- Biomedical Research (2008) Volume 19, Issue 1
Haematopoetic effect of methanol seed extract of Citrus paradisi Macfad (grape fruit) in Wistar rats
A.A. Adeneye*Department of Pharmacology, Faculty of Basic Medical Sciences, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria
- Corresponding Author:
- A.A. Adeneye
Departments of Pharmacology
Lagos State University College of Medicine
P.M.B. 21266, Ikeja, Lagos State, Nigeria
Mobile: +234-803-583-5589
e-mail: adeneye2001@yahoo.com
Accepted Date: December 29 2007
Abstract
The present study was undertaken to evaluate the blood-forming effects of (100% methanol seed extract) of Citrus paradisi Macfad in adult Wistar rats for 30 days as a way of evaluat-ing its traditional use in the treatment of blood deficiencies. Acute oral toxicity study was also conducted using limit dose test of the Up and Down Procedure statistical program (AOT425PgmStat, Version 1.0) at a dose of 2000 mg/kg body weight/oral route. Results showed significant (p<0.05) progressive and dose dependent elevations in total leucocyte count (TLC), lymphocyte differentials (Lymph.), red blood count (RBC), haemoglobin con-centration (Hb), packed cell count (PCV), mean corpuscular volume (MCV), mean corpus-cular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and platelet count (PL). Reversed effect was recorded for the neutrophil (Neutro.) and monocyte (Mono.) differentials which were significantly (p<0.05) decreased in the treated rats. Acute oral toxicity showed the extract to be relatively safe at 2000 mg/kg on acute exposure. Thus, the overall results lend support to its folkloric use
Keywords
Citrus paradisi Macfad, methanol seed extract, haematological parameters, Wistar rats
Introduction
Anaemia remains one of the major global health problems affecting more than 30% of world’s population, with higher prevalence among children and adults of low socio-economic class [1]. In developing countries, most common forms of anaemia are nutritional [2], pregnancy-related [3], secondary to acute febrile illness or parasitic infestation [4]. Hereditary haemoglobinopathies such as sickle cell disease and thalassemia which are associated with recurrent haemolysis are also important causes of childhood anaemia [4]. A few cases are drug-related particularly with the use of drugs such as sulphonamides, dapsone and methyl dopa. Other rare causes of anaemia include connective tissue disorders such as rheumatoid arthritis, polyarthritis nodosa, Wegener’s granulomatosis, progressive systemic sclerosis (scleroderma), etc. [5].
Despite availability of array of orthodox treatments for anaemia, there is still a heavy dependence on herbal alternatives for its treatment, particularly, the rural dwellers and urban residents of low socio-economic class [6]. While a good number of locally used herbal remedies have been scientifically evaluated and validated but a large number of these remedies remain scientifically unevaluated. One of this is the dried seeds of Citrus paradisi Macfad, which has ancestral use in the local management of diabetes, obesity, blood deficiency and as immune booster.
Citrus paradisi Macfad (Rutaceae) is popularly called grapefruit. Its tree grows up to 3–5 m high. Its fruit is mostly big and globular, with nipple at apex, and bright yellow or lemon coloured. Grapefruit juice is mildly acidic and has slight bitter taste [7]. In human, grapefruit seed extract has been documented to have broad spectrum antibacterial (through its bactericidal mechanism), anti-fungal, wound healing and antioxidant properties [8]. Also, there is a clinical evidence of its effectiveness in the treatment of urinary tract infections caused by Pseudomonas aeruginosa, Klebsiella spp, Staphylococcus aureus and Escherichia coli [9]. It has also been widely used in veterinary medicine as a panacea. Phytochemical studies showed the primary constituents present in grape seed extract to include the polyphenolic flavonone glycosides (hesperidin, neohesperidin, narirutin and naringin) that give the grapefruit extract its antioxidant activity, fructose, etc. [10]. Grapefruit extract has also been shown contain a high concentration of ascorbic acid (vitamin C) [11] and folate-polyglutamates [12].
The present study is aimed at evaluating the haematinic property of methanol seed extract of Citrus paradisi Macfad by investigating effects of its chronic administration at the oral dose of 100-600 mg/kg/day of the extract on the haematological parameters in normal young adult female Wistar rats.
