Research Article - Biomedical Research (2017) Volume 28, Issue 6
Growth of early or midterm human foetal skin in a burn model: establishment of the model, morphological and histological observations
Ji-xun Zhang1, Chuan Li2, Yao-nan Li3, Zheng-xue Dong1, Dao-jing Qiu1 and Du-yin Jiang1,3*1Department of Burns and Plastic Surgery, The Second Hospital of Shandong University, 247 Bei Yuan Road, Jinan, Shandong, PR China
2Peking Union Medical College, Plastic Surgery Hospital of Chinese Academy of Medical Science, Beijing, PR China
3Department of Emergency, Institute of Tissue Engineering of Shandong University, 247 Bei Yuan Road, Jinan, Shandong, PR China
- *Corresponding Author:
- Du-yin Jiang
Emergency Department
Institute of Tissue Engineering of Shandong University
Department of Burns and Plastic Surgery
The Second Hospital of Shandong University, PR China
Accepted on November 2, 2016
Abstract
Objective: To construct a burn model of early and midterm human Foetal Skin (hFS) in vitro, to observe its morphological and histological changes after transplantation, and to examine the expression of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinases (TIMPs), and further to analyse their roles in foetal wound healing preliminarily.
Methods: hFS harvested from aborted foetuses (gestational age 14-24 weeks) was transplanted into the backs of nude mice. Four weeks later, a burn wound was made in the transplanted foetal skin with a thermostatic electrical scald apparatus. To examine the role of exogenous mature immune cells, human Peripheral Blood Mononuclear Cells (hPBMCs) were injected into the subcutaneous layer of transplanted hFS. The histological characteristics and the expression of MMP-2, MMP-7, MMP-9, and TIMP-1 were examined during the wound-healing process above.
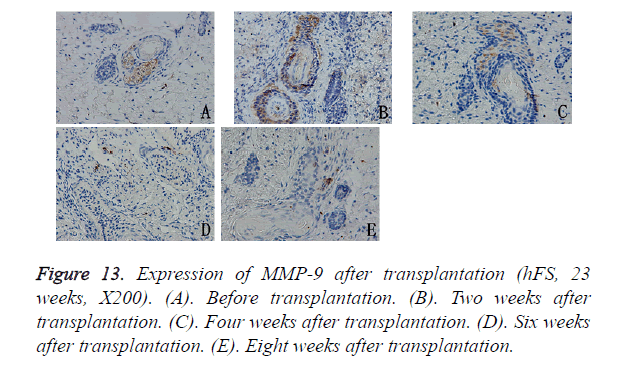
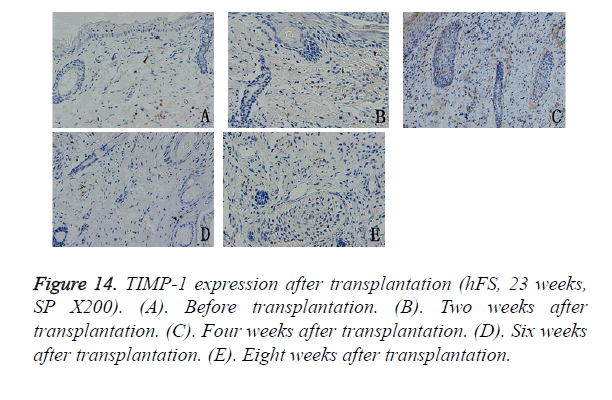
Results: The transplanted foetal skin survived on the backs of mice, the burn wounds healed rapidly and without scarring, and skin appendages regenerated after the epidermis were repaired. Injection of hPBMCs prolonged the burn wound-healing process and caused obvious scarring. MMP-2 expression was observed in the surviving grafts, but MMP-7 expression was not observed throughout the normal growth process after transplantation. MMP-7 expression was observed around the burn wound, but MMP-2 expression was observed only in surviving follicles. MMP-9 expression was observed in the cytoplasm of deep epithelial cells of skin appendages. TIMP-1 expression level was slightly lower than that of MMP-9, although its pattern of expression was similar to that of MMP-9. Injection of exogenous mature immune cells (hPBMCs) into the burn injury changed the patterns of MMP-9 and TIMP-1 expression.
Conclusion: Our method for constructing an early or midterm hFS burn model is simple and reliable. HFS maintains the ability for scarless wound healing and regeneration when transplanted into nude mice. This new animal model may be suitable for investigating the mechanisms responsible for hFS wound healing. Injection of hPBMCs prolonged burn wound healing and caused the formation of scars in the wound. MMPs and TIMPs expression might be important factors for scarless wound healing.
Keywords
Human foetal skin, Burn model, Wound healing, Human peripheral blood mononuclear cells (hPBMCs), Matrix metalloproteinases (MMPs).
Introduction
For adults, scar formation is an inevitable consequence of healing after severe wounds (including burns), and may cause significant disfigurement and dysfunction, and possible psychological problems. It is widely accepted that foetal skin of early or midterm gestational age can heal quickly without any scarring, which is called “scarless healing” [1-3]. Understanding the mechanisms underlying scarless healing may identify ways to help accelerate wound healing and prevent scar formation. After considering the results of previous studies [4], we designed an animal model of human Foetal Skin (hFS) of early or midterm gestational age and then developed a burn wound model. We observed the morphological changes to examine the mechanisms of scarless wound healing directly.
Both Matrix Metalloproteinases (MMPs) and their Inhibitors (TIMPs) play important roles in wound healing by regulating processes such as the proliferation and migration of keratinocytes, proliferation of fibroblasts, collagen synthesis, angiogenesis, and granulation tissue remodeling. We are interested whether the same processes occur in deep wounds of early or midterm hFS.
Using our new model, we observed the histological features of early and midterm wound healing in hFS in vitro and the healing process of early and midterm hFS subjected to a local deep burn after transplantation. We examined the changes of the expressions of MMPs (MMP-2, -7, and -9) and TIMP-1 in these processes. To confirm the role of the immune inflammatory response in wound healing of hFS, we introduced exogenous mature immune cells (human peripheral blood mononuclear cells, hPBMCs) during the growth process and wound-healing process. We examined the effects of hPBMC injection on the healing process and the expression of MMPs and TIMPs.
We want to identify important factors involved in scarless healing of hFS and ways to improve wound healing and scar prevention through these studies.
Materials and Methods
Procedure for transplantation of early or midterm gestational age hFS
The transplantation procedure is shown in Figures 1A-1D. First, human foetuses (gestational age 14-24 weeks) were obtained from patients undergoing uterine aspiration in the Obstetrics and Gynaecology Department of Second Hospital of Shandong University, Shandong Province, PR China.
