Research Article - Biomedical Research (2017) Volume 28, Issue 4
Growth inhibitory effect of stevioside on ovarian cancer through Akt/ERK pathway
Xiao-Yan Li1, Wen-Ming Lu2, Wen-Feng Shen1, Yan Wu1, Ya-Ping Liu3, Ya Tuo3, Yan-Long Liu1*1Department of Ultrasonography, The First Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
2Department of Ultrasonography, The First People’s Hospital of Huzhou City, Huzhou, Zhejiang Province, China
3Department of Gynaecology and Obstetrics, The First Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- *Corresponding Author:
- Yan-Long Liu
Department of Ultrasonography
The First Affiliated Hospital of Inner Mongolia Medical University
North Tongdao Road, Huimin District, Hohhot, Inner Mongolia, China
Accepted date: September 28, 2016
Abstract
Phytochemicals play a prominent role as cancer preventive agents. Stevioside is a phytochemical which is isolated from Stevia rebaudiana and is known for its medicinal properties including prevention against cancer. Therefore, the present work intended to explore the antiproliferative activity of stevioside on ovarian cancer cell line, OVCAR-3. The results elucidated that stevioside treatment repressed the growth of OVCAR-3 cells and induced cytotoxicity, both dose dependently and time dependently. Furthermore, stevioside has been found to be associated with increased ROS production in the cell which suggests the initiation of apoptosis. Additionally, the decrease in mitochondrial membrane potential indicated the involvement of stevioside induced intrinsic apoptotic pathway in the cell. These results were further confirmed by increased level of caspase-3 and caspase-9 in OVCAR-3 cancer cells after stevioside treatment. Moreover, flow cytometric study established the apoptotic behavior of stevioside and showed cell cycle arrest in G2/M phase. Additionally, the inactivation of P13K/AKT signaling pathway was also revealed. Thus, the results may summarize the therapeutic potential of stevioside against ovarian cancer and its role as chemotherapeutic agent in future clinical applications.
Keywords
Stevioside, Apoptosis, Growth inhibition, Ovarian cancer, Signaling pathway.
Abbreviation
MTT: 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazoliumbromide; LDH: Lactate Dehydrogenase; SDS: Sodium Dodecyl Sulphate; DMSO: Dimethyl Sulfoxide; PBS: Phosphate Buffer Saline; FBS: Fetal Bovine Serum; DMEM: Dulbecco’s Modified Eagle’s Medium; HRP: Horseradish Peroxidase; DCFH-DA: 2',7'-Dichlorofluorescin Diacetate; DiOC6(3): Dihexyloxacarbocyanine Iodide; ATCC: American Type Culture Collection; NAC: N-acetyl-L-Cysteine
Introduction
Ovarian cancer, a gynecological cancer, is the foremost reason of mortalities globally [1,2]. Although various advancements has been made in the field of cancer diagnosis and treatments, the difficulty in detecting ovarian cancer in its early stages still remains a challenge which owes to the high mortality rate among human population. Due to which, in most of the cases ovarian cancer is diagnosed during its advanced stages (approx. 70%), i.e. after metastasis [3]. Moreover, due to the heterogeneous property of ovarian cancer it also shows marked variations in histology, molecular profile and chemosensitivity [4]. Taxanes and platinum compounds, such as cisplatin, constitute the contemporaneous first line chemotherapy for ovarian cancer. Even though the patients respond well to first line chemotherapy, the chances for cancer recurrence prevail during ovarian cancer condition [5]. In addition, the limited efficacy and sensitivity to platinum drugs urges the demand for new chemotherapeutic molecules which acts through multiple signalling pathways and are able to overcome chemoresistance in women with