Materials and Methods
Plant material and its extraction
In the second week of December, 2006, fresh parts of Citrus paradisi tree were collected from a cultivated farm-land within the deciduous forest of Odorasanyi District, Ijebu-Igbo, Ogun State, Nigeria, for botanical identification and voucher specimen referencing (Voucher Specimen number: FHI 107460) at the Forestry Research Institute of Nigeria (FRIN), Ibadan, Oyo State, Nigeria. Plant taxonomy was done by Mr. T. K. Odewo, Chief Superin-tendent Officer, Taxonomy Section, FRIN, Ibadan, Oyo State, Nigeria. Plant authentication was done by Dr. A.B. Kadiri, The Herbarium, Botany and Microbiology Department, the University of Lagos, Akoka, Lagos State, Nigeria. Twenty ripe Grapefruit collected from the farmland was cut into pieces and the seeds were separated out. These were thoroughly but gently rinsed in distilled water. The seeds were completely dried at room temperature (28 ± 2 ºC) for four weeks, protected from direct sunlight and heat. The dried seeds were ground to powder using the Laboratory Hammer-mill. 100 g of the powdered sample was soaked in 250ml of 99.8% methanol (NAAFCO Scientific Supplies Ltd., Lagos, Nigeria) for 12 hours and intermittently shaken vigorously for another 6 hours before the solution was rapidly filtered through a piece of clean white handkerchief. The filterate was completely dried over a water bath until a yellow-to-brown, aromatic, oily residue was obtained. This was allowed to cool, stored in tight cap-fitted container at 4 ºC for 2 weeks before the commencement of the experiment. The extraction process was repeated two more times. The yield was 25.2 ± 1.2 % (w/w).
Experimental Animals
Experimental procedures involving the experimental animals and their care were conducted in conformity with international, national and institutional guidelines for Care and Use of Laboratory Animals in Biomedical Research as promulgated by the Canadian Council of Animal Care [13] and United States National Institutes of Health [14].
Thirty, adult inbred female Wistar rats, weighting 130-150 g, were obtained from the Animal House of the Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria, after approval was obtained from the ad hoc Ethical Committee of the College. The rats were fed standard rat chow (Livestock Feeds, Ikeja, Lagos State, Nigeria) and water ad libitum. The animals were maintained at standard laboratory conditions (12/12 hr dark/light cycle, 20 ± 2 ºC temperature, and 65 ± 5 % humidity). 12-16 hours before the experiment began, the rats were fasted but water was made available ad libitum.
Extract administration
Experimental animals were randomly divided into five groups of six rats each such that the average weight difference between and within groups does not exceed ± 20% of the average weight of the sample population. Group I served as the negative control and was orally given 10 ml/kg of body weight/day of distilled water, Group II was the positive control, orally administered 10 ml/kg of body weight/day of dimethyl sulphoxide (DMSO) (Prolabo, France) while Groups III, IV and V were orally administered 100, 300 and 600 mg/kg of body weight/day of the seed extract suspended in 10 ml/kg/day of DMSO, respectively, for 30 days.
Acute Oral Toxicity study
The acute oral toxicity study for the grapefruit seed extract was conducted using Preliminary limit dose test of the Up and Down Procedure statistical program - AOT425StatPgm [15] as adopted by Adeneye et al. [16].
Blood collection and bioassays
Prior to termination of the experiment on day 31, the rats were fasted overnight but distilled water was made available ad libitum. Blood samples were collected by cardiac puncture under halothane anesthesia, using 21 gauge (21G) needles mounted on a 5 ml syringe (Hindustan Syringes and Medical Devices Ltd., Faridabad, India) into Ethylene Diamine Tetraacetic Acid (EDTA)-coated sample bottles for full blood count (FBC), which included differential leucocyte counts (DLC), RBC, Hb, PCV, MCV, MCH, MCHC, PL and TLC. The collected blood samples were analysed using Automated Haematology System (Sysmex Haematology-Coagulation Systems®, Model KX-21N, Sysmex Incorporation, Kobe, Japan).