Patients were aware of the purpose of foetus donation and foetus donation consent forms were signed. We used surgical scalpels to separate the skin from the back of the human foetuses, and a thin slice of subcutaneous tissue was preserved together with the skin. Fresh hFS was maintained in cold saline (4°C) until transplantation.
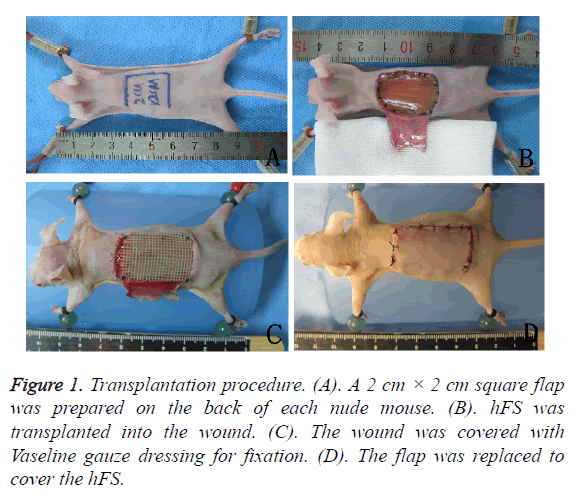
BALB/c nude mice weighing 20-25 g were purchased from the Department of Laboratory Animal Science, Peking University. Experimental animals were kept in an SPF environment, and fed with high-pressure steam-sterilized food and water. Nude mice were injected intraperitoneally with 4% chloral hydrate (0.75 ml/kg) for anaesthesia. A 2 cm × 2 cm square flap was prepared on the back of each nude mouse, hFS of the same size was transplanted into the wound and sutured, and the wound was covered with Vaseline gauze dressing for fixation. The flap was replaced to cover the transplanted hFS to maintain a moist and nutrient-rich environment. Two weeks after the operation, both the flap and gauze dressing were removed, and the hFS was exposed to the air.
Confirmation of the origin of the surviving graft
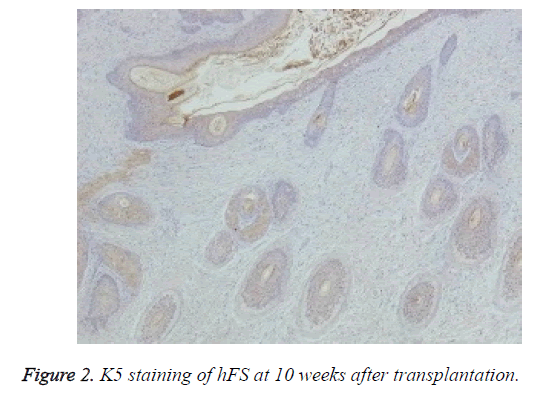
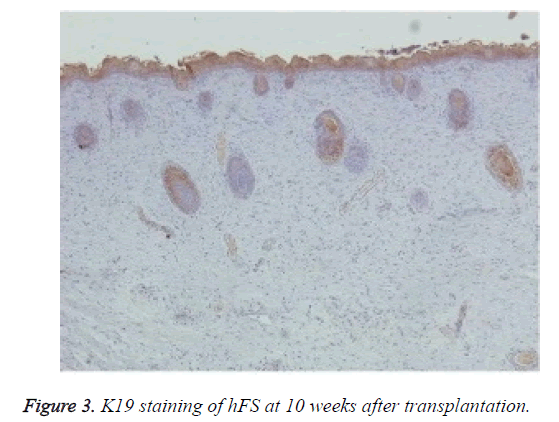
Immunohistochemical staining was used to confirm the origin of the surviving graft, especially of the skin and appendages. Rabbit anti-human K5 (bs-1060R) and rabbit anti-human K19 (bs-1028R) were purchased from Beijing Dingguo Biotech Co. Ltd. Rabbit hypersensitivity two-step immunohistochemical detection kits were purchased from Beijing Zhongshan Jinqiao Biological Technology Co. Ltd. Specimens taken from transplanted hFS were fixed, dehydrated, and embedded in paraffin, and 3-6 μm thick sections were cut. The sections were deparaffinised, rehydrated, and incubated with primary and secondary antibodies to K5 and K19 in two steps and finally stained with DAB. The nucleus was double stained with haematoxylin. The expression of K5 and K19 was then observed under an optical microscope in the surviving grafts.
Method for inducing the burn injury
Four weeks after the hFS was transplanted into the nude mice, we confirmed that the hFS had survived and continued growing. We chose this time point to cause the burn injury. Nude mice with transplanted hFS were injected intraperitoneally with 4% chloral hydrate (0.75 ml/kg) for anaesthesia. Using a thermostatic electrical scald apparatus with a copper head, we made a deep burn. The 1 cm × 1 cm square surface of the copper head was heated to 80°C and placed gently onto the transplanted hFS by its own weight for 5 s. The contact area on the hFS was burned and the wound size was controlled to remain a 1 cm × 1 cm square. After removal of the copper head, sterile saline-soaked gauze was applied to reduce the heat of the hFS for 3 min, after which the burn wound was exposed to the air. After this operation, the nude mice were injected intraperitoneally with 0.2 ml of penicillin in saline (20,000 U/ml) for 3 days to prevent infection. The nude mice were maintained in the SPF environment at room temperature, and their diet and activities were observed daily.
Intervention using exogenous mature immune cells
HPBMCs were separated and extracted from the peripheral blood of healthy adult persons and the cell concentration was adjusted to 5 × 106/ml with RPMI-1640 medium. Five nude mice were used as the experimental group each time. A total of 1 × 106 harvested hPBMCs were injected in a volume of 0.2 ml subcutaneously into the transplanted hFS from four different directions just after the burn injury; 0.05 ml containing 2.5 × 105 hPBMCs was injected into each side uniformly. The same amount of RPMI-1640 medium (0.2 ml) without cells was injected into the nude mice of control group. After the burn operation and hPBMC injection, the mice were injected intraperitoneally with 0.2 ml of penicillin in saline (20,000 U/ml) for 3 days to prevent infection. They were maintained in the SPF environment at room temperature, and their diet and activities were observed daily.
