Review Article - Research and Reports on Genetics (2023) Volume 5, Issue 6
Harlequin ichthyosis: A review paper.
Jayesh J Sheth*, Yash C Shah, Saumya A Patel
Department of Genetics, Institute of Human Genetics, Ahmedabad, Gujarat, India
- Corresponding Author:
- Jayesh J Sheth
Department of Sciences,
Institute of Human Genetics,
Gujarat,
India
E-mail: jayesh.sheth@frige.co.in
Received: 24-May-2023, Manuscript No. AARRGS-23-99888; Editor assigned: 26-May-2023, AARRGS-23-99888 (PQ); Reviewed: 09-June-2023, QC No. AARRGS-23-99888; Revised: 24-Jul-2023, Manuscript No. AARRGS-23-99888 (R); Published: 31-Jul-2023, DOI:10.35841/aarrgs.5.6.171
Citation: Sheth JJ, Shah YC, Patel SA. Harlequin ichthyosis: a review paper. J Res Rep Genet. 2023;5(6):1-6.
Abstract
Harlequin ichthyosis, is the most severe form of Autosomal Recessive Congenital Ichthyosis (ARCI) with distinctive phenotypic presentation. This arises due to the disruption of the lipid delivery system caused by a mutation in one of the several genes like ABCA12, NIPAL4, PNPLA1, SULT2B1, CERS3, SDR9C7, TGM1, ALOX12B, ALOXE3, and CYP4F22. A mutation in ABCA12 is responsible for causing harlequin ichthyosis in 93% of infants. The severity of the condition seems to be associated with the type of mutation; nonsense mutations and deletions being deleterious with increased mortality as compared to the missense mutations. The neonates born with this condition have hard, thickened armor like skin all over the body and are very prone to infections due to skin exposure. In the past, such infants would die within 2-3 months after birth but with advances in neonatal facilities and retinoid therapy, there is a better chance of survival for infants with the disorder. This review discusses the epidemiology, prenatal and clinical diagnosis, molecular genetics, and treatment for the respective disorder.
Keywords
Harlequin ichthyosis, Phenotypic, Autosomal Recessive Congenital Ichthyosis (ARCI), Genes, Clinical diagnosis
Introduction
Harlequin Ichthyosis (HI) also known as “ichthyosis congenita” or “harlequin fetus type” is a rare genodermatological disorder which results in a distinct appearance of an affected infant. Neonates born with this condition have a hard, thick armor like skin covering in the form of plates along with deep fissures that are present all over the body. Distorted facial features like ectropion, eclabium, and flattened ears are the distinctive features associated with HI. Among nine genes identified to be associated with HI, the ABCA12 gene is with the most common one located on chromosome 2. This gene is a member of ATP-binding cassette protein transporters and its main function is the transport of lipid glucosylceramides to lamellar granules which further move them to extracellular space. The loss of function of the ABCA12 disrupts lipid delivery system which ultimately leads to dehydration, breathing difficulty, and increased susceptibility to infection. The pathogenic variants on both the alleles of the ABCA12 gene have severe impact on the individual with the variant [1]. Electron microscopy study of the skin of HI patients has shown the abnormal shaped lamellar or they are scanty or absent. The details of skin microscopy are well documented in the paper by Milner ME and colleagues. This skin membrane is compromised due to the transport defect of lipids through lamellar granules leading to insufficient delivery of lipids, antimicrobial peptides increased Trans-Epidermal Water Loss (TEWL) ultimately leading to increased metabolic demand, risk of electrolyte imbalance, non-regulated body temperature, and agglomeration of stratum corneum. Previously, the disorder would prove to be fatal within a few days of birth for an affected infant largely due to bacterial infection but with the advancement in neonatal intensive care and treatment methods such as retinoid therapy with oral retinoids like etretinate, isotretinoin, and acitretin, patients have an increased chance of survival. Present review describes in details about the pathogenesis to molecular biology and treatment prevention of HI [2].
