Research Article - Biomedical Research (2021) Volume 32, Issue 6
Hepatoprotective activity and modulation of genes from lipid metabolism and inflammatory pathway in response to intervention of flax oil and fish oil against alcohol induced hepatotoxicity in rats
Tejaswi Chandrakant Chavan*, Abhijit Avinash Ghadge, Aniket Arun Kuvalekar
*Haymount Urgent Care,, 420 Owen Dr, Fayetteville, NC 28304, USA
Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth Deemed University, Pune-Satara Road, Pune, Maharashtra, India-411043
- Haymount Urgent Care
- 420 Owen Dr, Fayetteville, NC 28304
USA
Accepted date: November 01, 2021
Abstract
To evaluate hepatoprotective potential and molecular action on lipid metabolism, inflammation of flax oil and fish oil against alcohol induced hepatotoxicity in rats. Hepatic injury was induced by administering 30% alcohol (1 ml/100 g b.w./day, p.o.). Flax oil and fish oil were administered in the dose of 500 mg/kg b.w./ day orally for 15 days. Silymarin (100 mg/kg b.w./day) was used as standard drug. Biochemical parameters were analyzed from serum and liver tissue, and histological analysis was performed from liver tissue. The expressions of Fatty Acid Binding Protein 1 (FABP1), Peroxisome Proliferator Activated Receptor Gamma (PPARγ), Sterol Regulatory Element Binding Protein 1 (SREBP1), Nuclear Factor kappa β (NF-kβ) and Tumor Necrosis Factor alpha (TNF-α) genes from liver were assayed by semi-quantitative polymerase chain reaction. Administration of flax oil and fish oil prevented hepatic damage with marked improvement in hepatic function and normalization of lipid profiles in serum and liver. These interventions normalized oxidative stress through improvements in levels of antioxidant enzymes and oxidative stress markers. Expression of genes such as FABP1, PPARγ were deregulated and SREBP1, NF-kβ and TNF-α were up regulated in alcohol induced hepatotoxic rats while treatment with flax oil and fish oil showed improvement in these gene expression. Flax oil and fish oil showed a normal hepatic architecture. These results suggest that the flax oil and fish oil could protect the liver against alcohol-induced hepatotoxicity. However, further clinical studies are required to assess the safety and benefits of flax oil/fish oil in huma nbeings.
Keywords
Alcohol, Hepatoprotective activity, Fish oil, Flax oil, Silymarin
Abbreviations
PPARγ: Peroxisome Proliferator-Activated Receptor γ; SREBP: Sterol Regulatory Element Binding Protein; NF- κβ: Nuclear Factor kappa β; Acyl-CoA: Acetyl Coenzyme A; FAS: Fatty Acid Synthase; TNF-α: Tumour Necrosis Factor α; SGOT: Serum Glutamic Oxaloacetic Transaminase; SGPT: Serum Glutamic Pyruvic Transaminase; ALP: Alkaline Phosphatase; BIL: Total Bilirubin; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very Low Density Lipoprotein; TG: Triglycerides: CH: Total Cholesterol.
Introduction
The global status report on alcohol and health by world Health Organization (WHO) reported highest alcohol consumption in developed world including Western and Eastern Europe [1- 3]. A WHO study in 2016 reported about 3 million deaths of worldwide of which 5% were caused by alcohol consumption [3]. About 3.8% of global mortality is accounted for alcohol consumption [4]. Alcoholic liver disorders are frequently observed all through the world, including India [5,6], where alcohol is mostly consumed in the form of country made liquor (CML) [7].
Alcohol is one of the direct hepatotoxic agents and has intoxicating effects which produces oxidative stress and impairs tissues when consumed in high doses [8]. Metabolism of alcohol primarily occurs in liver through oxidation reactions through alcohol dehydrogenase pathway [8]. Studies on a variety of systems, cells and species including humans have demonstrated that acute and chronic ethanol treatment increases production of reactive oxygen species (ROS) like superoxide, hydroxyl radical and hydrogen peroxide in the hepatic cells that oxidize the glutathione [6], increase oxidative stress [8,9], by enhancing oxidation of lipids, proteins and DNA ultimately resulting in hepatic damage [8,9].
A balanced diet with suitable calories, carbohydrates, fats and proteins with good nutritive value helps in the regeneration of liver in patients with liver disease [10,11]. Herbal products, vitamins, minerals, and any product that is not a drug (medication) are dietary supplements [12]. Several Asian nations routinely use numerous food and nutrition supplements in diet that possess hepatoprotective activity [13]. Several nutritional supplements contain phytochemicals, which possess the potential ability to prevent or reverse different kinds of liver injuries10. Omega-3 fatty acids are also known to offer significant benefits a nutritional or dietary supplement for hepatoprotection [14]. Flax oil is one of the richest sources of Polyunsaturated Fatty Acids (PUFA) containing Alpha-Linolenic Acid (ALA)[15] and Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA), both commonly found in marine oils, are three physiologically important omega-3 fatty acids [15]. Several studies using different animal models have been reported on beneficial effects of omega-3 long-chain PUFA supplementation on liver injury [16]. Omega-3 fatty acids improve the expression of several key proteins related to inflammation, lipid metabolism and they help to reduce inflammation and lipid oxidation [13]. There are very few reports available on hepatoprotective activity of nutritional12 and the comparatively study of polyunsaturated fatty acids (Flax oil and fish oil) [15].
In the present hepatoprotective potential study of 15 days duration, we tested the hypothesis that the omega-3 polyunsaturated fatty acids (flax oil and fish oil) would be effective against alcohol induced liver injury in rats. Our specific objectives were to assess the effect of the nutritional supplement (omega-3 fatty acid) on alcohol induced hepatotoxicity Then, the blood biochemical parameters, liver biopsy, liver antioxidants enzymes and gene expression (molecular mechanism through the effect on lipid metabolism and inflammatory pathway) studies were assessed to evaluate the hepatoprotective properties of flax oil and fish oil.