advanced ovarian cancer [6]. Medicinal herbs, fruits and vegetables are rich in phytochemicals which are of great significance in medicinal treatments including chemotherapy against various cancers [7,8]. Some of these phytochemicals have also been demonstrated to perform growth inhibitory activity against ovarian cancer [9]. Recently, Ren et al. showed potential anticancer activity of quercetin, a flavonoid, on ovarian cancer SKOV-3 cells [10]. The anticancer activity of other phytochemicals, such as nobelitin [11], (-)-epigallocatechin-3-gallate (EGCG) [12], kaempferol [13], and apigenin [14], have also been investigated against ovarian cancer. Stevioside (a glycoside) is among such phytochemicals which is isolated from Stevia rebaudiana and consists of glucose and steviol [15]. Stevia rebaudiana is a shrub which is present worldwide and because of its medicinal values it is used in various industries including pharmaceutical and cosmetic industries [16,17]. It is a member of family Asteraceae and genus Stevia and is rich in terpenes and flavanoids. Due to its sweetening property it is also known as sugar leaf [18]. Furthermore, the growth inhibitory activity of stevioside against MCF-7, a human breast cancer cell line, has also been investigated by Paul et al. [19]. They found that stevioside stimulates ROS mediated apoptosis in MCF-7 human breast cancer cell line. Increased expression of apoptotic proteins like Bax, Bcl-2 and Caspase-9 was also observed in stevioside treated MCF-7 cells. However, the growth inhibitory potential of stevioside on ovarian cancer is yet unidentified. The effect of phytochemicals on cancer cells is highly modulated by the balance between cell apoptosis (programmed cell death) and cell survival [20]. Various mechanisms are well illustrated by which phytochemicals induce apoptosis, e.g downregulation of anti-apoptotic proteins, upregulation of pro-apoptotic members, and increase in intracellular reactive oxygen species (ROS) [21-23]. In addition, phytochemicals may also regulate signaling pathways essential for cell survival. PI3K/AKT [24] is among the most widely studied signaling pathways responsible for cell survival. Therefore, we aimed to exhibit the growth inhibitory effect of stevioside on ovarian cancer cells. Besides, the role of PI3K/AKT signaling pathway in regulating stevioside induced apoptosis is also investigated in the present study.
Materials and Methods
Chemicals
Stevioside hydrate of ≥ 98% purity, MTT, propidium iodide (PI), SDS and NAC (ROS scavenger) were acquired from Sigma-Aldrich, St. Louis, MO, USA. Stock solution of stevioside (50 mM) and stock solution of MTT (5 mg/ml) were prepared in DMSO (Sigma) and PBS (Sigma), respectively. Cytotoxicity detection kit for LDH assay was procured from Takara Bio Inc., Shiga, Japan. FBS and DMEM medium were attained from Invitrogen, Carlsbad, CA, while penicillin, streptomycin, 3,3’-DiOC6(3) and DCFH-DA were purchased from Thermo Scientific, Pierce, Rockford, IL, USA. Caspase activation kit was obtained from R&D Systems, Minneapolis, MN, USA. For western blot primary antibodies (pPI3K p85, PI3K p85, pAKT, AKT, and β-actin) and HRP conjugated rabbit anti-mouse secondary antibodies were bought from Santa Cruz Biotech, CA, USA. For transfection studies myrAktdeltaPH plasmid construct (mAKT) and empty plasmid pECE were obtained from Addgene, Cambridge. Additionally, Fugene HD (transfection reagent) was from Promega, Sunnyvale, CA.
Cell culture
The human ovarian cancer cell line OVCAR-3 was procured from the ATCC (Rockville, MD). Cells were cultured in DMEM medium containing antibiotics (100 U/ml each of penicillin and streptomycin) along with 10% FBS in a humidified incubator (95% air and 5% CO2) at 37°C.
