Editorial - Journal of Food Microbiology (2017) Journal of Food Microbiology(Special Issue-2017)
Growth inhibitory model of Cronobacter sakazakii.
Yabo Peng, Qiyang Ling, Lei Chen and Ting Fang*
Fujian Agriculture and Forestry University, School of Food Science, Fuzhou, 350001, China
- *Corresponding Author:
- Ting Fang
Fujian Agriculture and Forestry University
School of Food Science
Fuzhou, 350001, China
Tel: 15637846041
E-mail: fangting930@163.com
Accepted on September 26, 2017
Citation: Peng Y, Ling Q, Chen L, et al. Growth inhibitory model of Cronobacter sakazakii. J Food Microbiol. 2017;1(1):1-2.
Introduction
C. sakazakii belongs to Enterobacteriaceae, mainly parasitic in the intestine of humans and animals [1]. When humans and animals infected by C. sakazakii can cause a series of diseases, such as neonatal meningitis, necrotizing colitis, bacteremia [2,3]. Medical studies have shown that antibiotics can be used to treat diseases, but it may cause some side effects. The lowest mortality rate for such diseases is 40%, the highest can reach 80% [4]. In 1961, the United States for the first time found meningitis by the infection of Enterobacter sakazakii [5]. After that, Belgium, Greece and other countries occurred in the case of C. sakazakii [6]. By using mathematical methods to describe the relationship between changes in the number of bacteria and external environmental factors under different environmental conditions is now conventional and effective.
The concept of predictive microbiology was first proposed by Scott in 1937, and Ross proposed "microbial prediction techniques" in the 1980s. In 1983, a microbiologist used computers to predict the shelf life of food, established a database of spoilage growth. Predicting the microbial model and the corresponding model parameters will enable the growth state of the microorganisms to be displayed in mathematical models and corresponding parameter forms [7]. And provide some guidance for the processing of food, storage and key control in the process of circulation [8,9]. As the research on microbes continues to increase, the prediction model of microbes is also increased accordingly. There are new and better data simulation models, and some early development of the data model. At present, the microbial prediction model can be divided into three categories, primary, secondary and tertiary model.
Primary Model
The primary model is a description of the relationship between the number and duration of growth of microorganisms under different external conditions. The growth curve of microbes generally includes three parts, lag phase, logarithmic phase, stationary phase, and the decline phase is not manifested in the data model.
Three primary growth models were used to describe C. sakazakii growth curves, which were the Huang model, Baranyi model and Gompertz model [10-13].
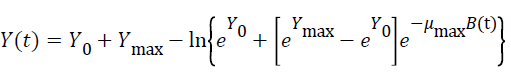 (1)
(1)
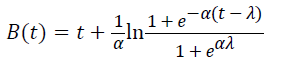 (2)
(2)
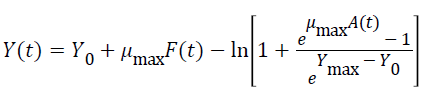 (3)
(3)
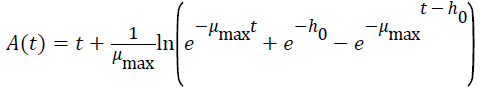 (4)
(4)
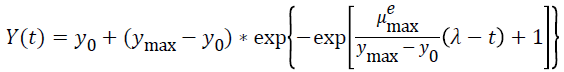 (5)
(5)
In equations 1-5, Y(t) represents the natural logarithm of bacterial counts (Ln CFU/g) at time t; Y0 is the initial bacterial counts (Ln CFU/g); Ymax is the bacterial counts (Ln CFU/g) at the stationary phase; μmax is the specific growth rate (h-1);λ is the lag phase duration (h). h0 reflectsthe physiological state of the microorganism. The parameter α(=4) is a coefficient used to define the transition from the lag phase to exponential growth curve [14].
Prospective
Food additives are often used in food processing to inhibit the growth of microorganisms. Traditional antimicrobial chemicals such as potassium sorbet have good antimicrobial utility, but they possess potential side effects, and their use is also limited. Scientists are increasingly accepting natural antibacterial agents as an alternative to the processing and production of food [15]. With the development of science, the level of medical progress, more and more antibiotics and drugs in the course of treatment has been widely used, leading to increased resistance to bacteria, seriously affecting the human and animal health [16]. Natural antibacterial agents are considered to be effective and safe antibacterial agents, with both nutritional value and other effects. Therefore, the search for antimicrobial substances from natural product is one of the hotspots of current international research. Predictive microbiology provides strong theoretical support for food production, processing, hazard analysis, and safety control [17-21]. The use of microbiology, mathematics, statistics, combined with computer software to build the model.