Materials and Methods
Chemicals
Flax oil (Alpha Lite) was purchased from EnSigns Diet Care Pvt. Ltd. (Pune, MS, India) that contained 50% Alpha- Linolenic Acid (ALA), 20% oleic acid and 12% linoleic acid. Fish oil (Maxepa) was purchased from Merck Limited (Goa, India) which contained 60% EPA and 40% DHA. Alcohol (Ethanol) was obtained from Changshu Yangyuan Chemical, China.
Alcohol (30%) was used as hepatotoxicant for animal administration. Silymarin (Silybon-140; Micro Labs) was purchased from local pharmacy and dissolved in sterile water to make the stock solution convenient for animal administration.
Ethical approval
This study was carried out as per CPCSEA guidelines and after approval of the experimental protocol by the Institutional Animal Ethical Committee (Ref. No: BVDUMC/443/2012-2013).
Experimental animals
Three months old male albino wistar rats, weighing between 150-200 g were obtained from the institutional animal house and used in the experiment. Animals were maintained under standard husbandry conditions (Temperature 25 ± 2ºC, 12-h light: 12-h dark cycle) and fed with standard pellet diet (Nutrivet Life Science, Pune, M.S., India) and tap water adlibitum.
Assessment of hepatoprotective activity
Rats were divided into five groups by random assignment of six animals per group. The variation in the average weight of the animals in and between the groups was less than 20%. Hepatotoxicity was induced by oral administration of alcohol for 15 days.
The treatment protocol is summarized below:
1. Group I: Healthy Control (n=6); fed on a normal diet and water for 15 days.
2. Group II: Negative Control (n=6); rats were administered 30% alcohol (1 ml/100g b.w./day, p.o.), for 15 days.
3. Group III: Positive Control (n=6); the rats in this group were treated daily with 30% alcohol (1 ml/100g b.w./day, p.o.), 30 minutes after administration of silymarin (100 mg/ kg b.w./day, p.o.), for 15 days.
4. Group IV: Treatment group 1 (n=6); the rats in this group were treated daily with 30% alcohol (1 ml/100g b.w./day, p.o.), 30 minutes after administration of flax oil (500 mg/kg b.w./day, p.o.), for 15 days.
5. Group V: Treatment group 2 (n=6); the rats in this group were treated daily with 30% alcohol (1 ml/100 g b.w./day, p.o.), 30 minutes after administration of fish oil (500 mg/kg b.w./day, p.o.), for 15 days.
During the experiment, animals were observed daily for any signs of infection and/or discomfort. After 15 days of the protocol, animals were fasted overnight and dissected. Blood samples were collected from cardiac puncher and allowed to clot at R.T. for 30 minutes and serum was separated by centrifugation at 2000 rpm for 15 minutes. The liver was excised, washed in saline, weighed and part of liver was stored in 10% neutral buffered formalin for histological studies while part of the liver tissue was snap frozen in liquid nitrogen for liver biochemical parameters and gene expression analysis.
Serum biochemical analysis
Biochemical markers of liver damage and dysfunction Serum Glutamic Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), Alkaline Phosphatase (ALP), total bilirubin and lipid profile (total cholesterol, High Density Lipoprotein (HDL), Low Density Lipoprotein (LDL), triglycerides) were estimated using commercial kits (Coral clinical system, Goa, India). Very Low-Density Lipoprotein (VLDL) was estimated by using the formula: Triglycerides/5.
Liver biochemical analysis
Liver tissue was homogenized with PBS buffer (pH 7.0) and the homogenate was centrifuged at 30,000 rpm for 15 minutes. Cell free supernatant was used for assay of total protein, total cholesterol, HDL-, LDL, triglycerides (Coral clinical system, Goa, India) and antioxidant parameters like lipid peroxidation (OxisResearchTM, U.S.A), Catalase (CAT) (Sigma-Aldrich, USA), Superoxide Dismutase (SOD) (Sigma-Aldrich U.S.A). Liver tissue homogenate was deproteinated with Metaphosphoric Acid (MPA) and Trietholamine (TEAM) reagent. Homogenate tissue used for estimation of reduced glutathione (GSH) by using commercially available kits (Cayman Chemical Company) as per instructions of the manufacturer’s and VLDL was estimated by using the formula: Triglycerides/5.
Histological analysis
The liver tissue was fixed in 10% formalin and embedded in paraffin. 4μm thick sections of paraffin embedded tissue was stained with haematoxylin and eosin (H&E staining), observed under a light microscope at 20X magnifying power and photographed using Image Pro Plus (v5.1.2.59).
RNA extraction and cDNA synthesis
The RNA was extracted by using TRIzol method (Sigma- Aldrich, USA) method. The total RNA was reverse transcribed using the Super Script First-Strand cDNA synthesis kit (Invitrogen, USA). The synthesized cDNA was stored at -80°C.
Semi-quantitative polymerase chain reaction
The cDNA was diluted with 1:40 Tris buffer (T10E1 buffer) (10 mM, Tris (pH 8.0), 1mM EDTA (pH 8.0) and used to perform the semi-quantitative polymerase chain reaction (PCR) study. To perform semi-quantitative PCR, normalized concentration of 1:40 diluted cDNA was used for amplification in a 25 μl reaction consisting of 2.5 μl 10X PCR buffer (Sigma-Aldrich, USA), 2 μl 2.5 mM dNTPs (GeNei, India), 0.3μl Taq DNA polymerase (5 U/μl, Sigma-Aldrich, USA), 0.5μl each of forward and reverse KiCqStart® primers (10pM/μl stock) (Sigma-Aldrich, USA). The temperature pro?le for semi-quantitative PCR was as below: Initial denaturation at 94°C for 10 minutes, followed by 25 cycles, each comprising 1-minute denaturation at 94°C, 30 seconds annealing temperature at 60°C and 1-minute extension at 72°C with a final extension at 72°C for 5 minutes followed by incubation at 4°C. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) gene was used as a control (housekeeping gene) for normalization. Expression analysis of fatty acid binding protein 1 (FABP1), peroxisome proliferatoractivated receptor gamma (PPARγ), sterol regulatory element binding protein 1 (SREBP1), nuclear factor kappa β (NF-κβ) and tumor necrosis factor alpha (TNF-α) was done from all the samples. Modulation of gene expression study was performed using Sigma KiCqStart® primers. The primer sequences are listed in (Table 1). The amplified 25 μl PCR products were resolved by electrophoresis on 1.5% agarose gel (Low EEO Genei). The images were captured under UV-trans illuminator (Image LabTM software 4.1, Bio-Rad Laboratories, Inc).