General and histological observations
Growth and development of hFS after transplantation: The size, color, thickness, and hair growth of the surviving hFS transplants were observed 2, 3, 4, 8, 12, and 16 weeks after transplantation. The length and width of the hFS transplant was measured with a calliper. Specimens of the hFS transplants were obtained, fixed, dehydrated, and embedded in paraffin, and 6-10 μm thick paraffin sections were cut and stained with Haematoxylin and Eosin (HE). The features of the epidermis and skin appendages of the hFS were observed carefully under the microscope after transplantation.
Burn wound healing and skin regeneration: On days 1, 3, 7, and 14 after the burn injury, the appearance of hFS was observed. We recorded changes in the wound size, color of the transplanted skin, wound-healing process, and scar formation. Specimens taken from the burned site were fixed, dehydrated, and embedded in paraffin, and 6-10 μm thick sections were cut and stained with HE. The proliferation and migration of keratinocytes and fibroblasts, skin structure restoration, and appendage regeneration through the burn wound healing were observed under the microscope.
Burn wound healing and skin regeneration after injection with exogenous mature immune cells: The same variables as described in above section were measured after hPBMCs were injected into the subcutaneous layer of the transplanted hFS immediately after the burn injury. The different processes of wound healing and changes were recorded.
Measurement of the expression of MMP-2, MMP-7, MMP-9, and TIMP-1: The primary antibodies rabbit antihuman MMP-2 and rabbit anti-human MMP-7 were purchased from Beijing Dingguo Biotech. Co. Ltd. Other primary antibodies rabbit anti-human MMP-9 and mouse anti-human TIMP-1 were purchased from Abcam Co. Ltd (Shanghai, China). Two-step immunohistochemical detection kits were purchased from Beijing Zhongshan Jinqiao Biological Technology Co. Ltd. Specimens taken from burned hFS were fixed, dehydrated, and embedded in paraffin, 3-6 μm thick sections were cut, and the sections were deparaffinised and rehydrated. The sections were incubated with the primary antibodies to MMP-2, MMP-7, MMP-9, and TIMP-1 and then the secondary antibodies were used for fluorescent staining in a two-step procedure and stained with DAB. The nucleus was double stained with haematoxylin. MMP-2, MMP-7, MMP-9, and TIMP-1 positivity in the transplanted hFS was observed using an optical microscope.
Results
Origin of the surviving graft
Macroscopically, the external features of the surviving graft resembled the foetal skin except that the surface was dry. Microscopic observation of HE staining showed normal hFS structure after transplantation and the subsequent development process. Immunohistochemical staining were positive for both human skin-derived K5 (Figure 2) and K19 (Figure 3) in the surviving grafts and was observed in the epidermis, follicles, and sebaceous glands. This confirmed that the surviving hFS-like structure was the transplanted hFS itself.
Growth and development of hFS
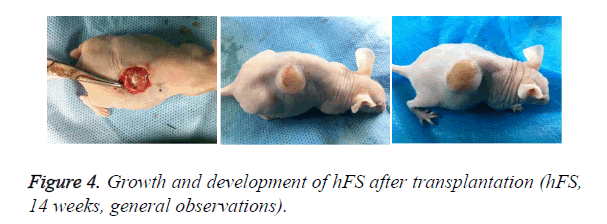
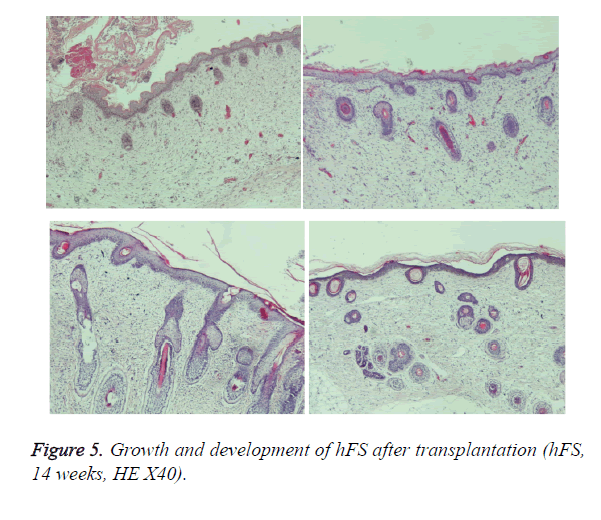
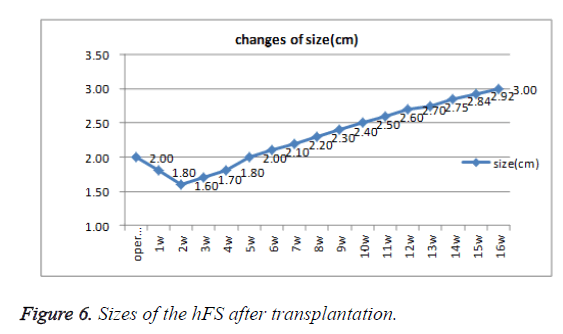
Early hFS (gestational age ≤ 16 weeks) appeared light red in color and the surface was smooth and there was not obvious stratum corneum. Few skin appendages appeared beneath the epidermis. Two weeks after transplantation, the hFS became thicker and the color was lighter. There were 4-5 layers of epithelial cells arranged neatly in a line, but no stratum corneum layer of the skin was observed. A few skin appendage primordia in various shapes could be seen with a microscope. Four weeks after transplantation, slight contraction of hFS was observed and the stratum corneum layer began to emerge, and the transplant could be exposed safely to the external environment. Microscopic observation showed more layers of epithelial cells, keratin formation, and more and larger skin appendage primordia. Primordial follicles began to develop into original hair shafts, and sweat gland primordia grew into the deep subcutaneous tissue from the basement of the epidermis. The hFS continued to grow quickly. Ten weeks after transplantation, the skin and subcutaneous tissue had become thicker, the epidermis had developed nearly to maturity, and obvious stratum corneum layers could be seen. The skin appendage primordia became larger and continued to differentiate. The secretory sections of sweat glands and hair shafts were observed. At 12 weeks after transplantation, development of hair follicles and sebaceous glands was completed and hair shafts protruded from the skin surface. At 16 weeks after transplantation, the hFS had grown to twice their original size. Development of sweat glands was almost completed; secretory sections appeared as bent loops with distinct lumen located in the deep tissue (Figures 4 and 5). The sizes of the hFS at various times after transplantation were measured with a calliper, and the results are showed in Figure 6.