Literature Review
Epidemiology
The first case of harlequin ichthyosis was reported by Reverend Oliver Hart in 1750. It is an autosomal recessive disorder with an incidence of 1 in 300,000 births globally. Technologies like single nucleotide polymorphisms and homozygosity mapping were used to identify homozygous regions at 2q35 in patients with HI. Several other mutations including insertions, deletions, termination, frame shift mutations were also observed which resulted in the absence or abnormalities of ABCA12 gene. But the mutations that allowed partial function of the gene ensured a higher survival rate in patients with the disease as compared to the patients having mutations with complete loss of gene function. Analysis of the mutations reveals that almost half of the survivors have compound heterozygous mutation and death is caused mainly by homozygous mutation. It affects individuals irrespective of their gender and ethnicity with more preponderance to females as compared to males in the ratio of 1.5:1. The severity of HI depends on the type of mutation in the ABCA12 gene with missense mutations likely to be milder than nonsense mutation [3]. Considering the incidence of HI, in India nearly twenty-five babies and globally around four hundred and sixty-six babies are born with HI every year. Due to rarity of the disease, it seems to be ignored as evident from the paucity of published literature. Table 1 depicts the number of HI cases studied genetically in different countries that include ten cases of infants of Scandinavian descent, ten infants of Japanese descent, six infants of Chinese descent, three infants of French descent, two Ecuadorian, one Spanish, one Italian, one infant of British descent, one infant of Malaysian descent, one Syrian infant, one Italian, one Hmong Laotian, one of Jamaican Eritrean, one Iranian, and three Indian have reported to be affected with HI. From the published literature, Japan and Scandinavia have a greater number of reported cases of HI. A survey was conducted in Iran, England, USA, Turkey, Sweden, New Zealand and forty-five cases of HI were noted with a survival rate of 56% (25/45). This report showed cases of people from various origins like ten of British Pakistani, five of British White, three of British Somali, two of British Bangladeshi, two of North American, two of Baluchistan, two of Swedish White, two of Turkish origin, two of Iranian, two of Swiss White, one of British Indian, one of Bangladeshi, one of Israeli, one of Jamaican Eritrean, one of Egyptian, one of Hmong Laotian, one of Kosavan, one of Afghani Iranian, one of South American, one of native American, one of Central American, one of Irish White and one of Arabian. This report stated that 83% of the individuals suffering from the disease when treated with retinoids were able to survive whereas the rate of survival was only 24% for infants who were not given the treatment [4]. A clinical survey was conducted in Japan for HI and questionnaires were distributed to pediatric centres. A clinical data for sixteen patients with HI was obtained where thirteen infants had survived and three died. Twelve individuals received retinoid therapy out of which eleven were able to survive which shows a survival rate of 91.7% whereas out of the four infants who did not receive the retinoid therapy, only two of them had survived showing a survival chance of 50%.
Results and Discussion
Clinical features
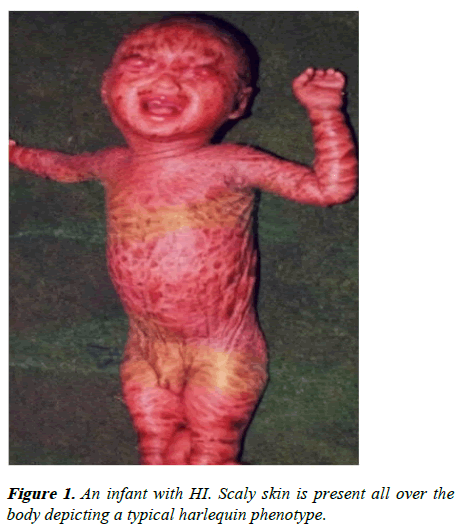
Harlequin ichthyosis is the most severe form of congenital ichthyosis presenting as a baby with scale like plates all over the body (Figure 1). The armor plating is in the form of diamond shaped hard plaques and is separated by deep fissures. Most of the babies suffering from this disease have a premature birth. Pseudo contractures may occur as a result of thick skin which may cease the movement of fingers and nails and may even lead to digital necrosis. The skin of infants with HI gets characterized by erythema and scaling of skin from the outer layer of epidermis once the baby moves out of the humid intrauterine environment to the extra uterine environment and in many cases, it is also associated with PPK (Palmo Plantar Keratoderma) [5]. Due to the compromised skin barrier function, the infants are not able to control and maintain water loss and temperature respectively and become more prone to infections. The prognosis of the HI neonates is poor mainly because of respiratory failure, infections from various pathogens, feeding problems, and loss of moisturization. Other generalized features include hyper keratinization of skin, deep fissures, yellow scale, large and flat nasal bridge, flattened philtrum. Syndactyly and camptodactyly of fingers and toes are also observed in some of the cases. This disorder is different from the collodion baby phenotype on the basis of clinical appearance. Common features of HI include armor like yellow skin, while in case of CBP the skin becomes waxier and more translucent. Conditions like ectropion and eclabium are visible in both the cases but are more severe in HI as compared to CBP (Figure 1) [6].