Cell viability and cell cytotoxicity assay
The effect of stevioside in growth inhibition of OVCAR-3 cells was analyzed using MTT assay. Initially, 5 × 103 cells per well were seeded into 96 well plate for 24 h and incubated at 37°C. Afterwards, stevioside (0, 2.5, 5 and 10 μM) was treated to the cells for additional 24, 48 and 72 h. After predetermined treatment time points, medium was aspirated and fresh medium along with MTT solution was supplemented to the wells for 2 h at same conditions. Supernatant was further discarded and the cells were rinsed with PBS. The formed mitochondrial succinate mediated MTT formazan in viable cells was then evaluated by adding DMSO to the wells and measuring absorbance at a wavelength of 570 nm. Furthermore, the amount of LDH produced from destructed cells was estimated via cytotoxicity detection kit to analyze the cytotoxic effect of stevioside on OVCAR-3 cells. For LDH assay, cells were seeded and treated similar to MTT assay. Following treatment, 10 μl of supernatant was collected at predetermined time points and mixed with 40 μl of PBS. LDH reagent was further added to the reaction solution in equal concentration and incubated in dark at 25°C. After 30 minutes, stop solution (50 μl) was mixed to the reaction mixture and absorbance was measured at 490 nm wavelength to determine cell cytotoxicity level. For accuracy both the assays were repeated thrice and expressed along with error bars.
Measurement of ROS production and mitochondrial membrane potential (ΔΨm) level
The ROS production was assayed using previously reported study with slight modification [25]. In this assay, cellular esterases deacetylates DCFH-DA into DCFH which further gets oxidized by ROS into 2',7'-dichlorofluorescin (DCF; a fluorescent compound). Therefore, it can be assumed that the level of fluorescence in the cells is directly proportional to the ROS level. To determine the level of ROS production within the cells, 2 × 105 OVCAR-3 cells/well was seeded in 24 wells plate. Thereafter, the cells were exposed to varying doses of stevioside (0 μM to 10 μM) for 72 h followed by cell harvesting as pellet and washing the pellet by PBS. The cells were stained by resuspending the pellet into DCFH-DA, a fluorescent probe. The reaction solution was incubated in the absence of light at 37°C for 30 min. DCF fluorescence levels in the stained cells were analyzed at 480 nm (excitation wavelength) and 525 (emission wavelength) using FACScan flow cytometer. Mean fluorescence intensity of three repeated experimental values was calculated. In addition, to confirm the role of ROS generation in stevioside induced apoptosis, OVCAR-3 cells were cultured in 48 wells plate till 70-80% confluence level. Cells were then pre-exposed to 5 mM of ROS scavenger (NAC) for 2 h. Later on, cells were treated with stevioside for 72 h followed by MTT assay.
As ROS production is associated with mitochondrial membrane potential (ΔΨm), the level of ΔΨm was also determined [26]. After seeding the cells, appropriate doses of stevioside were treated for 72 h. DiOC6(3) was then added to the harvested cells in dark for 20 min at 37°C and the fluorescent intensity was assayed by FACScan flow cytometer.
Caspase-3 and caspase-9 activity measurement
Caspase activation kit was used to measure caspase-3 and caspase-9 activity in stevioside treated OVCAR-3 cells in accordance to manufacturer’s protocol. Concisely, 72 h stevioside treated OVCAR-3 cells were collected as pellet after centrifugation. Cells were further mixed with lysis buffer (ice cold) for 15 min. After cell lysis total protein was collected from the supernatant and added with respective substrate and amount of p-nitroaniline was measured at 400 nm.
Cell cycle distribution and apoptosis analysis
In brief, stevioside was treated on seeded OVCAR-3 cells at a dose range of 0 μM to10 μM for 72 h. Afterwards, the treated cells were centrifuged at 1000 rpm for 15 min and the pellet was procured. The harvested cells were then fixed and washed followed by the addition of Triton X-100 (1% v/v) at 37°C for 15 min. DNA in the cells were stained by mixing PI solution (40 μg/ml) with the treated cells and incubate them in the dark at 4°C. Cell cycle distribution and apoptosis rate in OVCAR-3 cells induced by stevioside was then measured using FACScan flow cytometry (BD Biosciences, Calibur, USA). Experiment was performed thrice and shown as ± SD.
