) ROC curve showed the sensitivity and specificity of cut-off point of FAP expression regards to the outcomes of the gastric cancer patients; (B) Patients with high FAP expression had worse overall survival than the patients with low FAP expression (P value=0.026); (C) Gastric cancer patients with high FAP expression had shorter progression free survival than the patients with low FAP expression (P value=0.015).
| Features | Low FAP | High FAP | Chi-square test P value | ||
|---|---|---|---|---|---|
| n | Percentage | n | Percentage | ||
| Age | |||||
| <64 | 21 | 36.2 | 37 | 63.8 | 0.77 |
| ≥ 64 | 21 | 38.9 | 33 | 61.1 | |
| Gender | |||||
| Female | 15 | 42.9 | 20 | 57.1 | 0.43 |
| Male | 27 | 35.1 | 50 | 64.9 | |
| Grade | |||||
| High differentiation | 25 | 46.3 | 29 | 53.7 | 0.064 |
| Low differentiation | 17 | 29.3 | 41 | 70.7 | |
| T | |||||
| I+II | 20 | 50 | 20 | 50 | 0.042 |
| III+IV | 22 | 30.6 | 50 | 69.4 | |
| N | |||||
| No | 35 | 41.2 | 50 | 58.8 | 0.154 |
| Yes | 7 | 25.9 | 20 | 74.1 | |
| Distant metastasis | |||||
| No | 35 | 35 | 65 | 65 | 0.115 |
| Yes | 7 | 58.3 | 5 | 41.7 | |
| TNM stage | |||||
| I-II | 26 | 47.3 | 29 | 52.7 | 0.036 |
| III-IV | 16 | 28.1 | 41 | 71.9 | |
| Outcome of the patients | |||||
| Alive | 23 | 48.9 | 24 | 51.1 | 0.034 |
| Dead | 19 | 29.2 | 46 | 70.8 | |
Table 1. Correlation between FAP expression and clinicopathological features of gastric cancer.
Determination of cut-off point of FAP high and low expression
For further analysis, the patients were divided into two cohorts based on their FAP expression: The low FAP expression cohort and the high FAP expression cohort. Based on the ROC curve analysis, expression scores=2 was identified as the cut-off (greater than or equal to 2 means high FAP expression). ROC analysis also indicated that the Area Under the Curve (AUC) was 0.635 with 95% Confidence Interval (CI) from 0.527-0.743 (Figure 2A). 70 (62.5%) out of 112 patients showed high FAP expression.
Association between FAP expression and clinicopathological features of gastric cancer patients
The association between FAP expression and clinicopathological features was analyzed by Chi-square test. Our results indicated that patients with advanced TNM stages (III-IV stage) had a higher proportion of high FAP expression than the patients with low TNM stages (I-II stage) with the P value of 0.036. Also, the patients with advanced local invasion of the primary tumor (T I+II) at their diagnosis had higher FAP expression (P value=0.042). Other parameters, such as age, gender, lymph node metastasis, and distant metastasis were not correlated with FAP expression. These data suggested that the FAP positive cells play tumor-promoting roles during the development of gastric cancer.
The prognostic value of FAP expression in gastric cancer patients
Aiming to further explore the role of FAP expression in gastric cancer development, we evaluated its prognostic value by analyzing the archived follow-up data.
As shown in Figure 2B, the Overall Survival (OS) of the patients with high FAP expression was significantly lower than that of the patients with low FAP expression (P value=0.026).
Similar trend was seen in PFS (P value=0.015, Figure 2C). In the univariate HR analysis, tumor grade, lymph node status, TNM stage, and FAP expression were prognosticators of gastric cancer (Table 2).
| PFS | OS | |||
|---|---|---|---|---|
| Features | P value | HR (95% CI) | P value | HR (95% CI) |
| Age (≥ 64 vs. <64) | 0.449 | 1.268 (0.686, 2.346) | 0.699 | 1.101 (0.676, 1.793) |
| Gender (Male vs. Female) | 0.379 | 0.752 (0.398, 1.420) | 0.343 | 0.782 (0.470, 1.300) |
| Differentiation (Low vs. High) | 0 | 7.513 (3.713, 15.199) | 0 | 9.101 (4.885, 16.955) |
| T (III+IV vs. I+II) | 0.849 | 1.063 (0.567, 1.993) | 0.181 | 1.427 (0.847, 2.403) |
| N (Yes vs. No) | 0.026 | 2.200 (1.100, 4.401) | 0 | 2.786 (1.676, 4.631) |
| M (Yes vs. No) | 0.508 | 1.618 (0.389, 6.723) | 0 | 4.546 (2.356, 8.771) |
| TNM stage (III-IV vs. I-II) | 0 | 3.291 (1.721, 6.292) | 0 | 4.197 (2.424, 7.266) |
| FAP (High vs. low) | 0.02 | 2.158 (1.130, 4.121) | 0.03 | 1.811 (1.060, 3.095) |
Table 2. Univariate analysis of FAP expression regarding survival time of gastric cancer patients.
