Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2017) Volume 7, Issue 63
Hepatoprotective and Antioxidant Effect of Stem Barks Extracts: Methanolic and Aqueous of Pseudocedrela kotschyi (Meliaceae) on Paracetamol-Induced Hepatic Damage in Rats.
Moïse Legentil Nchouwet1, Sylvie Lea Wansi Ngnokam1*, Norbert Kodjio2, Sylviane Kamani Poualeu1, Pepin Alango Nkengeffouet3 and Albert Kamanyi1
1Department of Animal Biology, Faculty of Sciences, University of Dschang, Cameroon
2Departement of Biochemestry, Faculty of Sciences, University of Dschang, Cameroon
3Department of Chemistry, Faculty of Sciences, University of Dschang, Cameroon
- *Corresponding Author:
- Sylvie Lea Wansi Ngnokam
Department of Animal Biology
Faculty of Sciences
University of Dschang, Cameroon
E-mail: wansylvie@yahoo.fr
Accepted on August 21, 2017
Abstract
The present work has been designed to evaluate hepatoprotective and in vivo antioxidant effects of aqueous and methanolic of the stem barks extracts of Pseudocedrela kotschyi against paracetamol induced hepatotoxicity in experimental rats. Hepatic damage was induced by oral administration of paracetamol (2 g/kg) in rats after two weeks of pretreatment with plants extracts. The levels of transaminases (ALT and AST), total bilirubin (TB), alkaline phosphatase (ALP), Plasma total protein (TP), total cholesterol and HDL-Cholesterol were used to assess the integrity of the liver. Antioxidant status in liver was determined by measuring the activity of malonaldehyde (MDA), glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT). Histopathological study of isolated liver specimen was also carried out. The oral pre-treatment of rats with aqueous and methanolic extracts (50, 100 and 150 mg/kg bw) showed significant (p?0.001) hepatoprotective activity against paracetamol induced hepatotoxicity by decreasing the activities of transaminases (ALT and AST), total bilirubin, alkaline phosphatase, total cholesterol and MDA compared to negative control. It increase significantly (p?0.001) the plasma protein concentration, HDL-cholesterol, liver homogenate of GSH, CAT and SOD activities compared to negative control. The effects of aqueous and methanolic extracts were comparable to a standard drug, silymarin, which was confirmed by histopathological examinations where plants extracts reduced progressively the hepatic damage in rats. These findings, therefore supported the traditional believes on hepatoprotective effect of the Pseudocedrela kotschyi stem barks extracts.
Keywords
Pseudocedrela kotschyi; Hepatoprotective activity; Paracetamol; Antioxidants.
Introduction
Liver is the most important organ of the human body and plays many vital functions such as carbon hydrate metabolism, storage of glucose, secretion of bile, synthesis and detoxification [1]. Any injury to liver or impairment of its function may lead to many implications on one’s health. The liver disorders are the health problem throughout the world. Hepatocellular carcinoma is one of the most common tumors in the world with over many new cases each year. Paracetamol is the most widely used drug for its analgesic and antipyretic effects with reduce side effects when taken in sufficient doses [2]. Hepatotoxicity from drugs and chemicals is the commonest form of iatrogenic disease. Some of the inorganic compounds producing hepatotoxicity are arsenic, phosphorus, copper and iron. Excessive consumption of paracetamol or paracetamol overdose can lead to hepatic damage caused by the formation of a reactive metabolite N-acetyl-pbenzoquinone imine (NAPQI) by the action of cytochrome P450 enzyme system contributes to the hepatic damage of paracetamol. The covalent binding of NAPQI, an oxidation product of paracetamol to sulphydryl groups of protein, induces oxidative stress which leads to cell necrosis and lipid peroxidation [3]. Reactive oxygen species (ROS) produces during oxidative stress can react with the cell membranes and thus induce lipid peroxidation or cause inflammation, have been implicated as important pathological mediators in many clinical disorders such as heart disease, diabetes, fatty liver disease, liver necrosis, gout, hepatitis, cirrhosis and cancer [1]. Antioxidants are believed to play a very important role in the body defence system against reactive oxygen species (ROS). There are numerous plants and traditional formulations available for the treatment of liver diseases. In spite of tremendous advances made in allopathic medicine, no effective hepatoprotective medicine is available.
Many plants often contain substantial amount of antioxidants including vitamin C and E, carotenoids, flavonoids, tannins etc. and can be utilized to scavenge the excess of free radicals from human body [4]. Administration of antioxidants may be useful in protecting organs against pro-oxidative challenges. There are reports that antioxidants of natural origin obtained from various herbs are highly effective not only in quenching the free radicals but also in protecting various organs and organ systems [4]. Numerous medicinal plants and their formulations are used for liver disorders in ethnomedical practice as well as traditional system of medicine in Cameroon. In modern literature, the reports indicate that plant possesses flavonoids, tannins, carotinoids, steroid etc. and these constituents are known to be antioxidants and hepatoprotective.