| Gene | Primer sequence | Amplified fragment | Annealing temperature | |
|---|---|---|---|---|
| GAPDH | F 5’-AGTTCAACGGCACAGTCAAG-3’ R 5’-TACTCAGCACCAGCATCACC-3’ |
136 | 60?C | |
| FABP1 | F 5’-TGGAGGGTGACAATAAAATG-3’ R 5’-TCATGGTATTGGTGATTGTG-3’ |
86 | 60?C | |
| PPARγ | F 5’-AAGACAACAGACAAATCACC-3’ R 5’-CAGGGATATTTTTGGCATACTC-3’ |
195 | 60?C | |
| SREBP1 | F 5’- AAACCTGAAGTGGTAGAAAC-3’ R 5’-TTATCCTCAAAGGCTGGG-3’ |
142 | 60?C | |
| NF-κβ | F 5’- AAAAACGAGCCTAGAGATTG-3’ R 5’-ACATCCTCTTCCTTGTCTTC-3’ |
157 | 60?C | |
| TNFα | F 5’- CTCACACTCAGATCATCTTC-3’ R 5’-GAGAACCTGGGAGTAGATAAG-3’ |
194 | 60?C | |
GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase (Internal standard), FABP1: Fatty Acid Binding Protein1, PPARγ: Peroxisome Proliferator-Activated Receptor γ; SREBP: Sterol Regulatory Element Binding Protein; NF-κβ: Nuclear Factor kappa β; Acyl-CoA: Acetyl Coenzyme A; FAS: Fatty Acid Synthase; TNF-α: Tumor Necrosis Factor α
Table 1. List of primers used for the study.
Statistical analysis
The bands were quantified or compared by densitometry using ‘Image J’ analysis software V 1.41o (National Institute of Health, Washington). The data were presented as Mean ± Standard Error (SE). The Dunnett Multiple Comparison Test and One-Way Analysis of Variance (ANOVA) was done to estimate the statistical significance between groups. GraphPad Instat (Trial Version 3.06, GraphPad Software, San Diego, CA, USA) was used for statistical analysis while graphs were plotted using GraphPad Prism (Trial Version 5.0, GraphPad Software, San Diego, CA, USA).
Results
Serum biochemical parameters
In the present study, activities of serum SGOT, SGPT, ALP, bilirubin and lipid profile from negative control group showed a sharp and significant increase as compared to a healthy control group indicating successful induction of hepatic damage by repeated doses of 30% alcohol (Table 2). Administration of flax oil and fish oil had a favorable effect on the levels of these biochemical markers in the treatment groups. Animals treated with flax oil and fish oil exhibited improvements in SGOT, SGPT, ALP and bilirubin (P ≤ 0.01) levels, but the animals treated with the fish oil had statistically insignificant improvement in SGOT as compared to alcohol treated group. Treatment with silymarin helped to decrease the activities of these enzymes (Table 2). As compared to negative control group, flax oil and fish oil treatment exhibited improvement in lipid profiles in the animals with alcohol induced liver damage. Flax oil and fish oil treated groups displayed decrease in total cholesterol (P ≤ 0.01), LDL (P ≤ 0.01), VLDL (P ≤ 0.01), and triglycerides (P ≤ 0.01) as compared to the negative control group. Significant (P ≤ 0.01) increase in HDL was seen in the animals treated with flax oil and fish oil (Table 2).
| Group | SGOT (U/mL) |
SGPT (U/mL) |
ALP (U/mL) |
BIL (mg/dL) |
Lipid profile | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total Cholesterol (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
VLDL (mg/dL) |
TG (mg/dL) |
|||||
| I | 134.49 ± 3.0** | 154.67 ± 2.4** | 30.40 ± 1.3** | 1.13 ± 0.1** | 85.89 ± 1.6** | 19.31 ± 0.5** | 17.1 ± 1.3** | 27.71 ± 0.5** | 138.54 ± 2.5** |
| II | 162.31 ± 0.8 | 182.50 ± 2.5 | 55.89 ± 2.7 | 2.11 ± 0.2 | 203.36 ± 7.2 | 13.51 ± 1.0 | 40.2 ± 1.0 | 74.37 ± 0.8 | 371.87 ± 3.9 |
| III | 137.56 ± 1.5** | 174.16 ± 1.0* | 33.33 ± 0.7** | 1.14 ± 0.1** | 110.09 ± 2.6** | 14.02 ± 0.5 | 20.1 ± 0.6** | 39.9 ± 0.7** | 199.48 ± 3.3** |
| IV | 143.84 ± 1.2** | 143.67 ± 1.1** | 32.82 ± 1.0** | 0.95 ± 0.1** | 89.10 ± 1.3** | 19.65 ± 0.3** | 19.5 ± 1.1** | 49.42 ± 0.7** | 247.13 ± 3.4** |
| V | 156.15 ± 3.7 | 140.67 ± 2.2** | 32.87 ± 0.7** | 0.99 ± 0.2** | 113.62 ± 2.9** | 18.80 ± 0.3** | 20.1 ± 0.6** | 63.80 ± 0.3** | 319.01 ± 1.7** |
Results are a mean of measurements from six animals and are represented as Mean ± Standard Error. The data were subjected to Dunnett Multiple Comparison Test. *P ≤ 0.05; **P ≤ 0.01. Degree of freedom for one variable: Treatments 4; Residuals 25; Total 29. SGOT: Serum Glutamic Oxaloacetic Transaminase; SGPT: Serum Glutamic Pyruvic Transaminase; ALP: Alkaline Phosphatase; BIL: Total Bilirubin; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; TG: Triglycerides.