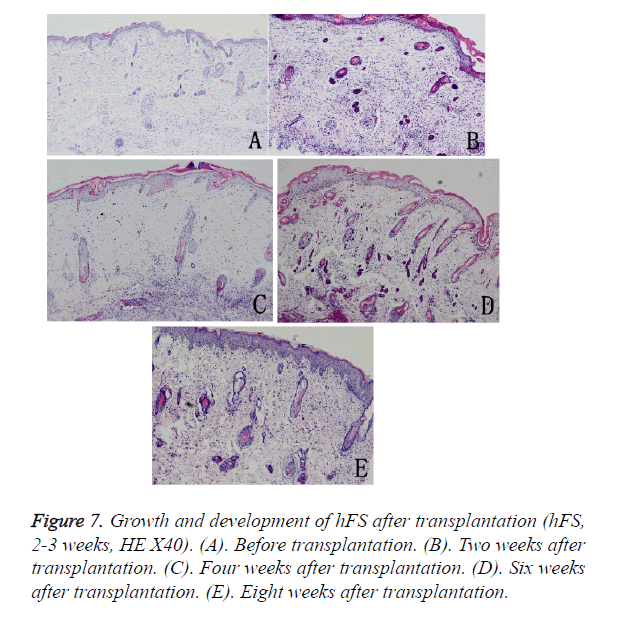
The growth process after transplantation of midterm hFS (gestational age 21-24 weeks) was similar to that of the early hFS (gestational age ≤ 16 weeks). One difference was that differentiation of skin appendage primordia was seen in the midterm hFS before transplantation (Figure 7). It is interesting that the hFS and skin appendages had the ability to grow and develop after surviving, and that the process was the same as that reported for foetuses in utero [5].
Burn wound healing and skin regeneration
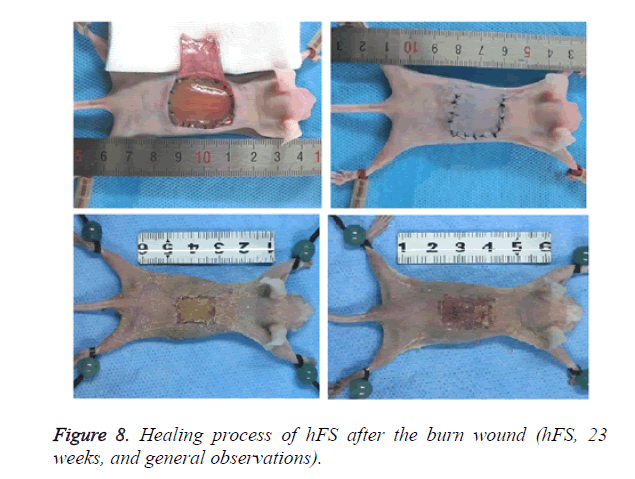
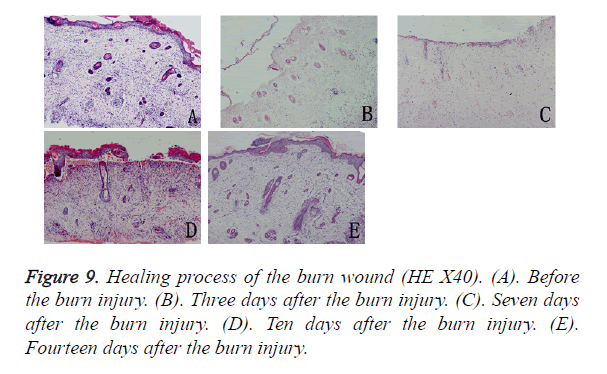
Macroscopically (Figure 8), the burned hFS healed in about 14 days and left no obvious scar. Right after the burn, the wound surface of hFS was yellow and hard, with a few translucent exudates. Twenty-four hours after the burn, a hard, 1 mm thick eschar formed. Three days later, the eschar began to shrink and 7 days later, the eschar began to soften and scab gradually. By 14 days, the eschar had scabbed completely and the wound was covered with flat and smooth new skin.
Microscopy confirmed that the depth of the burn reached the deep dermis and that most parts of the follicles had been destroyed (Figure 9). The entire epidermis and the upper two-thirds of the dermis and most appendages were necrotic, and an eschar covered the wound. There was no significant inflammatory cell infiltration in the wound. Three days later, the basal cells and epithelial cells of hair follicles had proliferated and migrated from the surrounding tissues. The morphology of the new epidermal cells resembled that of the basal cells and the new cells were arranged in a single layer covering the wound. Seven days after the burn injury, the single layer of new epidermal cells had changed into a stratified squamous structure, and 14 days after the burn injury, new skin appendage formation was observed from the basal layer of the new epidermis.
Burn wound healing and skin regeneration after injection of exogenous mature immune cells
Separation of hPBMCs: Density gradient centrifugation was used to separate hPBMCs from the peripheral blood of a healthy adult person [6]; 1 × 107 cells were gathered per 10 ml of peripheral blood. Trypan blue dye exclusion confirmed that living cells accounted for 95% of the cells.
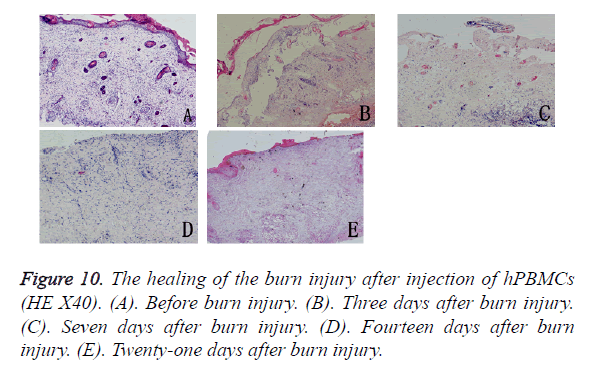
Histological observation after hPBMC injection: Three days after hPBMC injection into the burn wound, no visible immune rejection had occurred, but infiltration of inflammatory cells was seen in the subcutaneous and surrounding tissues. Seven days after injection, angiogenesis was observed in the tissues surrounding the wound, there was increasing evidence of inflammatory reactions, and infiltration of inflammatory cells was seen in the subcutaneous tissues. Fourteen days after the burn injury, the inflammatory reactions surrounding the wound appeared weaker, fibroblasts and cord-like epithelial cells proliferated vigorously, collagen fibers were gradually deposited on the defects but were arranged irregularly, and proliferating epithelial cells had begun to migrate into the wound. Twenty-one days after the burn injury, the healing of the burn wound was incomplete and was accompanied by obvious scarring. The epidermal basement zone was fully differentiated, and epithelial cells began to reconstruct the basal cell layer by moving through the wound and then covering the wound defect (Figure 10).