Pseudocedrela kotschyi (Meliaceae) is largely found in Cameroon. It is locally used by the population of Foumbam, West region of Cameroon to treat simple fevers, stomach pain and liver disorder. The literature survey of this plant revealed that traditionally it has been used for the treatment of colic, as febrifuge, ulcer, leprosy and epilepsy in Ghana [5]. The roots stem bark and the leaves are used in decoction or organic preparation for the treatment of some urogenital infections, rheumatism and dysentery [6], yellow fever, aphrodisiac and diuretic [7], nephroprotective activities and headache [8,9], antinociceptive and anti-inflammatory activities in mice and rats respectively [10,11]. However, there is no pharmacological study on the possible hepatoprotective activity of Pseudocedrela kotschyi stem bark. Therefore, the present study has been undertaken to investigate hepatoprotective activity and antioxidant properties of the aqueous and methanolic extracts of Pseudocedrela kotschyi stem bark against paracetamol induced liver damage in experimental rats.
Materials and Methods
Plant material
Pseudocedrela kotschyi (Meleacea) was collected from Foumban subdivision, West region of Cameroon during the dry season. The plant was taxonomically identified at national Herbarium of Cameroon by the taxonomists where it was registered under the number of 7007 (8563/SRFCam).
Preparation of the extracts
The stem barks was shade dried in the laboratory at the room temperature. The dried stem barks were powdered and 1 kg was obtained. It was extracted with distilled water and pure methanol.
Aqueous extract
To prepare the aqueous extract, 500 g of powder were macerated into 3 liters of distilled water for three days. After filtration using filter paper (whattman no 1), the filtrate was dried at 45-50°C using the oven (Titanox mark). The obtained product constituted the aqueous extract of the Pseudocedrela kotschyi stem bark (AEPK).
Methanolic extract
Powder (500 g) was subjected to successive extraction in methanol. The methanolic extract of Pseudocedrela kotschyi stem bark (MEPK) was prepared using soxhlet extractor, the concentrate was evaporated to dryness under reduced pressure and low temperature (40℃) on a rotary evaporator. The extract was concentrated and stored in desiccators, until further use for biological investigation. The resulting aqueous and methanolic extracts were then used for phytochemical screening, hepatoprotective and in vivo antioxidant.
Phytochemical screening
Standard test procedures were used to determine the presence of phytochemeical compound in AEPK and MEPK. The tannins, alkaloids, saponins, terpenoids, steroids, flavonoids, phenolic, carbohydrate and reducing sugar were determined according the protocol of (Harborne 1973) [12].
Drugs and chemicals
All the chemicals and solvents were of analytical grade and were purchased from either geochim laboratory Ltd, Bafoussam, Cameroon or Sigma Aldrich, German. Paracetamol was procured from local pharmacy of the hospital of Dschang, Cameroun. UV-Visible spectrophotometer was used to measure the absorbance.
Experimental animals
Both male and female Wistar rats (150-200 g) were used for the experiment. The inbreed colonies of rats were raised at the animal house of the Laboratory of Animal Physiology and Phytopharmacology (LAPHYPHA) of the University of Dschang, Cameroon. After randomization into various groups, animals were acclimatized for a period of 7 days under standard husbandry conditions at room temperature. All the animals were fed with rodent pellet diet and water allowed adlibitum under strict hygienic condition. All procedures described were approved by the University Animals Ethical Committee.
Evaluation of hepatoprotective activity in paracetamol induced hepatotoxicity
Fifty four (54) Wistar rats divided into nine groups of six per group were used;
Group I: Normal control rats (distilled water given p.o)
Group II: Distilled water+Paracetamol (2 g/kg bw, orally) (Negative control)
Group III: Silymarin (50 mg/kg/day p.o) (positive control) +Paracetamol (2 g/kg bw, orally)
Group IV-VI: Aqueous extract (50, 100 and 150 mg/kg/day respectively)+Paracetamol (2 g/kg bw, orally)
Group VII-IX: Methanolic extract (50, 100 and 150 mg/kg/day respectively)+Paracetamol (2 g/kg bw, orally)
All the animals were treated as shown above for a period of 14 days. Distilled water, Silymarin and plant extracts were administrated once a day. During this period of treatment the rats were maintained under normal diet and water. On the 15th day, paracetamol (2 g/kg p.o) was administered to all groups except group I. All the animals were anesthetized with ketamine/diazepam 24 h after the administration of paracetamol. Blood was collected by catheterism of abdominal artery in heparin tubes and was subjected to centrifugation (3000 rpm for 20 min) and plasma stored at -20℃ until it was used for the estimation of biochemical parameters (transaminase (ALT and AST), total bilirubin (TB), alkaline phosphatase (ALP), Total protein (TP), Total Cholesterol (TC) and HDL-C.
After collection of blood samples, the rats in different groups were sacrificed and their livers were excised immediately and washed in ice cold normal saline and divided in two parts. One part of liver was subjected for histological study and the other part of the liver was crushed in 0.15 M Tris (pH 7.4) to obtain homogenate. The homogenate was used either to estimate MDA [13] or measure GSH [14], SOD [15] and CAT [16] activities in the supernatants after subjecting homogenate to centrifugation (6000 rpm for 15 min) [17].
Histopathological study
Small pieces of the liver tissues in each group were collected and placed in 10% formalin solution dehydrated in graded alcohol and then embedded in paraffin. Microtome sections (5-6 μm thick) were prepared from each liver samples and stained with haematoxylin-eosin (H&E) dye. These sections were examined microscopically [18].