Histopathology
Severe keratosis and acanthosis (rare) of skin cells are associated with the histopathological findings of the individuals suffering from HI (Figure 1). A reduction in size of the elastic tissue is also observed. Investigations in the brain revealed an absence of normal laminar arrangement of neurons and also showed the presence of neurotic lesions along with severe inflammation of the alveoli associated with broncho-pneumonic changes that are prevalent in the lungs. Enlargement of the thymus is also reported in many cases [7]. A thick layer of stratum corneum with absence of lamellar structures is observed in the skin with the help of a light microscope. No major changes are observed in the spinous, basal and granulous cell layers. Migration of inflammatory cells is generally absent but the inflammation is prevalent in case of secondary infection. The lamellar granules are not seen in the upper spinous and granulous layers which are located in the cytoplasm of keratinocytes. There is also an absence of extracellular lamellar structures in the space between cornified cellular and granular cell layers. A small number of large shaped mitochondria are also prevalent in some keratinocytes of body regions like scalp, trunk, face. There is also prevalence of abnormal lipid droplets and vacuoles which are concealed into the intracellular spaces of cornified cells especially in the proximal and distal cell layers [8].
Molecular genetics of HI
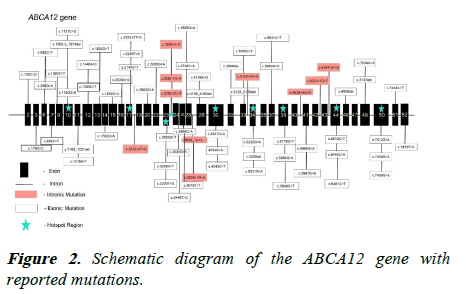
There are nine genes identified to be associated with HI that include ABCA12, NIPAL4, PNPLA1, SULT2B1, CERS3, SDR9C7, TGM1, ALOX12B, ALOXE3, and CYP4F22. Among these, the most common mutation in ABCA12 gene observed in 93% of cases making it the hot spot gene. ABCA12 is a large gene comprising of 22,000 kbp containing 53 coding exons and is located on chromosome 2 (Figure 2). This gene encodes for a protein of 2595 amino acids and is a member of ATP-binding cassette protein transporters. Its main function is to facilitate energy dependent transport of epidermal lipids mainly glucosylceramide and lamellar granules back and forth of some specialized organelles which are located in the upper layers of the epidermis [9]. This makes this protein as very crucial for the formation and functioning of the lamellar granules and lipid bilayer in the outermost part of the skin serving as an essential component of skin barrier. The loss of function of the ABCA12 gene disrupts lipid delivery system which ultimately leads to compromised skin barrier making the patient susceptible to other complications like dehydration, secondary infections, breathlessness, feeding problems, and more [10]. Many different pathogenic variants have been identified in the ABCA12 gene of the individuals affected by this disease. The effect of these variants is deleterious as they partially or completely destruct the function or production of transporter protein encoded by the gene. Many of the affected individuals suffering from the disorder are found to be homozygous for the variant with poor survival. A partial gene deletion ranging from 1-30 exons is also observed in some variants. The mutations are present on both introns as well as exons [11]. Since the protein of the gene is essential in lipid delivery, the mutation in the gene hampers the lipid delivery system leading to malformation of lamellar granules and incomplete or no secretion of epidermal lipids into the intercellular spaces. This prevents the formation of lipid bilayers into the stratum corneum hampering the skin barrier function. The transport of the proteolytic enzymes necessary for the normal desquamation of epidermis is also disrupted leading to a massive production of stratum corneum in the epidermis leading to harlequin ichthyosis. Though mutation occur in all exons and some introns also, exon 10, 17, 30, 34, 39, 44, 50 exon-intron 23 and exon-intron 26 seems to be the regions where maximum number of mutations are present on the ABCA12 gene (Table 1) [12].