Table 2. Effect of flax oil/fish oil on liver function markers and serum lipid profile.
Liver biochemical parameters
The lipid peroxidation (MDA levels) was significantly reduced in flax oil and fish oil (P ≤ 0.05 or P ≤ 0.01) treated animals. Flax oil and fish oil treatment did not show any effect in improving the levels of reduced Glutathione (GSH) as compared to alcohol treated animals (Table 3). SOD and catalase activities were found to be significantly increased in flax oil and fish oil treated animals (P ≤ 0.01) as compared to negative control group (Table 3). The total protein content of liver tissues was improved only in flax oil (P ≤ 0.01) treated group as compared to alcohol group. The lipid profile of liver also displayed improvements in flax and fish oil treated groups when compared with negative control. Flax and fish oil treated groups displayed decrease in total cholesterol, LDL, VLDL and triglycerides (P ≤ 0.01 for all lipid profile parameters) as compared to negative control. Significant increase in HDL (P ≤ 0.01) was seen in the animals treated with flax oil and fish oil (Table 3).
| Group | SOD (U/mg) |
CAT (mU/g tissue) |
MDA (μM) |
GSH (μM) |
Total Protein (mg/gtissue) |
Lipid profile | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cholesterol (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
VLDL (mg/dL) |
TG (mg/dL) |
||||||
| I | 66.23 ± 0.43** | 0.84 ± 0.03** | 5.74 ± 0.4** | 0.82 ± 0.01* | 4.51 ± 0.13** | 27.42 ± 1.4** | 14.36 ± 0.4** | 6.30 ± 0.4** | 4.64 ± 0.1** | 23.17 ± 0.70** |
| II | 14.56 ± 0.5 | 0.56 ± 0.03 | 17.17 ± 0.6 | 0.72 ± 0.01 | 3.38 ± 0.07 | 39.18 ± 1.7 | 11.79 ± 0.3 | 16.50 ± 0.5 | 10.47 ± 0.1 | 52.34 ± 0.70 |
| III | 15.45 ± 0.81 | 0.48 ± 0.03 | 14.00 ± 0.5** | 0.72 ± 0.01 | 4.27 ± 0.01** | 29.18 ± 0.4** | 13.67 ± 0.5* | 5.70 ± 0.7** | 7.13 ± 0.001** | 35.68 ± 0.48** |
| IV | 24.29 ± 0.30** | 0.74 ± 0.02** | 14.39 ± 0.6* | 0.70 ± 0.01 | 6.0 ± 0.1** | 29.11 ± 0.4** | 15.04 ± 0.4** | 6.30 ± 0.4** | 6.83 ± 0.1** | 34.11 ± 0.74** |
| V | 18.47 ± 0.34** | 0.71 ± 0.01** | 13.44 ± 0.6** | 0.61 ± 0.02** | 3.16 ± 0.1 | 31.45 ± 0.7** | 14.01 ± 0.4** | 7.50 ± 0.5** | 8.54 ± 0.1** | 42.71 ± 0.77** |
Results are a mean of measurements from six animals and are represented as Mean ± Standard Error. The data were subjected to Dunnett Multiple Comparison Test.*P ≤ 0.05; **P ≤ 0.01; Degrees of freedom for one variable: Treatments 4; Residuals 25; Total 29. SOD: Superoxide Dismutase; CAT: Catalase; MDA: Malondialdehyde; GSH: Reduced Glutathione TC: Total Cholesterol; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; TG: Triglycerides.
Table 3. Effect of flax oil/fish oil on hepatic oxidative stress markers, total protein and hepatic lipid profile.
Liver histology
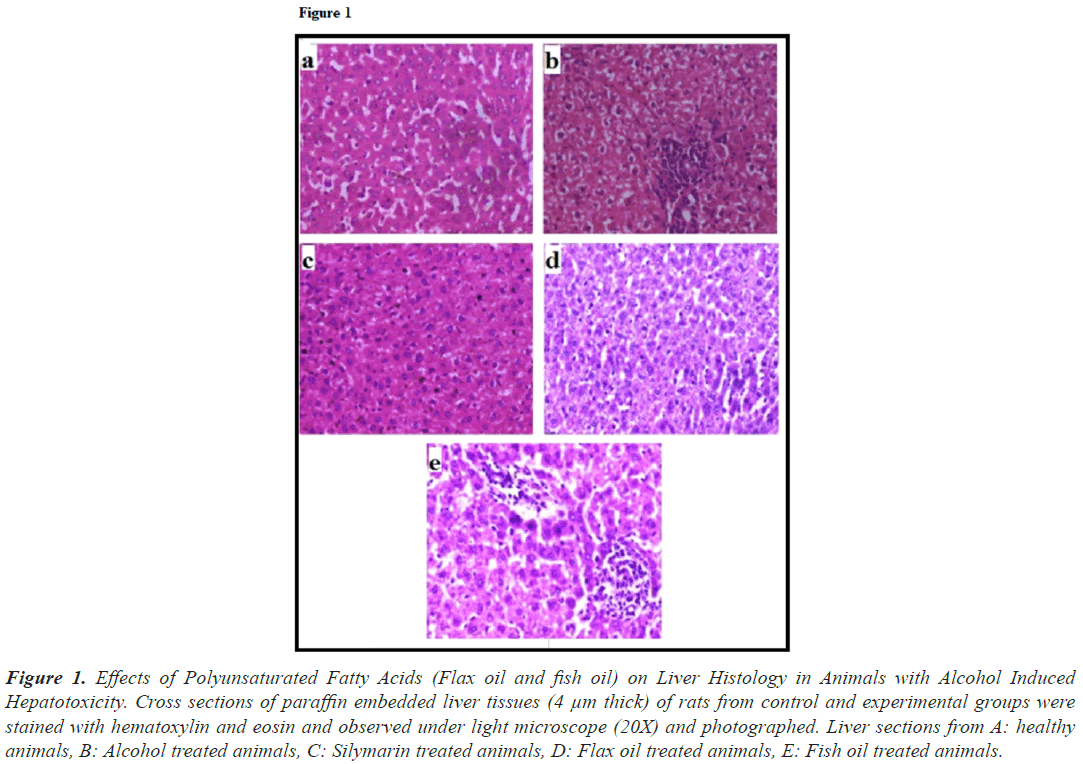
The effects of treatment with flax oil and fish oil were also visible at histological levels in animals. Liver from healthy (Figure 1a) group showed normal architecture. Sections of liver from alcohol treated group (Figure 1b) exhibited coarsely condensed cytoplasm in some cells with prominent coarse granularity in other cells, the sinusoid appeared compressed and hence the trabecular pattern of arrangement appeared disrupted. The hepatocytes were found to be swollen. Cytoplasmic borders were distinct. Liver from silymarin treated group (Figure 1c) had near normal liver architecture. The liver histology of the animals treated with flax oil (Figure 1d) exhibited normal histology. The liver histology of the group treated with fish oil (Figure 1e) exhibited improvements in the architecture except a few portal triad areas with collection of lymphocytes.