Statistical analysis
The experimental results were expressed as the Mean ± SEM. Comparison between groups were performed statistically using one-way analysis of variance (ANOVA), followed by Tukey- Krammer. P<0.05 was considered as statistically significant.
Results
Phytochemical composition of stem barks extracts of Pseudocedrela kotschyi
The results of qualitative analysis of AEPK and MEPK showed that each of extracts contains various phytochemical compounds such as alkaloids, tannins, terpenoids, flavonoids, steroids, saponins, phenolic, carbohydrates and reducing sugar.
Effect of aqueous and methanolic extracts on biochemical markers in paracetamol-induced liver damage
Rats subjected to the paracetamol challenge alone (negative control) developed significant liver injury as evident from a significant (p˂0.001) elevation in the biochemical markers like transaminases (ALT and AST), TB, ALP and TC in comparison with normal control rats. There was a significant (p˂0.01) decrease in the TP and HDL-C in comparison with normal control rats. Preventive oral administration of the AEPK and MEPK exhibited a significant (p˂0.01) reduction in the paracetamol induced increase in the biochemical levels, while significant (p˂0.01) increase in TP and HDL-C was observed in comparison with negative control rats. Treatment with the reference standard drug, Silymarin (50 mg/kg bw) also reversed significantly (p˂0.01) the increasing of ALT, AST, ALP, TB and TC levels while significant increase (p˂0.001) of TP and HDL-C levels was observed in comparison with negative control rats. These effects were summarized in Tables 1 and 2.
| Groups | ALT (U/L) | AST (U/L) | TB (mg/dL) | ALP (mg/dL) | Protein(g/dL) | Cholesterol (mg/dL) | HDL-C (mmol/L) |
|---|---|---|---|---|---|---|---|
| Normal | 1.103 ± 0.06 | 0.85 ± 0.1 | 3.075 ± 0.2 | 2.92 ± 0.32 | 0.17 ± 0.004 | 5.5 ± 1.06 | 0.41 ± 0.05 |
| Paracetamol | 1.881 ± 0.15c | 1.37 ± 0.03c | 4.5 ± 0.34b | 5.07 ± 0.34c | 0.092 ± 0.01c | 16.33 ± 1.6c | 0.18 ± 0.02c |
| Silymarin+Paracetamol | 1.15 ± 0.14** | 0.99 ± 0.03** | 2.94 ± 0.13** | 3.05 ± 0.43** | 0.16 ± 0.004** | 6.83 ± 1.25*** | 0.39 ± 0.03*** |
| AEPK150+Parcetamol | 1.18 ± 0.1** | 0.98 ± 0.07** | 3.16 ± 0.22* | 3.15 ± 0.42** | 0.16 ± 0.004** | 9 ± 0.73** | 0.35 ± 0.02* |
| AEPK100+Paracetamol | 1.32 ± 0.13* | 1.06 ± 0.8* | 3.2 ± 0.15* | 3.57 ± 0.27* | 0.15 ± 0.01* | 9.5 ± 1.2** | 0.31 ± 0.01 |
| AEPK50+Paracetamol | 1.35 ± 0.06 | 1.12 ± 0.04 | 3.72 ± 0.27 | 3.82 ± 0.26 | 0.13 ± 0.02 | 11.5 ± 1.26 | 0.24 ± 0.02 |
Table 1: Effect of aqueous extract of P. kotschyi (AEPK) on enzyme AST, ALT, Total Bilirubin, Total Protein, ALP, Chlesterol and HDL-C levels in blood plasma of paracetamol induced hepatotoxicity (Each values represent the Mean ± SEM (n=6). *P<0.05; **P<0.01; ***P<0.001 compared with paracetamol rats (negative control). bP<0.01, CP<0.001 compared with normal rats.).
| Groups | ALT (U/L) | AST (U/L) | TB (mg/dL) | ALP (mg/dL) | Protein (g/dL) |
Cholesterol (mg/dL) | HDL-C (mmol/L) |
|---|---|---|---|---|---|---|---|
| Normal | 1.103 ± 0.06 | 0.85 ± 0.1 | 3.075 ± 0.2 | 2.92 ± 0.32 | 0.17 ± 0.004 | 5.5 ± 1.06 | 0.41 ± 0.05 |
| Paracetamol | 1.881 ± 0.15c | 1.37 ± 0.03c | 4.5 ± 0.34b | 5.07 ± 0.34c | 0.092 ± 0.01c | 16.33 ± 1.6c | 0.18 ± 0.02c |
| Silymarin+Paracetamol | 1.15 ± 0.14** | 0.99 ± 0.03** | 2.94 ± 0.13** | 3.05 ± 0.43** | 0.16 ± 0.004** | 6.83 ± 1.25*** | 0.39 ± 0.03*** |
| MEPK 150+Paracetamol | 1.21 ± 0.14** | 0.96 ± 0.08** | 3.18 ± 0.22* | 3.37 ± 0.27* | 0.17 ± 0.01*** | 8.85 ± 0.74** | 0.33 ± 0.04 |
| MEPK 100+Paracetamol | 1.23 ± 0.14* | 0.94 ± 0.09** | 3.02 ± 0.21** | 3.12 ± 0.18** | 0.16 ± 0.01** | 9.13 ± 1.32** | 0.35 ± 0.02* |
| MEPK 50+Paracetamol | 1.4 ± 0.11 | 0.93 ± 0.03** | 3.26 ± 0.38* | 3.83 ± 0.22 | 0.14 ± 0.003 | 8.5 ± 0.92*** | 0.24 ± 0.05 |
Table 2: Effect of Methanol extract of P. kotschyi (MEPK) on enzyme AST, ALT, Chlesterol, HDL-C, Total Bilirubin, ALP, and Total Protein levels in blood plasma of paracetamol induced hepatotoxicity (Each values represent the Mean ± SEM (n=6). *P<0.05; **P<0.01; ***P<0.001 compared with paracetamol rats (negative control). bP<0.01, CP<0.001 compared with normal rats).