| Location | DNA change | Protein change | Ethnicity in which reported |
|---|---|---|---|
| Exon 2 | c.130C>G | p.(Arg44Gly) | Not known |
| Exon 3 | c.179G>C | p.(Arg60Pro) | Not known |
| Exon 6 | c.596G>A | p.(Trp199*) | Scandinavian |
| Exon 7 | c.859C>T | p.(Arg287*) | Chinese |
| Exon 10 | c.1062–3_1074del | p.(Leu355Lysfs*12) | Japanese |
| Exon 10 | c.1160G>A | p.(Ser387Asn) | Japanese |
| Exon 11 | c.1194_1221del | p.(Gln400Phefs*18) | Japanese |
| Exon 12 | c.1300C>T | p.(Arg434*) | Not known |
| Exon 12 | c.1446A>C | p.(Glu482Asp) | Not known |
| Exon 14 | c.1782G>A | p.(Glu594=) | Scandinavian |
| Exon 16 | c.2033A>G | p.(Asn678Ser) | Not known |
| Intron 17 | c.2332+2T>G | - | British |
| Exon 22 | c.3085G>A | p.(Glu1029Lys) | Chinese |
| Exon 23 | c.3265G>T | p.(Val1089Phe) | Scandinavian |
| Intron 23 | c.3295-2A>G | - | Japanese |
| Intron 23 | c.3295-1G>A | - | Malaysian |
| Intron 23 | c.3295-2A>G | - | Japanese |
| Exon 24 | c.3535G>A | p.(Gly1179Arg) | Hmong/Laotian |
| Exon 26 | c.3746C>A | p.(Ser1249*) | French |
| Intron 26 | c.3829+1G>A | - | Not known |
| Intron 26 | c.3829+1G>A | - | Scandinavian |
| Exon 28 | c.4158_4160del | p.(Thr1387del) | Japanese |
| Exon 28 | c.4139A>G | p.(Asn1380Ser) | Spanish |
| Exon 30 | c.4541G>A | p.(Arg1514His) | Scandinavian |
| Exon 30 | c.4554G>A | p.(Trp1518*) | Scandinavian |
| Exon 30 | c.4543C>T | p.(Arg1515*) | Japanese |
| Exon 32 | c.4896del | p.(Ser1633Hisfs*30) | Scandinavian |
| Exon 33 | c.5005C>T | p.(Gln1669*) | Japanese |
| Exon 33 | c.5125_5128del | p.(Asp1709Thrfs*4) | Syrian |
| Intron 33 | c.5128+3A>G | - | Scandinavian |
| Exon 34 | c.5232G>A | p.(Trp1744*) | Chinese |
| Exon 34 | c.5229del | p.(Trp1744Glyfs*24) | Italian |
| Exon 37 | c.5690G>C | p.(Arg1897Thr) | Eritrean/Jamaican |
| Exon 39 | c.5779G>T | p.(Val1927Leu) | Ecuadorian |
| Exon 39 | c.5884G>A | p.(Gly1962Ser) | Chinese |
| Intron 40 | c.5939+4A>G | - | Indian |
| Exon 41 | c.5985G>A | p.(Met1995Ile) | Japanese |
| Intron 42 | c.6233+1G>T | - | Iranian |
| Intron 43 | c.6394-2A>G | - | Spanish |
| Exon 44 | c.6610C>T | p.(Arg2204*) | Ecuadorian |
| Exon 44 | c.6443C>A | p.(Pro2148Gln) | Chinese |
| Exon 46 | c.6858del | p.(Phe2286Leufs*6) | Chinese |
| Exon 48 | c.7137del | p.(Met2380Cysfs*25) | Scandinavian |
| Exon 48 | c.7239G>A | p.(Leu2413=) | Japanese |
| Exon 50 | c.7412G>A | p.(Gly2471Glu) | Scandinavian |
| Exon 50 | c.7436G>A | p.(Arg2479Lys) | French |
| Exon 51 | c.7444C>T | p.(Arg2482*) | French |
Table 1. Some of the reported mutations in ABCA12 causative of Harlequin ichthyosis.