Figure 1: Effects of Polyunsaturated Fatty Acids (Flax oil and fish oil) on Liver Histology in Animals with Alcohol Induced Hepatotoxicity. Cross sections of paraffin embedded liver tissues (4 μm thick) of rats from control and experimental groups were stained with hematoxylin and eosin and observed under light microscope (20X) and photographed. Liver sections from A: healthy animals, B: Alcohol treated animals, C: Silymarin treated animals, D: Flax oil treated animals, E: Fish oil treated animals.
Genes involved in lipid metabolism
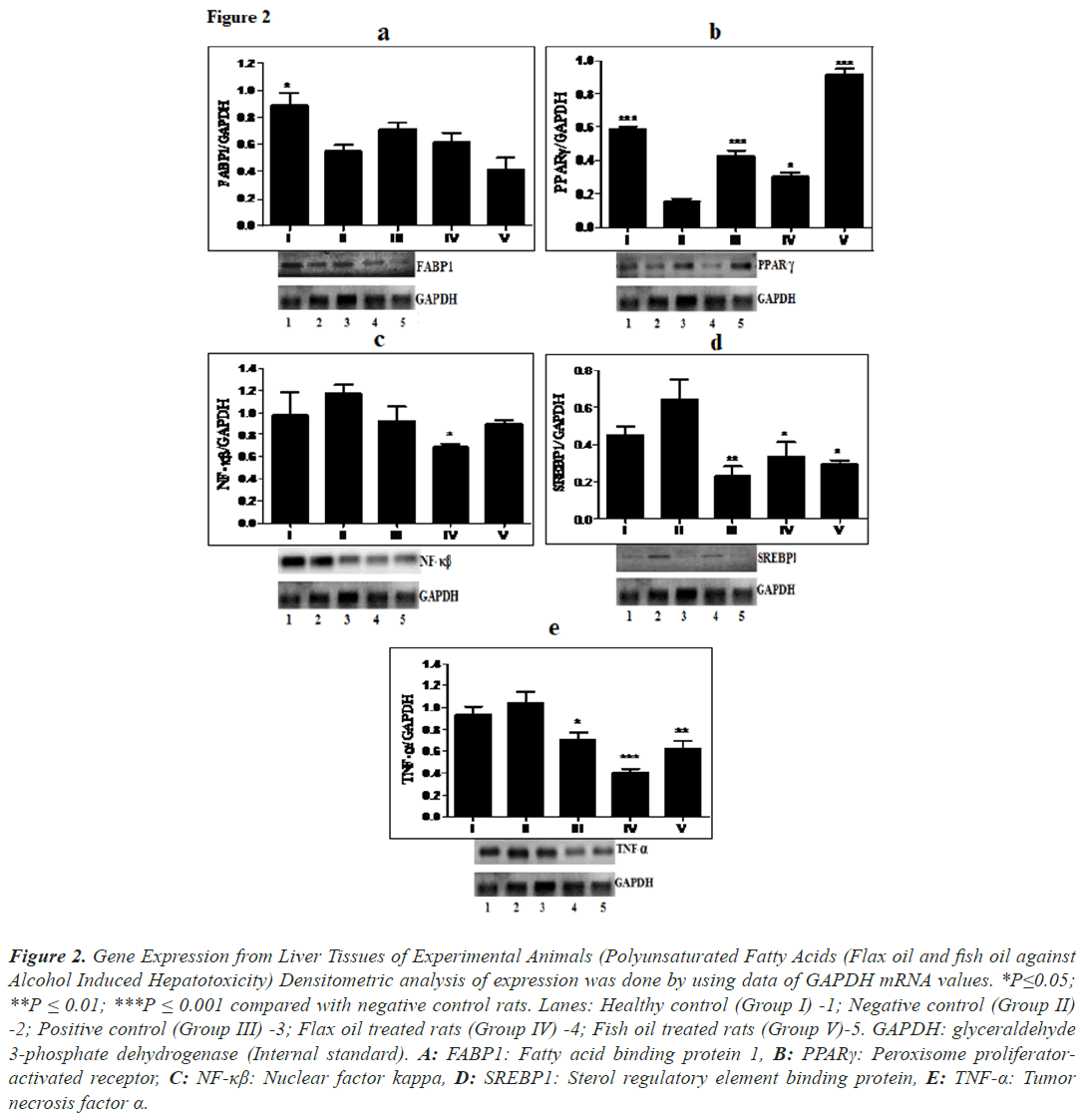
Expression of FABP1 (Figure 2a) and PPARγ (Figure 2b) decreased in alcohol treated animals than in healthy animals. Treatment with flax oil showed marginal improvement in the expression of FABP1 but it was found to be statistically insignificant. PPARγ was significantly up-regulated (P ≤ 0.001, 2.16-fold) in animals treated with fish oil and in animals treated with flax oil (P ≤ 0.05). SREBP1 (Figure 2d) were up regulated in alcohol treated animals than in healthy animals while expression of SREBP1 was significantly down regulated in both flax oil and fish oil treated groups (P ≤ 0.05). Treatment with fish oil exhibited 2.18-fold decrease in SREBP1.