Effect of aqueous and methanolic extracts on oxidative stress parameters in paracetamol-induced liver damage
Malondialdehyde MDA: Hepatotoxicity rats presented significantly (p˂0.001) increased in MDA levels compared to normal control rats. Pre-treatment with different tests doses of AEPK, MEPK and Silymarin (50 mg/kg) significantly (p˂0.01) abolished the increase in MDA levels induced by paracetamol compared to negative control rats (Tables 3 and 4).
| Groups | MDA (nmol/g of tissue) | SOD(nmol/mg of protein) | CAT(nmol/mg of protein) | GSH (nmol/mg of protein) |
|---|---|---|---|---|
| Control | 0.02 ± 0.002 | 0.18 ± 0.02 | 36.5 ± 1.21 | 0.35 ± 0.04 |
| Paracetamol | 0.04 ± 0.003c | 0.05 ± 0.02c | 22.67 ± 1.41c | 0.10 ± 0.01c |
| Silymarin+Paracetamol | 0.02 ± 0.002** | 0.16 ± 0.02** | 35.1 ± 2.25*** | 0.32 ± 0.03*** |
| AEPk150+Paracetamol | 0.024 ± 0.002** | 0.13 ± 0.02* | 32.88 ± 1.84** | 0.26 ± 0.02** |
| AEPk100+Paracetamol | 0.026 ± 0.002* | 0.12 ± 0.01 | 31.43 ± 1.19* | 0.28 ± 0.02*** |
| AEPk50+Paracetamol | 0.03 ± 0.002 | 0.11 ± 0.02 | 23.82 ± 0.94 | 0.25 ± 0.04** |
Table 3: Effect of Aqueous extract of Pseudocedrela kotschyi (AEPK) on antioxidant activity of the levels of MDA, SOD, CAT and GSH levels in liver serum of paracetamol induced hepatotoxicity (Each values represent the Mean ± SEM (n=6). *P<0.0; **P <0.01; ***P<0.001 compared with paracetamol rats (negative control). CP<0.001 compared with normal rats).
| Groups | MDA (nmol/g of tissue) | SOD(nmol/mg of protein) | CAT(nmol/mg of protein) | GSH(nmol/mg of protein |
|---|---|---|---|---|
| Control | 0.02 ± 0.0016 | 0.18 ± 0.02 | 36.5 ± 1.21 | 0.35 ± 0.04 |
| Paracetamol | 0.04 ± 0.003c | 0.05 ± 0.02c | 22.67 ± 1.41c | 0.10 ± 0.01c |
| Silymarin+Paracetamol | 0.02 ± 0.002** | 0.16 ± 0.02** | 35.1 ± 2.25*** | 0.32 ± 0.03*** |
| EMPk150+Paracetamol | 0.025 ± 0.003* | 0.15 ± 0.02** | 32.85 ± 1.5** | 0.25 ± 0.03** |
| EMPk100+Paracetamol | 0.023 ± 0.003** | 0.15 ± 0.02* | 35.5 ± 1.82*** | 0.23 ± 0.01* |
| EMPk50+Paracetamol | 0.024 ± 0.002* | 0.11 ± 0.01 | 28.78 ± 2.35 | 0.21 ± 0.02 |
Table 4: Effect of Methanolic extract of Pseudocedrela kotschyi stem bark (MEPK) on antioxidant activity of the levels of MDA, SOD, CAT and GSH in liver serum of paracetamol induced hepatotoxicity (Each values represent the Mean ± SEM (n=6). *P<0.0; **P<0.01; ***P<0.001 compared with paracetamol rats (negative control). CP<0.001 compared with normal rats).
Liver antioxidant enzymes glutathione (GSH): Hepatotoxicity rats revealed significantly (p˂0.001) decreased levels of GSH in comparison to normal control rats. Pretreatment with different doses of plant extracts (50, 100 and 150 mg/kg) and Silymarin (50 mg/kg) showed significant (p˂0.001) improvement in GSH levels in comparison with negative control rats (Tables 3 and 4).
Catalase: Hepatotoxicity rats exhibited significantly (p˂0.001) decreased levels of CAT compared to the normal control rats. Pre-treatment of rats with doses of AEPK and MEPK (100 and 150 mg/kg) and Silymarin (50 mg/kg) significantly (p˂0.001) augmented the levels of CAT activity compared to negative control rats (Tables 3 and 4).