Statistical analysis
Results were presented as mean ± standard error of mean (S.E.M.) of six observations and statistically analyzed using two-way analysis of variance on statistical computer software program, SYSTAT 10.6. Post hoc test was conducted using Student-Newman-Keuls test and the level of significance was considered at p<0.05.
Results
Acute oral toxicity
Table 1 shows the sequence and results of the preliminary limit dose test of the Up-and-down procedure of the test extract. As shown in the table, there was no death recorded among the 5 sequentially treated rats. Also, the high dose was not associated with obvious physical or behavioural toxicities.
Effect of extract on haematological parameters
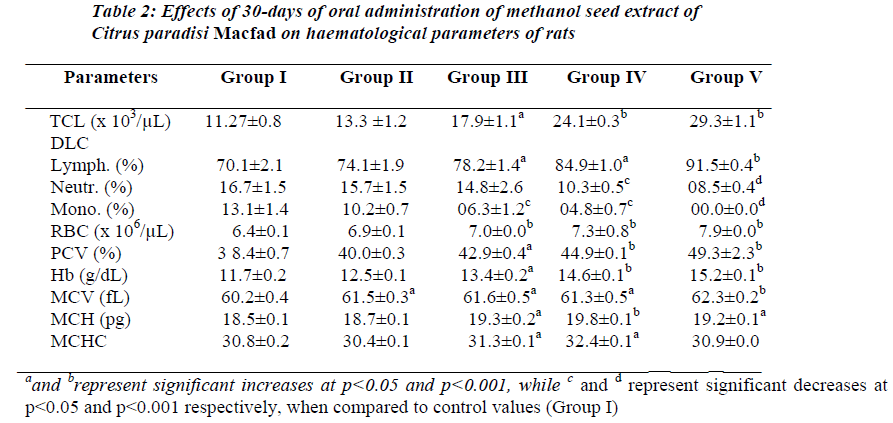
Table 2 shows effect of 100–600 mg/kg of body weight/day of the extract on haematological parameters in treated rats. Results showed that 30-days of oral treatment with graded doses of the extract significantly (p<0.05) increased RBC, PCV, Hb, MCV, MCH, MCHC, TLC, lymphocyte differential, and PL in dose related fashion. Reverse effect was recorded for neutrophil and monocyte differentials.
Discussion
Results of the present study showed that chronic administration of methanol seed extract of Citrus paradisi Macfad to treated rats resulted in significant (p<0.05), dose related elevations in the haematological parameters investigated except the neutrophil and monocyte differentials. Literature has shown that oral ingestion of medicinal compounds or drugs can alter the normal range of haematological parameters [17,18]. These alterations could either be positive or negative. In this study, most of the effects recorded for the extract were positive except for its suppressive effects on the neutrophil and monocyte differentials. This suggests that the extract may be selectively toxic to these leucocyte lineages. This postulation, however, requires validation.
The significant increases (p<0.05) in the total leucocyte count from 11.27 ± 0.8 x 103/μL in distilled water-treated group to 29.3 ± 1.1 x 103/μL in 600 mg/kg-treated group and lymphocyte differential from 70.1 ± 2.1 (%) in distilled water-treated group to 91.5 ± 0.4 (%) in 600 mg/kg/day-treated group, reflect leucopoetic and possible immunomodulatory effects of the extract. It is possible that the extract possesses active principle(s) containing haematopoetin-like principle(s) or contains active biological principle(s) stimulating haematopoeitins (erythropoietin, leucopoetin, thrombopoetin) synthesis or release. The active biological principle(s) contained in the extract may be responsible for its haematopoetic effect. Literature has equally shown grapefruit to be a rich natural source of ascorbic acid [11] and folates [12]. Ascorbic acid or vitamin C is known to be essential for body tissue (including blood, blood vessels and bone) formation and maintenance [19,20]. Folate has equally been documented to be very important in haematopoeisis [12]. The presence of these two vitamins in the extract may also account for the significant improvement in the haematological indices recorded in this study.
Result of acute oral toxicity of the seed extract suggests that the extract could be relatively safe on acute exposure to it, even at a high dose.
In conclusion, the overall results of this study lend support to the folkloric use of methanol seed extract of Citrus paradisi Macfad in the treatment of blood deficiency. However, isolation of the active principles in the extract and elucidation of their mechanisms of inducing the observed effects would constitute areas of further studies.