Figure 2: Gene Expression from Liver Tissues of Experimental Animals (Polyunsaturated Fatty Acids (Flax oil and fish oil against Alcohol Induced Hepatotoxicity) Densitometric analysis of expression was done by using data of GAPDH mRNA values. *P≤0.05; **P ≤ 0.01; ***P ≤ 0.001 compared with negative control rats. Lanes: Healthy control (Group I) -1; Negative control (Group II) -2; Positive control (Group III) -3; Flax oil treated rats (Group IV) -4; Fish oil treated rats (Group V)-5. GAPDH: glyceraldehyde 3-phosphate dehydrogenase (Internal standard). A: FABP1: Fatty acid binding protein 1, B: PPARγ: Peroxisome proliferatoractivated receptor, C: NF-κβ: Nuclear factor kappa, D: SREBP1: Sterol regulatory element binding protein, E: TNF-α: Tumor necrosis factor α.
Genes involved in inflammation
NF-κβ (Figure 2c), and TNF-α (Figure 2e) were up regulated in alcohol treated animals than in healthy animals. Treatment with flax oil normalized the expression of NF- κβ (P ≤ 0.05). Expressions of TNF-α were significantly down regulated by both flax oil and fish oil (P ≤ 0.001 and P ≤ 0.01, respectively). Treatment with flax oil showed 2.56-fold decrease in TNF-α.
Discussion
Alcohol is one of the most used hepatotoxic agents in the experimental study of liver related disorders [1,2,4]. Type of liver injury is determined by measuring the presence of hepatocellular enzymes in liver like SGOT, SGPT and ALP [1,10,15] and increased levels of these enzymes indicate mitochondrial damage and cell membrane damage [17]. The number of reports shows elevated levels of liver function markers like SGOT, SGPT, ALP and bilirubin in ethanol-induced hepatoxicity in rats [1,10,15]. Reports showed treatment of rats with fish oil showed significant decrease in serum SGOT, SGPT, ALP and total bilirubin [15]. Reported flaxseed have been shown to be effective in restoration of increased activities of liver function enzymes [18]. Reported that rats treated with flaxseed oil showed protective effect against hypercholesterolemia induced hepatotoxicity [19]. In present study also, treatment of rats with flax oil and fish oil treatment significantly reduced levels of SGOT, SGPT, ALP and bilirubin as compared to alcohol treated group but flax oil intervention in alcohol treated rats produced significant normalization of liver function.
Many nutritional, physiological, biochemical and pharmacological research focused on the effects of flaxseed extract, raw flax seeds as well as dietary flaxseeds as baked products due to the health properties (hypolipidemic, hypoglycaemic and hypocholesterolaemia) in several animal and human intervention studies indicate that flaxseed oil has beneficial effects [14,19]. Demonstrated that supplementation of flaxseed oil resulted in significant reduction in serum total cholesterol, LDL, VLDL in rats treated with carbon tetrachloride19. Administration of 15% flaxseed chutney or flaxseed oil to rats showed improvements of the lipid profile (Increased levels of HDL, decreased level of total cholesterol and LDL) in serum and liver homogenates [20]. High cholesterol diet-induced hypercholesterolemic rats exhibited lower serum total cholesterol, triglycerides, LDL, VLDL, phospholipids, and increase in HDL after flaxseed oil intervention [21]. In the present study also, flax oil showed normolipidemic effects with decreased levels of total cholesterol, LDL, triglycerides, VLDL and increased HDL in serum as well as liver homogenates. Hepatoprotective and anti-oxidant activity of flaxseed oil has been demonstrated through restoration of antioxidant enzymes in the liver of flaxseed pre-treated animals challenged with carbon-tetrachloride [22]. Treatment of the hull fraction of flaxseed also resulted in a significant increase in hepatic anti-oxidant enzymes such as SOD, CAT, peroxidase as compared to carbon tetrachloride treated group which is attributed to Secoisolariciresinol Diglycosidic (SDG) content of the flaxseed hull [23]. The present study also reports significant increase in activity of SOD, CAT, reduced glutathione and reduced levels of lipid peroxides in flax oil treated animals as compared to alcohol treated group.
There are several reports which indicate almost normal liver architecture with minor histological anomalies after treatment with omega-3 fatty acids like flax or fish oil [13]. In the present study, PPARγ level was significantly increased in fish oil treatment animals and SREBP-1 was significantly decreased in flax oil and fish oil as compared to alcohol treated animals. Fish oil has been reported to increase the expression of PPARγ mRNA in mice liver [24]. Treatment of rats with flax oil and fish oil showed up regulation PPARγ and down regulation of SREBP-1 expression along with decreased serum triglyceride levels [25]. Fish oil also has effects on reduction of SREBP-1c and increase in the PPARα expression in ethanol induced fatty live [26].