Super oxide dismutase (SOD): Treatment of rats with paracetamol significantly (p˂0.001) reduced the level of SOD activity when compared with normal control rats. While, significant (p˂0.01) increase of the level of SOD activity were found when rats were pre-treated with of the AEPK, MEPK and Silymarin during two weeks compared to negative control (Tables 3 and 4).
Effect of aqueous and methanolic extracts of Pseudocedrela kotschyi stem bark on histopathological profile study of the liver
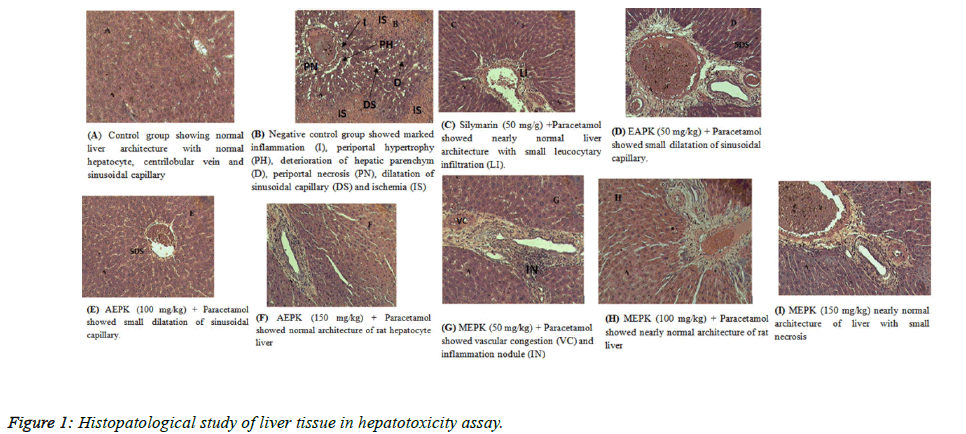
Histological study of the liver tissue revealed normal histology structures of the liver of rats in normal control with normal hypatocyte, centrilobular vein and sinusoidal capillary (Figure 1A). Animals from negative control showed histological structures with marked inflammation, peripotal hypertrophy and necrosis, dilatation of sinusoidals capillaries and ischemia (Figure 1B). Protective effect of tests extracts was established by histopathological examination of liver section. Pretreatment of the animal during a period of two weeks with AEPK and MEPK revealed small dilatation of sinusoidal capillary and nearly normal architecture and preservation of parenchyma structure of hepatocyte respectively at doses 50, 100 and 150 mg/kg of AEPK (Figure 1C-1F), vascular congestion and inflammation nodule, nearly normal architecture of rats liver and small necrosis respectively at doses 50, 100 and 150 mg/kg of MEPK (Figure 1G-1I). Pretreated rats with silymarin showed small leucocytes infiltration but the general structure of the liver was nearly similar to the structure of the animal in normal control (Figure 1C).
Discussion
Human beings constantly struggle against the changing environmental condition to maintain optimum health and vigour throughout their life, during all the seasons. In addition, they are also exposed to various xenobiotics including drugs, chemical substances; toxins etc. and other stress [4]. Physiological homeostasis is preserved in spite of exposure to several such external challenges and endogenous aberrations.
Such challenges or aberrations cause various diseases. It is increasingly being realized now that a majority of the disease/ disorders are mainly due to imbalance between pro-oxidant (free radicals) and antioxidant homeostatic phenomenon. Liver is an organ that its physiological role and its self-protective mechanism are well developed. In spite of such balanced internal milieu, hepatic aberration, damage and necrosis commonly occurring due to over exposure to hepatotoxic causes to such an extent that it over powers the mechanism. Hepatotoxicity has been reported as one of the damage caused by free radicals.
The present study has been undertaken to investigate hepatoprotective activity and antioxidant role of the aqueous and methanolic extracts of Pseudocedrela kotschyi stem bark on paracetamol-induced liver damage in rats on the basis of its traditional use.