References
- World Health Organisation. National strategies for overcoming micronutrients malnutrition: Executive Board. W.H.O. (EB 89/27) Geneva, Switzerland. 1991
- Rebecca JS, Hababu MC, James MT, Kerry JS, Marco A, Lorenzo S. Epidemiology of iron deficiency anaemia in Zanzibasi school children: The importance of hookworms. American Journal of Clinical Nutrition 1997; 65: 153-159.
- Hossain MM, Bakir M, Pugh PN, Sheekh HM, Bin Oshaq SA, Lindbland BS. The prevalence and correlation of anaemia among young children and women of child-bearing age in Al-Ain, United Arab Emirates. Annals of Tropical Paediatrics 1995; 15: 227.
- Adetuyibi A. Companion to Clinical Medicine in the Tropics. Macmillan International College Edition, Macmillan Education Ltd, Hong Kong 1990; 202-228.
- Falase AO, Akinkugbe OO. A Compendium of Clinical Medicine. Spectrum Books Limited, Ibadan 1999; 545-604
- Agbor AG, Odetola AA. Haematological studies of Parquetina nigrescens on haemorrhagic anaemic rats. African Journal of Medicine and Medical Sciences 2001; 30: 105-109.
- Monsef-Esfahani HR, Amanzade Y, Hajiaghaee R, Mahdavi F. Concentration of Grapefruit Essential oil by Fractional Distillation. Planta Medica 2006; 72: 1078.
- Supplement news. Grapefruit Seed Extract. 2006. An electronic journal available online: www. Supplement- news.org/ grapefruit-seed
- Oyelami OA, Agbakwuru EA, Adeyemi LA, Adedeji GB. The Effectiveness of Grapefruit (Citrus paradisi) Seeds in Treating Urinary Tract Infections. The Journal of Alternative and Complementary Medicine 2005; 11 (2): 369 - 371
- Rouseff RL, Martin SF, Youtsey CO. Quantitative Survey of Narirutin, Naringin, Hesperidin, and Neohesperidin in Citrus. J. Agriculture and Food Chemistry 1987; 35: 1027 ? 1030
- Marcus R, Coulston AM. Water-soluble vitamins: the Vitamin B Complex and Ascorbic Acid. In Goodman and Gilman?s The Pharmacological Basis of Therapeutics (Hardman JG and Limbird LE eds.). 10th edition. New York McGraw-Hill Medical Publishing Division 2001; 63: 1753-1791
- Hoffbrand AV. Megaloblastic anaemia. In: Hoffband AV, Lewis SM, Tuddenham EGD, ed. Postgraduate Haematology. 4th edition. New York: Oxford University Press 2001; 47-67.
- Canadian Council of Animal Care. Guide to the handling and Use of experimental animals. Ottawa: Ont. 1984; 2 United States NIH publication, 1985. no. 85 ? 23.
- Acute Oral Toxicity (OECD Test Guideline 425) (AOT). Statistical Programme (AOT425StatPPgm), version 1.0., 2001. Available online at http://www. oecd. org/ OECD/ pages/home/ displaygeneral/ 0,3380, EN-document-524-nodirectorate-no-24-6775-8,FF.html
- Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. Journal of Ethnopharmacology 2006; 105: 374-379
- Abatan MO, Arowolo RO. Toxicity of Eugenia uniflora to rats. Nigerian Journal of Animal Production 1989; 16: 16-19
- Ajagbonna OP, Onifade KI, Suleiman U. Haematological and Biochemical changes in rats given extract of Calotropis procera. Sokoto Journal of Veterinary Sciences 1999; 1: 36-42
- Ofuya ZM, Ebong OO. Plasma ascorbic acid levels in adult females in Port-Harcourt, South Eastern Nigeria. West African Journal of Pharmacology and Drug Research 1996; 12: 32-36
- Osilesi O, Adeiyi A, Ogunyemi EO, Fakunle JB. The glycaemic response to selected fruits and vegetables in Nigerian diabetics. African Journal of Medicine and Pharmaceutical Sciences 1997; 1: 1-6