Reported that the expression of FABP decreases with an increase in the extent of fatty liver in ethanol intoxicated rats [27]. In the present study also, near normal liver function and histology was associated with flax oil treatment which showed increase in the level of FABP expression as compared to alcohol treated animals. Various animal study reports suggest that omega-3 fatty acid supplementation lowered the expression of inflammatory cytokines like TNF-α, IL-1 and IL-6 and NF-kβ [28,29]. Treatment of mice with omega-3 fatty acids reduced Serum Glutamic Pyruvic Transaminase (SGPT) levels and decreased inflammatory response with decreased plasma TNF-α levels and this treatment also led to reduced hepatic expression of TNF-α, IL-1β, IFN-γ and IL-6 as compared to lipopolysaccharide/D-galactosamine-induced hepatitis model [28]. In the present study, expression of NF-kβ and TNF-α was significantly decreased in flax oil treated animals as compared to alcohol treated animals.
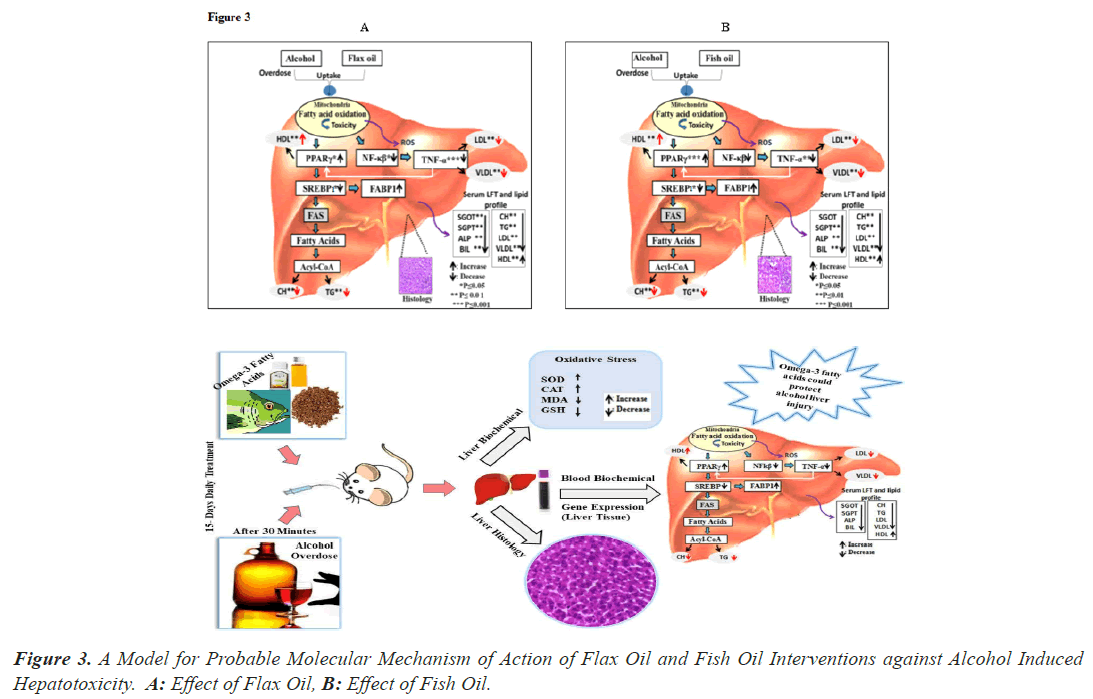
The probable molecular mechanism of action of flax oil and fish oil against alcohol intoxicated rats is shown in (Figure 3). Long term overdose of alcohol results into liver injury through mitochondria damage, increase in oxidative stress, enhance peroxidation of lipids and oxidation of protein and DNA [6,8,9] PPARγ [30] and SREBP1 [24,25] are transcription factors and regulators of lipid metabolism. Alternation of PPARγ and SREBP1 further activates the genes involved in lipid synthesis such as FABP, FAS [30] and Acetyl-CoA Carboxylase Alpha (ACACA) [30] which lead to the synthesis of cholesterol, triglyceride [29]. FABP which are involved in the fatty acid uptake, intracellular transport and in regulating lipid metabolism, cellular signaling pathways and other lipid ligands [30]. NF-κβ is one of the most important transcription factors and it is activated by inflammatory cytokines like TNF-α [29,30]. These alternations in liver function lead to variation in lipid profile which may be risk factor other clinical implication hence aggressive treatment is required to correct lipid profile and liver function test [30]. As per our omega-3 intervention study [Figure 3] shown to have protective effects against alcohol induced hepatotoxicity by improving liver function test marker, lipid profile, oxidative stress markers and the expression level of PPARγ, FABP1 showed an increase and SREBP, NF-kβ and TNF-α decreased in animals treatment with intervention. These improvements in transcription factors and genes involved in lipid metabolism and inflammatory cytokines, which improve liver function test marker and lipid profile level along with hepatic architecture. Based on these studies hepatoprotective activities of flax oil was found to be higher than that of fish oil in alcohol induced hepatotoxicity [Figure 3A].
Conclusion
In conclusion, the current study clearly demonstrates and provides novel information on the protective mechanisms of the ameliorating effects of polyunsaturated fatty acids (flax oil and fish oil) on hepatic injury caused by repeated sub-acute alcohol dosing in rats. The protective effects of flax oil and fish oil are mainly attributed to long chain polyunsaturated fatty acids like ALA, EPA and DHA. These fatty acids have been shown to have biochemical, histological, gene modulation properties and play an important role in overall metabolism. The flax oil and fish oil exhibited differential hepatoprotective activity, because their actions have different physiological targets. The dietary supplements of these oils may prove to be beneficial, especially in clinical cases where the prescribed drugs are known to be hepatotoxic on long term use. Our findings suggest that these fatty acids attenuated inflammation and improved lipid metabolism in alcohol intoxicated rats. This study is the first report of analysis of hepatoprotective potential and modulation of genes from lipid metabolism and inflammatory pathway in response to intervention of polyunsaturated fatty acids (flax oil and fish oil).
Acknowledgments
The authors sincerely thank Prof. S. Mahadik, Medical College of Georgia, USA for his kind support and suggestions. The authors are also grateful to Bharati Vidyapeeth Deemed University for providing financial support.