Measurement of the liver activity is considered as a good marker of hepatic function in animals and human in general. Assessment of liver damage can be made by evaluation of the plasmatic biochemical parameters such as transaminases (ALT and AST), TB, ALP, TP, TC and HDL-C. These substances leak into bloodstream during hepatopathy, which confirms the extent liver damage [19]. In this study, rats treated only with paracetamol developed a significant hepatic damage, which was observed from a substantial increase in the biochemical markers enzyme like transaminases (ALT and AST), Bilirubin, ALP, TC. This result is an indicative of cellular leakage and loss of functional integrity of cell membrane in liver which is evident in paracetamol treated groups because these substances leak into the bloodstream when hepatic membrane damage occurs [4,17]. Paracetamol is a drug who is largely used for its antipyretic, anti-inflammatory and analgesic effects on human [20]. Exposure of liver cell membrane to hepatotoxin like paracetamol can cause destruction of hepatocyte cell’s membrane and induce the release of variety of enzymes located in cytosol into blood circulation [20]. Excessive intake of paracetamol cause acute liver damage. This drug is metabolized in liver in excretable glucuronide and sulfate conjugates [1]. The hepatotoxicity activity of paracetamol has been attributed to the formation of toxic metabolite produced from the activation of paracetamol by hepatic cytochrome P450, to N-acetyl-p-benzoquinone imine (NAPQI) who is highly toxic metabolite. The produced NAPQI is firstly detoxified by conjugation with reduce glutathione (GSH) to form mercapturic acid which is excreted in urine [1]. However, the excessive production of NAPQI due to the overdose of paracetamol breaks the SH-group of proteins, nucleic acids and membranes leading to loss of architectural integrity and functions of liver cells, increase lipids peroxidation (MDA) resulting in membrane damage or necroses and alters homeostasis of calcium after depletion of GSH, SOD and CAT concentration [21]. Pre-treatement of the rats with AEPK and MEPK during two weeks significantly decreases the levels of ALT and AST. These results indicated that a plant extracts have a possibility to fixe toxic metabolite produce during over consumption of paracetamol and protect liver hepatocyte to NAPQI damage. This effect was in agreement with the commonly accepted view that the plasma levels of transaminases save to normal with a normal hepatic parenchyma and hepatocytes [4].
Total protein, bilirubin and ALP levels are correlated to the liver function of hepatocyte [17]. Reduction of total protein levels can be deemed as a useful index of the severity of cellular dysfunction in chronic liver diseases while stimulation of biosynthesis of protein has been suggested as a hepatoprotective mechanism which enhances the regeneration process of the production of hepatocyte [22]. Bilirubin and ALP levels are the most useful clinical clues to the severity of necrosis, biliary pressure and its accumulation is a measure of binding, conjugation and excretory capacity. Any abnormal increase of total bilirubin traduces hepatobiliary disease and severe disturbance of hepatocellular architecture while increase in ALP is due to increase synthesis; in the presence of increasing biliary pressure [21]. Significant decrease (p˂0.05) of TP observed in negative control rats compared with the normal control rats, indicates hypoproteinemia activity of paracetamol while rats pretreated with plants extracts showed significant increase (p˂0.05; p˂0.01) of TP in comparison with the negative control rats. These results is similar to that obtained with Silymarin treated groups and suggest that, plant extracts as Silymarin stimulated protein biosynthesis in hepatocyte, accelerates the generation process and the production of liver cells. Administration of paracetamol showed significant increase (p˂0.05; p˂0.01) of bilirubin and ALP in negative control rats group compared with normal control rats group. This result suggests that paracetamol induces hepatobiliary disease while pre-treatment of animal with plant extracts during two weeks showed significant decrease (p˂0.01) of total bilirubin in comparison with negative control rats group. This is similar to that obtained with Silymarin treated goups. These finding suggest that the plants extracts have hypobilirubin activity and defective hepatocellular uptake, conjugation and excretion of bilirubin due to the failure of hepatic cell function [23].
Treatment of rats only with paracetamol showed a significant increase of the level of TC while a significant decrease of HDL-C in liver was observed. These results suggest that paracetamol enhance cholesterol accumulation that has negative effects on the liver tissues and cardiovascular system [24]. Pretreatment of animals with silymarin and plant extracts showed significant decrease of the level of total cholesterol while increasing level of HDL-C was observed. These finding suggest that these substances are capable to enhance the capacity of endogenous enzyme system to destroy or capture ROS and prevent the occurrence of certain pathologies [24].
Aqueous and methanolic extracts of the Pseudocedrela kotschyi stem bark were subjected to In-vivo antioxidant activity. A major defense mechanism involves the antioxidant enzymes and non-enzyme, including CAT, GSH, SOD, LPO or MDA which convert reactive oxygen species into non- toxic compounds when assayed by estimation [25]. Reactive oxygen species (ROS) or oxygen containing free radicals that are generated endogenously during normal physiological functioning or exposure to stress play vital role in causing oxidative stress and tissue damage. An antioxidant is a molecule stable enough to donate an electron to a rampaging free radical and neutralize it, thus reducing its capacity to damage. These antioxidants delay or inhibit cellular damage mainly through their free radical scavenging property. Several endogenous antioxidants that are playing vital role as organs protectants are GSH, CAT and SOD [26]. These antioxidants constitute a mutually supportive team of defense against ROS.
In paracetamol induced hepatotoxicity, the balance between ROS production and these antioxidant defenses may be lost, oxidative stress results leading to hepatic necrosis: The covalent binding of NAPQI, an oxidation product of paracetamol, to sulphydryl groups of protein results in cell necrosis and lipid peroxidation. This induces reduction in GSH levels, which attributed to be the cause of hepatotoxicity [3].
Lipid peroxidation has been postulated as being the destructive process in liver injury due to paracetamol administration [27]. Malondialdehide (MDA) is the end product of lipid peroxidation. Augmentation level of MDA in cells liver signified that lipid peroxidation has taken place and resulting from failure of antioxidant defense system to prevent formation of many free radicals, hence liver tissue damage [27]. The process of lipid peroxidation increases during inflammatory condition and pre-treatement with Silymarin or plant extracts is found to inhibit this process. Any oxidative stress to a cell induces lipid peroxidation of cell-membrane lipids. In the present study, rats group treated only with paracetamol showed significant increase (p˂0.01) of MDA level when compared with normal control group rats. This effect was significantly reduced (p˂0.05; p˂0.01) in rats pre-treated with Sylimarin or AEPK and MEPK. Hepatoprotective drugs are characterized by their index of protective effects, defined as the capacity to reduce the injurious effects or to preserve the normal hepatic physiological mechanisms or architecture, which have been disturbed by a hepatotoxin [20].