Prenatal diagnosis of HI
Harlequin ichthyosis can be prenatally diagnosed using different techniques like 3D ultrasonography, amniocentesis, Chorionic Villus Sampling (CVS), and fetal skin biopsy. 3D ultrasonography can detect abnormalities like a large open mouth, a flat nose, ectropion, eclabium, flattened ears, short feet, and abnormal limb position. Other intrauterine abnormalities that are linked with HI include growth restriction, polyhydramnios, increased echogenicity of amniotic fluid, and floating membranes. These malformations can be identified without the use of any invasive technique. A limitation to sonography is that it cannot detect any abnormality until the second trimester which minimizes the option of early termination of pregnancy. The earliest diagnosis using a 3D ultrasonography occurred at twenty-two weeks. Delayed prenatal diagnosis of HI leads to several neonatal complications at birth [13]. Therefore, in order to detect HI in the early stages of pregnancy amniocentesis and Chorionic Villus Sampling (CVS) is the preferred choice. Both of these techniques use the fetal tissue or amniotic fluid obtained using an invasive technique to confirm a mutation in the ABCA12 gene. The prerequisite of this approach is the confirmed mutation study of the index case or availability of its DNA for the analysis followed by mutation study in parents and prenatal tissue. This approach provides almost 100% accuracy of the prenatal diagnosis. Skin biopsy is not recommended to pregnant females because of the related risk of preterm pregnancy, miscarriage, and possible haemorrhage [14].
Treatment
Timely and proper care is very critical for the survival of a harlequin baby. At birth there are high chances of contracting a fetal infection and therefore, the baby must receive proper care as early as possible after birth. First of all, these baby a need multidisciplinary approach from different medical fields like dermatology, pediatrics, neonatology, genetics, ophthalmology, otolaryngology, orthopaedic, plastic surgery, nutrition, physical therapy, and nursing. This will maximize the chance of survival by minimizing the high risk of respiratory failure, electrolyte imbalance, temperature dysregulation, malnutrition, and Transepidermal Water Loss (TEWL). In case of the presence of severe hyperkeratosis bands, a surgical escharotomy is performed to release the hyperkeratotic bands under local anaesthesia [15]. This should be done within a day or two of birth to avoid any complications and improve the digital functions. The major cause of death for most HI patients is found to be infection in both the lungs and hence intubation at an early stage is suggested. Application of petroleum and gentle bleach bath is advised to heal the incisions formed due to escharotomy. Skin care also involves application of petroleum jelly, extra virgin coconut oil, and sunflower seed oil once or twice daily as have been suggested to have antibacterial properties. Buffered dilute sodium hypochlorite baths are also recommended to promote shedding of stratum corneum by deep cleaning and hydrating the skin. The procedure for this bath includes creating a mixture of 0.125% sodium hypochlorite mixed with 1:10 warm sterile water, maintained at a pH 8 to 8.5, and gently applying it on the infant’s body with the help of roll gauze and a plastic wrap for ten to twenty minutes [16]. The application of a bland emollient is advised after removing the wet wrap. However, the application of keratolytic containing emollient like salicylic acid is discouraged because it possesses the risk of percutaneous toxicity. Nutritional care of the HI neonates needs special attention as they have high caloric demand because of TEWL and large body surface to weight ratio [17]. Nutritional balance order to reduce pain, adequate narcotics are prescribed to the neonate. If the pain is not controlled and surgery is not performed on time, there are chances of developing tissue necrosis and autoamputation. The severity of pain reduces upon removal of the surface layer either by natural shedding or by surgical removal. Infants have a high risk of developing eye related infections like conductivities because of ectropion. Therefore, the application of lubricant ophthalmic ointment should be promoted every six to twelve hours. The use of retinoid therapy for treating patients with HI has reported an 83% to 86% survival rate when treated with oral retinoids [18]. On the other hand, 76% babies who did not receive retinoid therapy died, and of the 76%, 64% died by day three of life. Some of the common retinoids used in the treatment of HI are etretinate, isotretinoin, and acitretin. The first successful neonatal use of acitretin in HI was reported in the year 2001 at a dose of 1 mg/kg per day [19].
Gene therapy
Gene therapy has also shown a positive result in patients with HI. In this process, the autologous keratinocyte stem cells are cultured and modified genetically to form sheets of the disease free epithelium with the help of an integrating vector. The genetic modification is done by amplifying overlapping regions of ABCA12 cDNA by PCR using human keratinocyte cDNA as a template. As a result, a full length cDNA was constructed and sub-cloned into the pCMV-tag4B vector. This newly constructed vector was used to transfect the HI keratinocytes using lipofectamine reagent [20]. This has shown a restore of lipid secretion in lamellar granules in HI keratinocytes. Staining procedures showed an increase in the normal distribution of glucosylceramide staining of 16.7% keratinocytes after corrective ABCA12 gene transfer as compared to 7.0% staining in cells having defective ABCA12 gene. Even though corrective gene therapy has shown positive results, there are chances of occurrence of insertional mutagenesis.
Conclusion
HI is the fatal genodermatological disorder with higher mortality and morbidity in neonatal period. Its early diagnosis and multidisciplinary care provide better survival chances. Genetic diagnosis provides a useful clue to the severity of the disease and helps in the prenatal diagnosis during pregnancy. It will also identify the carrier status of other unaffected family members thereby providing a precise genetic counselling to the affected families. In addition to retinoid therapy, gene therapy holds a promise that restores lipid secretion in lamellar granules in HI keratinocytes.
Acknowledgement
We sincerely thank Aadhira Nair and Jhanvi Shah for providing guidance in writing our manuscript.
References
- Sheth JJ, Bhavsar R, Patel D, et al. Harlequin ichthyosis due to novel splice site mutation in the ABCA12 gene: Postnatal to prenatal diagnosis. Int J Dermatol. 2018;57(4):428-33.
[Crossref] [Google Scholar] [PubMed]
- Kelsell DP, Norgett EE, Unsworth H, et al. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet. 2005;76(5):794-803.
[Crossref] [Google Scholar] [PubMed]
- Akiyama M, Sugiyama-Nakagiri Y, Sakai K, et al. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115(7):1777-84.
[Crossref] [Google Scholar] [PubMed]
- Elias PM, Williams ML, Feingold KR. Abnormal barrier function in the pathogenesis of ichthyosis: Therapeutic implications for lipid metabolic disorders. Clin Dermatol. 2012;30(3):311-22.
[Crossref] [Google Scholar] [PubMed]
- Montalvan-Suarez M, Esperón-Moldes US, Rodríguez-Pazos L, et al. A novel ABCA12 pathologic variant identified in an Ecuadorian harlequin ichthyosis patient: A step forward in genotype-phenotype correlations. Mol Genet Genomic Med. 2019;7(5):e608.