Expression of MMP-2, MMP-7, MMP-9, and TIMP-1 after transplantation and during burn wound repair
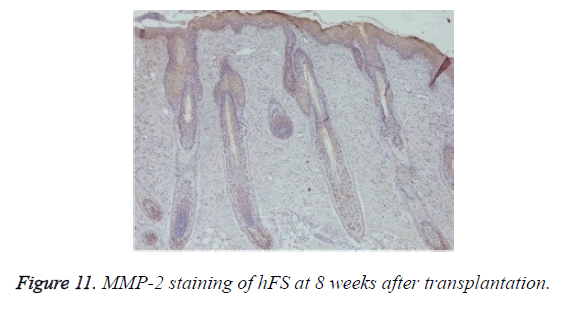
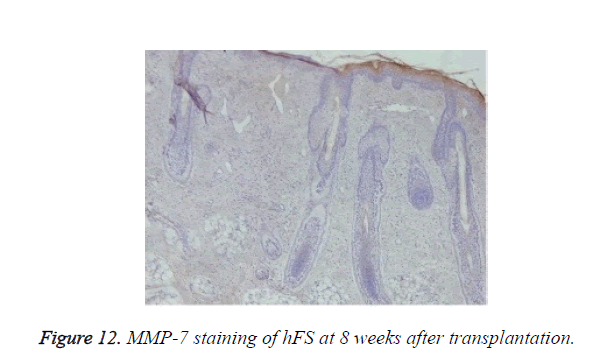
Growth and development of hFS after transplantation: MMP-2 expression was observed after transplantation in the surviving grafts and was located in follicles, sebaceous glands, and sweat glands (Figure 11). Two weeks later, MMP-2 expression began to increase and reached a peak at 4 weeks. Although still positive, MMP-2 expression decreased gradually from 6-8 weeks and reached its lowest level 10 weeks after transplantation. By contrast, MMP-7 expression was not observed throughout the entire process (Figure 12).
MMP-9 expression was observed in the cytoplasm of deep epithelial cells in the skin appendages, such as incompletely developed follicles, sebaceous glands, and sweat glands. During repair, MMP-9 expression decreased progressively with the development of the skin appendages. 6 to 8 weeks after transplantation, MMP-9 expression in the hFS grafts tended to remain stable, which was sporadically expressed in the cytoplasm of fibroblasts (Figure 13).
The TIMP-1 expression level was slightly lower than that of the MMP-9, but its expression pattern was similar to that of MMP-9. The peak in TIMP-1 expression was about 4 weeks after transplantation, after which its expression decreased gradually to a low level and scattered distribution, mainly in the cytoplasm of fibroblasts around the widened skin appendages (Figure 14).
Burn wound-healing process in hFS
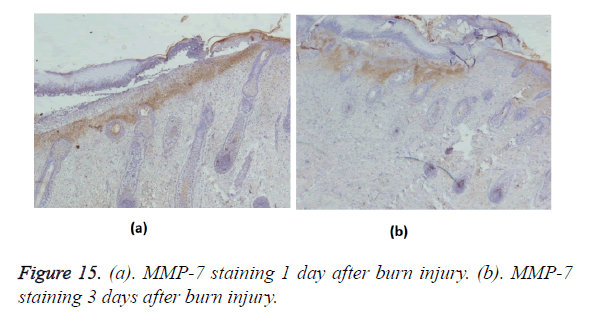
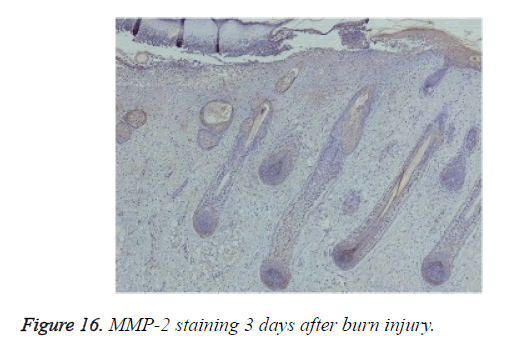
Positive immunohistochemical staining for MMP-7 was distributed in the tissues under the burn eschar at 24 h and appeared as a belt (Figure 15a). Three days after burn injury, MMP-7 staining had expanded to deeper tissues (Figure 15b). MMP-7 staining lessened markedly during the wound-healing process. At 13 days after burn injury, MMP-7 staining became negative. MMP-2 staining was negative in the wound, but positive in surviving follicles (Figure 16).
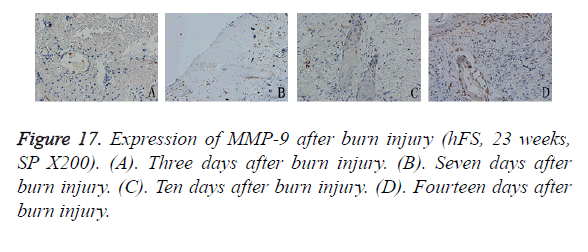
Three days after burn injury of the transplanted hFS, MMP-9 expression appeared to be scattered in the denatured collagen around the extended blood vessels. As the necrotic tissues were removed and cells migrated into the area of wound repair, MMP-9 staining appeared in the cytoplasm of epithelial cells in the surviving skin appendages. Seven days after burn injury, MMP-9-positive particles increased slightly in collagen surrounding the wound and these particles remained up to 10 days after burn injury. After 14 days, the general healing process came to an end, and MMP-9 expression was observed widely throughout the cytoplasm of epithelial cells in the skin appendages and in new basement cells (Figure 17).
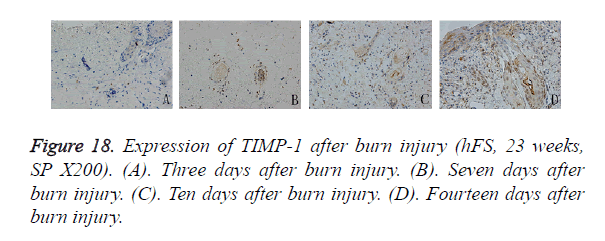
Compared with MMP-9, the distribution of TIMP-1-positive particles was more irregular, and was scattered mainly in the cytoplasm of the skin fibroblasts. TIMP-1-positive staining began to appear on day 7 after burn injury and continued to increase until the wound-healing process was completed. At the beginning, positive particles of TIMP-1 were expressed mainly in fibroblasts around the surviving skin appendages. Ten days after burn injury, TIMP-1 began to be expressed widely in the cytoplasm of fibroblasts around the burn injury. Fourteen days after burn injury, widely and obviously positive expression of TIMP-1 could be seen in the cytoplasm of proliferative epithelial cells and fibroblasts around the regenerated basement (Figure 18).