Glutathion (GSH) is a non-enzymatic biological antioxidant and one of the most abundant naturally occurring tripeptides present in the liver. GSH is capable to remove free radicals such as H2O2, superoxide radicals and alkoxy radicals produced during oxidative stress, maintenance of membrane protein thiols detoxification of foreign chemicals and biotransformation of drugs [26]. Hepatotoxicity induced by paracetamol in rats by excessive consumption, created hepatic GSH depletion, as NAPQI reacts fastly with GSH and damage the hepatocyte membrane. A significant decrease (p˂0.05) of the level of GSH was observed in negative control rats when compared with the control rats group. This result shows that paracetamol causes depletion of GSH and damage liver membrane. However, pre-treatment of rats with AEPK and MEPK, significantly increase (p˂0.05) the level of GSH compared with negative control rats group. This result is similar to that obtain with silymarin during assessment and suggest that plant extracts as silymarin prevented paracetamolinduced liver damage and indicate antioxidative activities of Pseudocedrela kotschyi stem bark by inhibition the hepatotoxicity induced by paracetamol.
Superoxide dismutase (SOD) and Catalase (CAT) are antioxidant enzymes used to scavenge ROS which cause oxidative stress responsible to produce liver injury or damage [22]. SOD is an antioxidant enzyme, responsible of catalytic dismutation of highly reactive and potentially toxic superoxide radicals to H2O2 [22]. CAT protects the tissue from highly reactive hydroxyl radicals by decomposing the hydrogen peroxide to form oxygen and water [25]. The reduced activities of CAT and SOD observed point out the hepatic damage in rats administered with paracetamol. In this study, pre-treatment with plant extracts significantly increase (P˂0.05) SOD and CAT activities. The restoration of tissue activities of SOD and CAT levels by the pre-treatment with test extracts is similar to that obtained with silymarin and indicating that the protective mechanism is being saved. This hepatoprotective activity may be attributed to the anti-oxidant activity of the plant extracts.
These observations were confirmed by the histological studies suggest that, the AEPK and MEPK progressively restored doses dependant manner the normal architecture of the liver tissue and possessed hepatoprotective and antioxidant activities against paracetamol-induced hepatotoxicity. Silymarin is a standard drug which generally showed the protection of hepatocyte membrane or regeneration of damage of hepatocytes. Silymarin is a flavonolignan that has been introduced recently as a hepatoprotective agent. The possible mechanism of silymarin hepatoprotection is due to its higher phenolic compound which has been known to contribute to the antioxidant activity. Since the preliminary phytochemical analysis of the AEPK and MEPK showed the presence of flavonoids, tannins, saponin, alkaloids, phenolic, terpenoids, steroids, carbohydrates and reducing sugar which have been known for their antioxidant and hepatoprotective properties. These phytochemical analyses are similar to that obtained by Ojewale et al. [9] on the roots of the same plant. Our results can be assimilated to these compounds well known to their antioxidant activity by scavenging free radical, enhancement of antioxidant defense system and their anti-inflammatory activity.
Conclusion
At the end of this study, it can be concluded that possible mechanism of hepatoprotective activity of Pseudocedrela kotschyi stem bark may be due to its antioxidant activity. The hepatoprotective and antioxidant activity of aqueous and methanolic extracts of Pseudocedrela kotschyi stem bark was confirmed by biochemical, antioxidant and histopathological studies. There is area for further study to characterize the active principle responsible for the hepatoprotective activity of the plant.
Acknowledgements
The autors are greatful thanks Dr NGOUPAYE T. Gwladys and Dr OUMAR Mahamat, Lecturers at Animal Biology Department of the Faculty of Sciences, University of Dschang and University of Bamenda, Cameroon respectively to helpful discussion and critical reading of this manuscript.
References
- Dash S, Pattanaik S, Rout SS, Bose A. Evaluation of hepatoprotective activity of aerial parts of Phyllanthus reticulates against paracetamol induced hepatic damage in rats. Asian J Pharm Clin Res. 2015; 8: 221-224.
- Lalit SR, Jagruti P. Antioxidant and hepatoprotective activity of ethanolic extracts of Bark of Zanthoxylum armatum DC in Paracetamol-induced hepatotoxicity. Int J Pharm Sci Drug Res. 2013; 5: 120-124.
- Abeer H, Hibah MA, Jihan MB, Amany KI, Seham EA. Evalution of hepatoprotective activity of Adansonia digitata extract on acetaminophein-induced hepatotoxixity in rats. Evidence-Based complementary and Alternative Medicine. 2016; 1-7.