Author Contributions
All authors have given approval to the final version of the manuscript.
Acknowledgment
This work was financially supported by the Natural Science Foundation of China (Grant No. 31401597 and 31601393).
References
- Fang T, Gurtler JB. Growth Kinetics and Model Comparison of Cronobacter sakazakii, in Reconstituted Powdered Infant Formula. J Food Sci. 2012;77:247-55.
- Bar-Oz B, Preminger A, Peleg O, et al. Enterobacter sakazakii infection in the newborn. Acta Paediatr Suppl. 2001;90(3):356-58.
- Muytjens H L, Kollée L A. Neonatal meningitis due to Enterobacter sakazakii. Tijdschrift voor Kindergeneeskunde. 1982;50:110-2.
- Chandarkant P, Kensuke Shima. Cronobacter spp. (previously Enterobacter sakazakii) invade and translocate across both cultured human intestinal epithelial cells and human brain mecrovascular endothelial cells. Microb Pathog. 2012;52:140-47.
- Block C, Peleg O, Minster N, et al. Cluster of neonatal infections in Jerusalem due to unusual biochemical variant of Enterobacter sakazakii. Eur J Clin Microbiol. 2002;21:613-16.
- Acker J V, Smet F D, Muyldermans G, et al. Outbreak of Necrotizing Enterocolitis Associated with Enterobacter sakazakii in Powdered Milk Formula. J Clin Microbiol. 2001;39:293-7.
- Yu C, Davidson V J, Yang S X. A neural network approach to predict survival/death and growth/no-growth interfaces for Escherichia coli O157: H7. Food Microbiol. 2006;23:552-60.
- Mcmeekin T, Bowman J, Mcquestin O, et al. The future of predictive microbiology: strategic research, innovative applications and great expectations. Int J Food Microbiol. 2008;128:2-9.
- Mcmeekin T A, Ross T, Olley J. Application of predictive microbiology to assure the quality and safety of fish and fish products. Int J Food Microbiol. 1992;15:13-32.
- Huang L. Growth kinetics of Escherichia coli O157:H7 in mechanically -tenderized beef. Int J Food Microbiol. 2010;140:40-48.
- Baranyi J, Mcclure P J, Sutherland J P, et al. Modeling bacterial growth responses. J Ind Microbiol Biotechnol. 1993;12:190-94.
- Baranyi J, Roberts T A. Mathematics of predictive food microbiology. Int J Food Microbiol. 1995;26:199-218.
- Buchanan R L, Whiting R C, Damert W C. When is simple good enough: A comparison of the Gompertz, Baranyi, and Three-phase linear models for fitting bacterial growth curves. Food Microbiol. 1997;14:313-26.
- Huang, L. Optimization of a new mathematical model for bacterial growth. Food Control. 2013;32:283-88.
- Lucas D L, Were L M. Anti–Listeria monocytogenes Activity of Heat-Treated Lyophilized Pomegranate Juice in Media and in Ground Top Round Beef. J Food Prot. 2009;72:2508-16.
- Su X W, Howell A B, D'Souza D H. Antiviral effects of cranberry juice and cranberry proanthocyanidins on foodborne viral surrogates - a time dependence study in vitro. Food Microbiol. 2010;27:985-91.
- Nganje W E, Siaplay M, Kaitibie S, et al. Predicting food safety losses in turkey processing and the economic incentives of hazard analysis and critical control point (HACCP) Int J Agric. 2006;22:475-489.
- Dampawan P, Huntrakul C, Reutrakul V, et al. Constituents of Clinacanthus nutans and the crystal structure of Lup-20 (29)-ene-3-one. J Sci Soc. 1997;3:14-26.
- Teshima K I, Kaneko T, Ohtani K, et al. Sulfur-containing glucosides from Clinacanthus nutans. Phytochem. 1998;48:831-35.
- Tu S F, Liu R H, Cheng Y B, et al. Chemical Constituents and Bioactivities of Clinacanthus nutans Aerial Parts. Molecules. 2014;19:20382.
- Sakdarat S, Shuyprom A, Ayudhya TDN, et al. Chemical composition investigation of the Clinacanthus nutans Lindau leaves. Thai J Phytopharm. 2006;13:13-24.