Expression of MMP-9 and TIMP-1 during burn wound healing after hPBMC injection
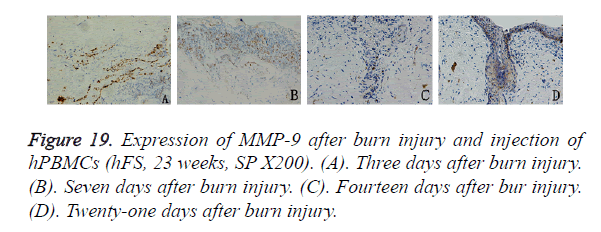
In the mice injected with hPBMCs, 3 days after burn injury, MMP-9 was expressed widely and diffusely in the cytoplasm of inflammatory cells that had infiltrated into the subcutaneous fat tissue and tissues around the burn wound. Seven days after burn injury, MMP-9 expression began to decrease in the subcutaneous tissue layer and to increase markedly in the cytoplasm of inflammatory cells that had infiltrated into tissues around the wound. MMP-9-positive particles were also observed in the cytoplasm of epithelial cells in the surviving skin appendages. Fourteen days after burn injury, MMP-9 expression was not observed in subcutaneous tissues, and the expression level began to decrease in tissues around the wound. MMP-9-positive particles were deposited mainly in the cytoplasm of proliferating epithelial cells that had migrated into the wound. Twenty-one days after burn injury, MMP-9 expression was not observed except in the basal cell layer, which had become completely differentiated. No obvious MMP-9 distribution was observed in other areas (Figure 19).
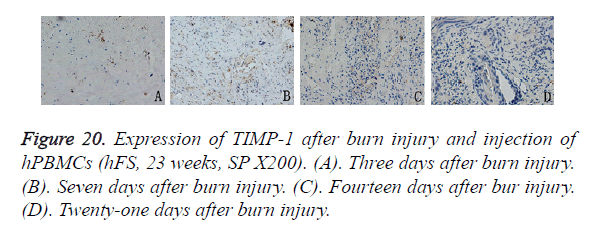
The expression pattern of TIMP-1 after hPBMC injection differed from that of MMP-9. Three days after burn injury, TIMP-1 expression appeared in the periphery of the wound, but the expression level was low. Seven days after burn injury, TIMP-1 expression peaked and was expressed widely in the cytoplasm of new capillary endothelial cells and proliferating fibroblasts in and around the wound. Fourteen days after burn injury, the TIMP-1 expression level decreased slightly, and expression was observed mainly in the cytoplasm of epithelial cells and peripheral fibroblasts. Twenty-one days after burn injury, TIMP-1 was not expressed in either the non-healing wound or the new basal cells (Figure 20).
Discussion
Scientists and clinical practitioners have made great efforts to promote wound healing and reduce scar formation. Earlier studies have shown that wounds on embryonic skin of early or midterm gestational age can restore skin structure and function completely without scarring, which is not achievable in the adult [7-9]. The result for adult might be related to factors such as the lack of inflammatory reactions, low level of procollagen synthesis, up regulated Interleukin-10 (IL-10) and Transforming Growth Factor-β (TGF-β1) expression, or the Wnt signalling pathway [10-14]. A stable wound-healing model using hFS is needed to study this issue.
Our model involves transplanting hFS to nude mice and causing burn injury directly to the transplanted hFS. The main questions asked when we created our model were how to transplant the fragile hFS into nude mice in a dry environment and what gestational age of hFS is best for transplantation without inducing teratoma formation. Thus, we implanted hFS under the recipient’s skin flap to avoid direct contact between the hFS and the external environment and, 2 weeks later, after we confirmed that the transplanted hFS had survived, we exposed the transplanted hFS to dry air by removing the skin flap. At gestational 14 weeks, the horny layer starts to grow to protect hFS and the skin structure is still developing without visible teratoma formation. At gestational 24 weeks, most development processes of the skin and appendages, including the epidermis, hair follicles, sebaceous glands, and sweat glands, have reached the end stage, so we considered gestational age 14-24 weeks as suitable for our research. In our transplantation experiments, hFS continued to grow and develop after being transplanted into nude mice and these processes could be observed visually and under the microscope. We also confirmed that the transplanted hFS could heal quickly and restore its structure within 14 days without scar formation. Interestingly, new skin appendage primordia were observed in the regenerated epidermis. We believe that the hFS of early or midterm gestational age retains its growth, developmental, and regenerative characteristics after transplantation. This type of new model can be used to study both the growth and repair after burn injury of transplanted hFS and will help researchers to study the mechanisms underlying the effects of hFS on wound healing and regeneration of skin appendages.
Using this burn injury model, we confirmed by experimental observation that early or midterm hFS can survive after transplantation and can achieve rapid wound healing without scarring after receiving a deep burn wound. There was no obvious evidence of acute inflammatory immune reactions in the early stage after burn injury. The whole repair process finished within about 14 days and the new collagen was arranged neatly and without excessive deposition. The new skin resembled the skin before the burn injury. The speed and quality of the repair process observed for this hFS model were better than that observed in adult skin, which needs a longer time for repair and develops scarring. The mechanism underlying this ability of hFS to repair burn injury might be related to lack of acute inflammatory immune reactions [15], rapid migration of repair cells to the wound, and strong abilities of foetal skin cells to proliferate and differentiate [16].
To test this hypothesis, we intervened in the wound-healing process by injecting hPBMCs locally beneath the burned skin. HPBMCs have a single nucleus and comprise mainly T lymphocytes (25-60% CD4+ and 5-30% CD8+ cells) and monocytes (10-30%). T cells and monocytes are the major immune effector cells in the human body and play important roles in regulating the reactions mediated by inflammation. In our study of healing of hFS after deep degree burn, we noted that there was no evidence of inflammatory reactions in the early stage of healing. By contrast, there was significant infiltration of inflammatory cells after injection of hPBMCs into the tissues surrounding the wound and the subcutaneous fat. This observation suggests that, although the transplanted hFS was immature, blood platelets coming from it were activated by the burn injury and were able to release inflammatory cytokines such as TGF-β1, TGF-β2, and Platelet- Derived Growth Factor (PDGF), which may have initiated inflammatory reactions and induced inflammatory cells to migrate to the wound. The healing time became longer in transplants that received hPBMCs; the process had not finished by 21 days after burn injury and significant scarring occurred. Our findings suggest that the infiltration of local inflammatory cells mediated by immune cells is an important factor in wound healing after burn injury. The intervention induced by exogenous immune cells caused fibroblasts and endothelial cells to proliferate and migrate into the wound, which seemed to have induced the growth of new capillaries and the formation and deposition of collagen in the damaged areas. The presence of hPBMCs made the migration process slower and more complex by reducing the migratory ability of the proliferating epithelial cells in the surviving skin appendages and was ultimately manifested as a prolonged wound-healing time and scar formation caused by the growth and organization of granulation tissues.