Funding Sources
The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Cui Y, Ye Q, Wang H, Li Y, Yao W, Qian H. Hepatoprotective potential of Aloe vera polysaccharides against chronic alcohol-induced hepatotoxicity in mice. J Sci Food Agric 2014; 94(9): 1764-1771.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. J Hepatol 2014; 60(6): 2099-2108.
- World Health Organization. Global status report on alcohol. 2018.
- Marroni CA, Fleck AM Jr, Fernandes SA, Galant LH, Mucenic M, de Mattos Meine MH, Mariante-Neto G, Brandão ABM. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J Gastroenterol 2018; 24(26): 2785-2805.
- Ramos LO, Martinez LE, Roman S, Fierro NA, Panduro A. Genetic, metabolic, and environmental factors involved in the development of liver cirrhosis in Mexico. World J Gastroenterol 2015; 21(41): 11552-11566.
- Bruha R, Dvorak K, Petrtyl J. Alcoholic liver disease. World J Hepatol 2012; 4(3):81-90.
- Kumar G, Banu GS, Pandian MR. Biochemical activity of selenium and glutathione on country made liquor (Cml) induced hepatic damage in rats. Indian J Clin Biochem 2007; 22(1): 105-108.
- Ohashi K, Pimienta M, Seki E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res 2018; 2(4): 161-172.
- Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 2015; 16(11): 26087-26124.
- Chavan TC, Aniket AK. A Review on Drug Induced Hepatotoxicity and Alternative Therapies. J Nutrition Health Food Sci 2019; 7(3): 1-29.
- Shukla SK, Kumar V. Bioactive foods and supplements for protection against liver diseases. Bioactive Food as Dietary Interventions for liver and Gastrointestinal Disease. 1st ed. Academic Press; 2013; 557-567.
- De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-arterogenic and pro-inflammatory genes. J Membr Biol 2005; 206(2): 103-116.
- Baker MA, Nandivada P, Mitchell PD, Gillian LF, Amy P, Bennet SC, Denis JDLF, Lorenzo Anez-Bustillos, Duy TD, Vania N, Mark. Omega-3 fatty acids are protective in hepatic ischemia reperfusion injury in the absence of GPR120 signaling. J Pediatr Surg 2019; 54(11): 2392-2397.
- Chavan T, Khadke S, Harke S, Ghadge A, Karandikar M, Pandit V, Ranjekar P, Kulkarni O, Kuvalekar A. Hepatoprotective effect of polyunsaturated fatty acids against repeated subacute acetaminophen dosing in rat. J Pharma and BioSci 2013; 4(2): 286-295.
- Abo El-Magd NFA, El-Karef A, El-Shishtawy MM, El-Magd LAE. Hepatoprotective effects of glycyrrhizin and omega-3 fatty acids on nuclear factor-kappab pathway in thioacetamide-induced fibrosis in rats. Egypt J Basic Appl Sci 2015; 2(2): 265-274.
- Deckelbaum RJ, Worgall TS, Seo T. n-3 Fatty acids and gene expression1 - 4. Am J Clin Nutr 2006; 83(6):1520-1525.
- Chavan T, Ghadge A, Karandikar M, Pandit V, Ranjekar P, Kulkarni O, Kuvalekar A, Mantri N. Hepatoprotective activity of stawa, an ayurvedic formulation, from three forms of Tinospora against alcohol-induced liver injury in rats. Altern Ther Health Med 2017; 23(4): 34-40.
- Essam FAl-J, Ahmed HAL-A. Antioxidant and Hepatoprotective activity of purelignan from flaxseed (linumusitatissimum l.) onacetaminophen induced toxicity in male rabbit. Int Res Stu Bio sci. 2015; 3(5): 8-16.
- Mian AA, Rashid A, M. Ehsanul H, Nawab MK. Hepatoprotective effect of flaxseed oil on hypercholesterolemia induced hepatotoxicity. P J M H S 2017; 11(3): 1159-1162.
- Yari Z, Rahimlou M, Eslamparast T, Ebrahimi-DN, Poustchi H, Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study. Int J Food Sci Nutr 2016; 67: 461–469.
- Rajesha J, Chidambara Murthy KN, Kumar MK, Madhusudhan B, Ravishankar GA Antioxidant potentials of flaxseed by in vivo model. J Agric Food Chem 2006; 54(11): 3794-3799.
- Rajesha J, Rao AR, Kumar KM, Ravishankar GA. Hepato-protective potential of hull fraction from indian flaxseed cultivar. Asian J Med Sci 2010; 1(2): 20-25.
- Newairy AA. and Abdou HM. Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food and Chem Toxicol 2009; 47: 813-818.
- Ghadge A, Harsulkar A, Karandikar M, Pandit V, Kuvalekar A. Comparativeanti-inflammatory and lipid normalizing effects of metformin and omega-3 fatty acids through modulation of transcription factors in diabetic rats. BMC Genes and Nutrition 2016; 11: 10.
- Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J. Hepatol 2008; 49(3): 441-450.
- Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-(Ppar) regulated genes and is ameliorated by ppar-activation. J Pharmacol Exp Ther 2004; 310(1): 417-424.
- Poirier H, Niot I, Degrace P, Monnot MC, Bernard A, Besnard P. Fatty acid regulation of fatty acid-binding protein expression in the small intestine. Am J Physiol Gastrointest 1997; 273(2): 289-295.
- Calder PC. Omega-3 fatty acids and inflammatory processes. Nutr 2010; 2(3): 355-374.
- Tan A, Sullenbarger B, Prakash R, McDaniel JC. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids 2018; 132: 23-29.
- Chavan TC, Ghadge AA, Kuvalekar AA. Effect of satwa from three tinospora species on lipid metabolism and inflammatory markers in acetaminophen and alcohol-induced hepato-toxicity in rats. Int J Res Rev Pharm Appl Sci 2020; 11(8): 3876-3890.