In the multivariate HR analysis (Table 3), high FAP expression was identified as an independent adverse prognosticator of the gastric cancer patients with a HR of 2.257 (95% CI: 1.154-4.413).
| PFS | OS | |||
|---|---|---|---|---|
| Features | P value | HR (95% CI) | P value | HR (95% CI) |
| Age (≥ 64 vs. <64) | 0.625 | 1.180 (0.608, 2.292) | 0.934 | 1.022 (0.608, 1.719) |
| Gender (Male vs. Female) | 1.186 | 0.623 (0.309, 1.256) | 0.232 | 0.524 (0.301, 0.913) |
| Differentiation (Low vs. High) | 0 | 6.719 (3.242, 13.926) | 0 | 8.752 (4.534, 16.896) |
| T (High vs. Low) | 0.334 | 0.689 (0.324, 1.465) | 0.377 | 0.759 (0.411, 1.399) |
| N (Yes vs. No) | 0.835 | 0.921 (0.423, 2.006) | 0.369 | 0.741 (0.386, 1.424) |
| M (Yes vs. No) | 0.781 | 1.263 (0.245, 6.521) | 0.006 | 4.021 (1.504, 10.747) |
| TNM stage (III-IV vs. I-II) | 0.005 | 2.843 (1.372, 5.892) | 0 | 3.371 (1.729, 6.573) |
| FAP (High vs. low) | 0.065 | 2.126 (0.956, 4.729) | 0.017 | 2.257 (1.154, 4.413) |
Table 3. Multivariate analysis of FAP expression regarding survival time of gastric cancer patients.
Discussion
The tumor stroma consists of various non-malignant cells, including endothelial cells, fibroblasts, immune cells, and so on [1,2]. Co-evolution and “reprogramming” of the stromal compartments are necessary for human cancer development [1,2]. Among the stromal cells, CAFs make up the major cell types, and reactive CAFs frequently accumulate in gastric cancer tissues [1,2]. FAP was known to be expressed in the reactive CAFs and predicted adverse prognosis in various cancers, such as colon cancer and pancreatic cancer [8,9]. However, its role in gastric cancer is unclear. In the present study, we investigated the clinical significance of FAP expression in gastric cancer patients and highlighted the potential tumor promoting effects of FAP in gastric cancer.
It was widely accepted that cancer progression depends on dysregulation of protein expression, i.e. upregulation of tumor promoting proteins and down-regulation of tumor suppressing proteins. Via comparing the gastric tumor tissues and adjacent normal gastric tissues, we noticed that FAP was up-regulated in most gastric cancer patients, suggesting that FAP might be a tumor promoting factor in gastric cancer. Meanwhile, we analyzed the correlation between FAP expression and clinicopathological parameters of gastric cancer. Importantly, we found that high expression of FAP was correlated with primary tumor invasion and TNM stage. These data were in line with the previous study, which indicated that FAP significantly enhanced invasion and migration of gastric cancer cells [13].
Proteins expressed in tumor stromal cells have shown critical values in predicting cancer patient’s survival. In breast cancer and gastric cancer, down-regulation of caveolins-1 expression in CAFs, but not in tumor cells, was correlated with poor survival [12,14]. In our study, we observed that FAP was predominantly expressed in the CAFs of gastric cancer tissue. Clinically, patients with high FAP expression showed shorter survival time than patients with low FAP expression. In the HR analyses, FAP expression was identified as the independent prognosticator of OS and PFS. A recent study also investigates the expression and clinical significance of FAP in gastric cancer patients by analyzing The Cancer Genome Atlas [15]. Mengmou et al. found that the highest FAP-α expression was observed in the poorly differentiated gastric cancer and elevated FAP-α expression correlated with adverse clinicalpathological characteristics and poor survival [15]. Meanwhile, FAP-α upregulation was associated with activation of tumor progression related pathways. Our major results were in line with the previous study. Also, our study provided a protein level validation of Mengmou’s report, further confirming the tumor promoting effects and clinical significance of FAP expression in gastric cancer.
To validate our findings, a prospective study is highly recommended regarding the clinical significance of measuring FAP expression in gastric cancer patients.
In conclusion, our study indicated that FAP was overexpressed in gastric cancer tissues compared with the adjacent normal gastric tissues. Furthermore, high expression of FAP in gastric cancer tissues is an independent adverse prognosticator, suggesting the tumor promoting effects of FAP in gastric cancer. This study affirmed the rationale for on-going clinical investigations using FAP as a therapeutic target to disrupt FAP-driven tumor progression in gastric cancer patients.
References
- Wagner EF. Cancer: Fibroblasts for all seasons. Nature 2016; 530: 42-43.
- Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016; 16: 582-598.
- Park JE. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem 1999; 274: 36505-36512.
- Pineiro-Sanchez ML. Identification of the 170-kDa melanoma membrane-bound gelatinase (seprase) as a serine integral membrane protease. J Biol Chem 1997; 272: 7595-601.
- Cheng JD. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res 2002; 62: 4767-4772.
- Santos AM. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 2009; 119: 3613-3625.
- Kraman M. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Sci 2010; 330: 827-830.
- Henry LR. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 2007; 13: 1736-1741.
- Cohen SJ. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 2008; 37: 154-158.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7-30.
- Chen W. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115-132.
- Zhao X. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One 2013; 8: e59102.
- Wang RF. Effects of the fibroblast activation protein on the invasion and migration of gastric cancer. Exp Mol Pathol 2013.
- Witkiewicz AK. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol 2009; 174: 2023-2034.
- Hu M. Biomarkers in tumor microenvironment? Upregulation of fibroblast activation protein-alpha correlates with gastric cancer progression and poor prognosis. OMICS 2017; 21: 38-44.