The MMP superfamily is an important group of proteases that plays an important role in wound healing and scarring [17,18]. Their biological activities are regulated precisely by pH [19,20]. MMPs are involved in keratinocyte migration and granulation tissue remodeling during wound healing [21-25]. Of the known MMP family members, MMP-2, MMP-7, and MMP-9 have strong effects on the processes of wound healing and skin growth [26-32]. TIMPs are endogenous inhibitors of MMPs which are secreted mainly by fibroblasts, and they can bind irreversibly to the activated active centers of MMPs to inhibit their activities and to prevent excessive degradation of the Extracellular Matrix (ECM). The homeostasis of MMPs/ TIMPs is required for maintaining the normal physiological structure of the skin [33], and disorders of MMPs/TIMPs expression can cause chronic wounds [34-36] or hypertrophic scarring and keloids [37-44].
MMP-2 and -7 are expressed in adult wounds and participate in the development of sweat glands [45]. Overexpression of MMP-2 or -7 hinders wound healing by reducing angiogenesis and the migration of keratinocytes. We observed the expression pattern of MMP-2 and -7 in hFS during the process of normal growth and partial deep degree burn wounds. MMP-2 was expressed in normal developing skin appendages, whereas MMP-7 expression was not seen during the entire process in the skin appendages. By contrast, only MMP-7 was expressed in the burned area, and MMP-2 expression was low in the deep surviving skin appendages. These findings suggest that MMP-2 expression may be involved in the regulation of skin appendages and that MMP-7 expression is related to wound healing [46]. The different expression patterns of MMP-2 and -7 between adult skin and hFS might be an important reason why hFS can heal without scar formation after deep degree burn injury.
MMP-9 is another gelatinase and is closely involved in the development of skin appendages and the activities of inflammatory cells and repair cells in wound healing. Early and midterm hFS is immature, and MMP-9 is expressed mainly in the cytoplasm of epithelial cells in skin appendages (follicles and sweat glands). In our study, the early expression level of MMP-9 was higher and decreased gradually with the development of skin appendages. TIMP-1 is an inhibitor of MMP-9, and TIMP-1 expression lags behind that of MMP-9. After injection of hPBMCs, MMP-9 expression was observed in the inflammatory cells that had infiltrated into the subcutaneous fat layer at the injection site. This result suggests that the injected hPBMCs released MMP-9 in response to the release of chemotactic inflammatory factors from the burned hFS and that MMP-9 may degrade the ECM on the migration route and induce the migration of monocytes to the burn wound. At 7 days after burn injury, the MMP-9 expression level began to decrease in the subcutaneous tissue layer, but increased significantly in the cytoplasm of inflammatory cells in tissues around the wound and epithelial cells of the surviving skin appendages. These changes suggest that lymphocytes within the hPBMC population may migrate into the wound under induction by macrophages and may be involved in regulation of the wound-healing process. Epithelial cells in the surviving skin appendages may begin to migrate into the wound, where they participate in damage repair. Under the induction by macrophages and lymphocytes, fibroblasts may begin to proliferate and infiltrate into the wound, where they secrete TIMP-1, which can inhibit MMP-9 from degrading the ECM. The massive release of TIMP-1 would prevent excessive degradation of collagen fibers in the wound and, at the same time, deposition of collagen would change the loose structure of the ECM in the hFS, which would reduce the migration of epithelial cells and, finally, prolong the time needed for wound healing. Thus, the expression of and interaction between MMP-9 and TIMP-1 may be closely related to the migration of epithelial and inflammatory cells into the burned skin tissue. An imbalance between MMP-9 and TIMP-1 expression may lead to destruction of the normal structure of hFS and alter the wound-healing process.
We have described the various wound-healing processes involved in our burn model, including the development of skin structures and appendages after transplantation of early or midterm hFS into nude mice and the wound-healing processes within the transplanted skin after deep burn with or without injection of exogenous mature immune cells. The experimental results suggest that the low-level immune and inflammatory reactions and loose structure of the ECM may be important environmental factors for ensuring scarless healing of burns and that the strong proliferative ability of cells and flexible migration ability of epithelial repair cells may be key factors for rapid healing [47]. The presence of exogenous mature immune cells can change the immune inflammatory environment and the migration of epithelial keratinocytes, which may interfere with wound healing, prolong healing time, and cause scar formation. Our experimental results indicate that the expression of MMPs in hFS differs from that in adult skin; this may be an important reason for the scarless healing of hFS, but not of adult skin. Homeostasis between MMPs and TIMPs is required for maintaining the normal physiological structure of the skin, and disorders that alter MMPs or TIMPs expression may prolong wound healing and lead to scar formation [48].
This study observed the dynamic morphological changes during the growth and development process of transplanted hFS under different conditions. Although immunohistochemical staining was used to detect changes in the expression of MMPs and TIMPs in the transplanted hFS, and we have speculated on the possible mechanisms related to these changes, quantitative analysis of these substances at the protein and molecular level is still needed. Further studies are also needed to understand the migration, differentiation, and phenotypic changes in immune cells induced by injection of hPBMCs. All of these factors may be important to understanding the mechanisms responsible for scarless healing.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No.30772258, 81071560, 81372074 and 81401608), the Scientific and Technological Project of Shandong Province (2009GG10002078, 2015GSF118041), Jinan Young Star Project of Science and Technology (grant no. 2013031), and the Youth Foundation of the Second Hospital of Shandong University (Y2013010036).
References
- Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol 2010; 63: 866-881.
- Larson BJ, Longaker MT, Lorenz HP. Scarless foetal wound healing: a basic science review. Plast Reconstr Surg 2010; 126: 1172-1180.
- Wang Z, Liu X, Zhang D, Wang X, Zhao F, Shi P, Pang X. Co-culture with human foetal epidermal keratinocytes promotes proliferation and migration of human foetal and adult dermal fibroblasts. Mol Med Rep 2015; 11: 1105-1110.
- Lane AT, Scott GA, Day KH. Development of human foetal skin transplanted to the nude mouse. J Invest Dermatol 1989; 93: 787-791.