[Crossref] [Google Scholar] [PubMed]
- Glick JB, Craiglow BG, Choate KA, et al. Improved management of harlequin ichthyosis with advances in neonatal intensive care. Pediatrics. 2017;139(1).
[Crossref] [Google Scholar] [PubMed]
- Ahmed H, O’Toole EA. Recent advances in the genetics and management of harlequin ichthyosis. Pediatr Dermatol. 2014;31(5):539-46.
[Crossref] [Google Scholar] [PubMed]
- Rajpopat S, Moss C, Mellerio J, et al. Harlequin ichthyosis: A review of clinical and molecular findings in 45 cases. Arch Dermatol. 2011;147(6):681-6.
[Crossref] [Google Scholar] [PubMed]
- Akiyama M. ABCA12 mutations and autosomal recessive congenital ichthyosis: A review of genotype/phenotype correlations and of pathogenetic concepts. Hum Mutat. 2010;31(10):1090-6.
[Crossref] [Google Scholar] [PubMed]
- Shruthi B, Nilgar BR, Dalal A, Limbani N. Harlequin ichthyosis: A rare case. Turk J Obstet Gynecol. 2017;14(2):138-40.
[Crossref] [Google Scholar] [PubMed]
- Kataria S, Ajmani SN. Harlequin Ichthyosis: A rare case of congenital ichthyosis. J Obstet Gynaecol India. 2019;69(3):292-3.
[Crossref] [Google Scholar] [PubMed]
- Shibata A, Akiyama M. Epidemiology, medical genetics, diagnosis and treatment of harlequin ichthyosis in Japan. Pediatr Int Off J Jpn Pediatr Soc. 2015;57(4):516-22.
[Crossref] [Google Scholar] [PubMed]
- Haftek M, Cambazard F, Dhouailly D, et al. A longitudinal study of a harlequin infant presenting clinicallyas non-bullous congenital ichthyosiform erythroderma. Br J Dermatol. 1996;135(3):448-53.
[Crossref] [Google Scholar] [PubMed]
- Moskowitz DG, Fowler AJ, Heyman MB, et al. Pathophysiologic basis for growth failure in children with ichthyosis: an evaluation of cutaneous ultrastructure, epidermal permeability barrier function, and energy expenditure. J Pediatr. 2004;145(1):82-92.
[Crossref] [Google Scholar] [PubMed]
- Kun Darbois JD, Molin A, Jeanne Pasquier C, et al. Facial features in Harlequin ichthyosis: Clinical findings about 4 cases. Rev Stomatol Chir Maxillo Faciale Chir Orale. 2016;117(1):51-3.
[Crossref] [Google Scholar] [PubMed]
- Jilumudi UBR. Harlequin ichthyosis: A medico legal case report and review of literature with peculiar findings in autopsy. J Forensic Leg Med. 2012;19(6):352-4.
[Crossref] [Google Scholar] [PubMed]
- Buxman MM, Goodkin PE, Fahrenbach WH, et al. Harlequin ichthyosis with epidermal lipid abnormality. Arch Dermatol. 1979;115(2):189-93.
[Google Scholar] [PubMed]
- Dale BA, Holbrook KA, Fleckman P, et al. Heterogeneity in harlequin ichthyosis, an inborn error of epidermal keratinization: Variable morphology and structural protein expression and a defect in lamellar granules. J Invest Dermatol. 1990;94(1):6-18.
[Crossref] [Google Scholar] [PubMed]
- Akiyama M, Dale BA, Smith LT, et al. Regional difference in expression of characteristic abnormality of harlequin ichthyosis in affected fetuses. Prenat Diagn. 1998;18(5):425-36.
[Google Scholar] [PubMed]
- Milner ME, O’Guin WM, Holbrook KA, et al. Abnormal lamellar granules in harlequin ichthyosis. J Invest Dermatol. 1992;99(6):824-9.
[Crossref] [Google Scholar] [PubMed]