- Mohammed R, Kulkarni P, Syed A, Karigar A, Koshy S, Chandur V, Pande SS, Parveen S. Hepatoprotective and antioxidant effect of whole plant extract of polygonum glabrum willd. on CCl4 induced hepatic damage in rats. Unique J Pharmac Biolog Sci. 2013; 01: 25-32.
- Neuwinger HD. Afrikanische Arzneipflanzen und Jagdgifte: Chemie, correction noted Pharmakologie, Toxikologie Eine afrikanisch verlagsgesells chaft; Auflage, 1998.
- Adekunle MF. Survey of non-timber forestproducts and their uses in Ogun State Nigeria. A case study of Omo reserve M. Phil. Thesis Univ. Of Agriculture Abeokuta Nigeria; 1998.
- Boyom FF, Fotio D, Zollo PHA, Agnaniet H, Menut C, Bessière JM. Aromatic plants of tropical Central Africa. Part XLIV. Volatile components from Pseudocedrela kotschyi (Schweinf.) Harms growing in Cameroon. Flavour Fragr J. 2004; 19: 9–11.
- Ahua KM, Ioset JR, Ioset KN, Diallo D, Mauël J, Hostettmann K. Antileishmanial activities associated with plants used in the Malian traditional medicine. J Ethnopharmacology. 2007; 110: 99–104.
- Ojewale AO, Adekoya AO, Faduyile AF, Yemitan OK, Odukanmi AO. Nephroprotective activities of ethanolic roots extract of Pseudocedrela kotschyi against oxidative stress and nephrotoxicity in alloxan-induced Diabetic albino rats. Br J Pharmacol Toxicol. 2014; 5: 26-34.
- Musa YM, Haruna AK, Ilyas M, Yaro A, Ahmadu AA, Usman H. Analgesic and anti-inflammatory activities of the leaves of Pseudocedrela kotschyi Harms (Meliaceae). Books of abstracts of the 23rd National Scientific Conference of the Nigerian Society of Pharmocognosy. 2005; 88-89.
- Abubakar K, Danjuma NM, Maiha BB, Anuka JA, Yam MF, Bello I, Naisiba US, Zaini MA. Antinociceptive activity of the crude methanolic extract of Pseudocedrela kotschyi and its chloroform and n-butanol fractions in mice. J Pharm Biomed Sci. 2016; 06: 158-164.
- Harborne JB. Phytochemical methods. London Chapman and Hall Ltd. 1973; 49-188.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351-358.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82: 70-77.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984; 21: 130-132.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972; 47: 389-394.
- Mutiat AO, Oyinlade CO, Adeteju OL. Hepatoprotective effect of Mangifera-Indica stem bark extracts on paracetamol-induced oxidative stress in albino rats. Eur Sci J. 2015; 11: 299-309.
- Damjanov I, Baltimare, Williams, Nilkins. Histopathology A colour atlas and text book. 2nd ed. 1996; p. 211.
- Dinesh KG, Jayaseelan T, Senthil J, Mani P. Evaluation of hepatoprotective and antioxidant activity of Avicennia alba (Blume) on paracetamol-induced hepatotoxicity in rats. Innov J Ayruvedic Sci. 2016; 4: 19-22.
- Effiong GS, Udoh IE, Udo NM, Asuquo EN, Wilson LA, Ntukidem IU, Nwoke IB. Assessment of hepatoprotective and antioxidant activity of Nauclea latifolia leaf extract against acetaminophen induced hepatotoxicity in rats. Int Res J Plant Sci. 2013; 4 (2): 55-63.
- Monira A, Naima ZM. Evaluation of protective and anioxidant activity of Thyme (Thumus vulgaris) extract on paracetamol-induced toxicity in rats. Aust J Basic Appl Sci. 2012; 6: 467-474.
- Taju G, Jayanthia S, Majeed A. Evaluation of hepatoprotective and antioxidant activity of Psidiumguayava extract against acetaminophen induced liver injury in rats. Int J Toxicol App Pharm. 2011; 1: 13-20.
- Das S, Sarma G. Study of hepatoprotective activity of ethanolic extract of the pulp of Eugina jambolana (JAMUN) in albino rats. J Clin Diagn Res. 2009; 3: 1466-1474.
- Ottu OJ, Atawodi SE, Onyike E. Antioxidant, hepatoprotective and hypolipidemiceffetcs of methanolic root extract of Cassia singueana in rats following acute and chronic carbon tetrachloride intoxication. Asian Pac J Trop Med. 2013; 609-615.
- Amol NM, Pradeeo BP, Kishori GP. Evaluation of activity of whole stem extracts of Oroxylum Indicum on paracetamol-induced hepatotoxicity. Int J Phama Biol Sci. 2013; 4: 255-265.
- Anil KVK, Satish R, Rama T, Anil K, Babul D, Samhitha J. Hepatoprotective effect of FlemingiaStrobilifera R.Br. on Paracetamol induced hepatotoxicity in rats. Int J Pharm Res. 2010; 2: 1924-1931.
- Ojo OO, Kabutu FR, Bello M, Babayo U. Inhibition of paracetamol-induced oxidative stress in rats by extracts of lemongrass (Cymbropogon citrates) and green tea (Camellia sinensis) in rats. Afr J Biotechnol. 2006; 5: 1227-1232.