- Ersch J, Stallmach T. Assessing gestational age from histology of foetal skin: an autopsy study of 379 foetuses. Obstetrics Gynecol 1999; 94: 753-757.
- DAngelo F, Bernasconi E, Schafer M, Moyat M, Michetti P, Maillard MH, Velin D. Macrophages promote epithelial repair through hepatocyte growth factor secretion. Clin Exp Immunol 2013; 174: 60-72.
- Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol 2011; 50: 85-93.
- Somasundaram K, Prathap K. Intra-uterine healing of skin wounds in rabbit foetuses. J Pathol 1970; 100: 81-86.
- Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg 1971; 6: 523-528.
- Naik-Mathuria B, Gay AN, Zhu X, Yu L, Cass DL. Age-dependent recruitment of neutrophils by foetal endothelial cells: implications in scarless wound healing. J Pediatr Surg 2007; 42: 166-171.
- Goldberg SR, Quirk GL, Sykes VW, Kordula T, Lanning DA. Altered procollagen gene expression in mid-gestational mouse excisional wounds. J Surg Res 2007; 143: 27-34.
- King A, Balaji S, Marsh E, Le LD, Shaaban AF. Interleukin-10 regulates the fetal hyaluronan-rich extracellular matrix via a STAT3-dependent mechanism. J Surg Res 2013; 184: 671-677.
- Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits TGF-b1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns 2009; 35: 527-537.
- Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP. Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in foetal and postnatal fibroblasts. Plast Reconstr Surg 2010; 125: 74-88.
- Bullard KM, Longaker MT, Lorenz HP. Foetal wound healing: current biology. World J Surg 2003; 27: 54-61.
- Larijani B, Ghahari A, Warnock GL, Aghayan HR, Goodarzi P. Human foetal skin fibroblasts: Extremely potent and allogenic candidates for treatment of diabetic wounds. Med Hypotheses 2015; 84: 577-579.
- Lazaro JL, Izzo V, Meaume S, Davies AH, Lobmann R. Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. J Wound Care 2016; 25: 277-287.
- Ayuk SM, Abrahamse H, Houreld NN. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res 2016; 2016: 2897656.
- Vuotila T, Ylikontiola L, Sorsa T, Luoto H, Hanemaaijer R. The relationship between MMPs and pH in whole saliva of radiated head and neck cancer patients. J Oral Pathol Med 2002; 31: 329-338.
- Sakimoto T, Ohnishi T, Ishimori A. Simultaneous study of matrix metalloproteinases, proinflammatory cytokines, and soluble cytokine receptors in the tears of noninfectious corneal ulcer patients. Graefes Arch Clin Exp Ophthalmol 2014; 252: 1451-1456.
- Baker EA, Leaper DJ. Proteinases, their inhibitors, and cytokine profiles in acute wound fluid. Wound Repair Regen 2000; 8: 392-398.
- Rayment EA, Upton Z, Shooter GK. Increased Matrix Metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008; 158: 951-961.
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3: 27-45.
- Jansen PL, Rosch R, Jansen M, Binnebosel M, Junge K. Regulation of MMP-2 gene transcription in dermal wounds. J Invest Dermatol 2007; 127: 1762-1767.
- Saarialho-Kere UK, Crouch EC, Parks WC. Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol 1995; 105: 190-196.
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3: 27-45.
- Agren MS. Gelatinase activity during wound healing. Br J Dermatol 1994; 131: 634-640.
- Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD. Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen 1999; 7: 166-171.
- Okada A, Tomasetto C, Lutz Y. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol 1997; 137: 67-77.
- Inkinen K, Turakainen H, Wolff H, Ravanti L, Kahari VM. Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. APMIS 2000; 108: 318-328.
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 2005; 31: 674-686.
- Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 2005; 87: 287-297.
- Ja G, Mw RDB. Matrix metalloproteinase activity and immunohistochemical profile of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 during human dermal wound healing. Wound Repair Regen 2004; 12: 295-304.
- Zhang J, Huang X, Wang L. Pioglitazone inhibits the expression of matrix metalloproteinase-9, a protein involved in diabetes-associated wound healing. Mol Med Rep 2014; 10: 1084-1088.
- Simonetti O, Lucarini G, Cirioni O, Zizzi A, Orlando F, Provinciali M, Di Primio R, Giacometti A, Offidani A. Delayed wound healing in aged skin rat models after thermal injury is associated with an increased MMP-9, K6 and CD44 expression. Burns 2013; 39: 776-787.
- Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg 2005; 116: 539-545.
- Muller M, Trocme C, Lardy B, Morel F, Halimi S. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med 2008; 25: 419-426.
- Salonurmi T, Parikka M, Kontusaari S, Pirilä E, Munaut C. Overexpression of TIMP-1 under the MMP-9 promoter interferes with wound healing in transgenic mice. Cell Tissue Res 2004; 315: 27-37.
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, vant Veer LJ, Bartelink H, van de Rijn M, Brown PO, van de Vijver MJ. Robustness, scalability, and integration of a wound response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA 2005; 102: 3738-3743.
- Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg 2010; 63: 1015-1021.
- Yeh FL, Shen HD, Tai HY. Decreased production of MCP-1 and MMP-2 by keloid-derived fibroblasts. Burns 2009; 35: 348-351.
- Dasu MR, Barrow RE, Spies M, Herndon DN. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns 2003; 29: 527-531.
- Dasu MR, Spies M, Barrow RE, Herndon DN. Matrix metalloproteinases and their tissue inhibitors in severely burned children. Wound Repair Regen 2003; 11: 177-180.
- Ulrich D, Noah EM, von Heimburg D, Pallua N. TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg 2003; 111: 1423-1431.
- Li J, Fu X, Sun X, Sun T, Sheng Z. The interaction between epidermal growth factor and matrix metalloproteinases induces the development of sweat glands in human fetal skin. J Surg Res 2002; 106: 258-263.
- Hayden DM, Forsyth C, Keshavarzian A. The role of matrix metalloproteinases in intestinal epithelial wound healing during normal and inflammatory states. J Surg Res 2011; 168: 315-324.
- Walraven M, Talhout W, Beelen RH, van Egmond M, Ulrich MM. Healthy human second-trimester foetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen 2016; 24: 533-541.
- Ren Y, Gu G, Yao M, Driver VR. Role of matrix metalloproteinases in chronic wound healing: diagnostic and therapeutic implications. Chin Med J (Engl) 2014; 127: 1572-1581.