Western blot analysis
Stevioside treated OVCAR-3 cells were collected by centrifugation and rinsed with PBS. Ice cold RIPA buffer and protease inhibitor cocktail (Roche) mixture was added to lyse the cells and to collect total cell extract. Proteins were collected as supernatant by first vortexing the extracts and then centrifuging them at 12,000 rpm for 15 min at 4°C. The total amount of protein in the samples was measured using BCA protein assay reagent (Pierce). Equal quantity (50 mg) of each protein samples were loaded on SDS polyacrylamide gel (10%-12%) and SDS-PAGE (SDS polyacrylamide gel electrophoresis) was performed to separate the protein bands. The separated protein bands were transferred to nitrocellulose membrane which was further incubated with 5% skim milk in Tween 20-Tris buffered saline (TBST). After blocking the nonspecific sites on the membrane, required quantity of primary antibodies (pPI3K p85, PI3K p85, pAKT, AKT, and β-actin) was added to the membrane and incubated overnight. Membrane was washed and incubated with secondary antibody (horseradish peroxidase (HRP) conjugated rabbit anti-mouse secondary antibody). The signals from the membrane were then detected using enhances chemiluminescence detection system (FUJFILM Las-3000 mini, Tokyo, Japan).
Overexpression of AKT allele
Overnight seeded OVCAR-3 cells were transfected with mAKT plasmid construct (0.50 μg) along with transfection reagent Fugene HD. Empty plasmid pECE was taken as control. After 4 h of incubation medium was aspirated and cells were treated with stevioside for 72 h followed by MTT assay.
Statistical analysis
Two-tailed student t test and one way ANOVA (significance value was P<0.05) was performed on the results based on the experiments which were conducted in triplicate.
Results
Stevioside affects proliferation of OVCAR-3 cells and induces cell cytotoxicity
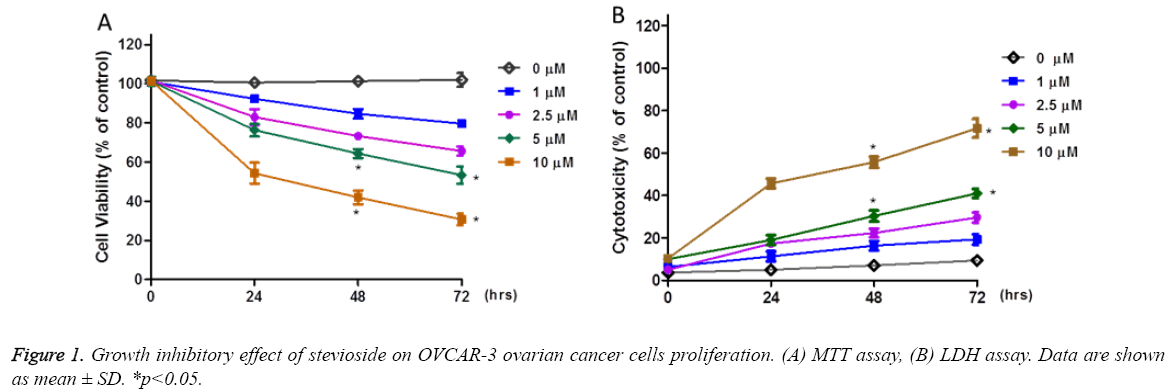
To investigate the antiproliferative effect of stevioside on OVCAR-3 cells, MTT and LDH assays were performed to determine the cell viability and cell cytotoxic effect of stevioside on OVCAR-3 cells, respectively. For this, OVCAR-3 cells were exposed to stevioside at concentrations ranging from 0 μM to 10 μM from 0 h to 72 h. Both MTT and LDH results showed dose dependent anti-proliferative effect of stevioside on OVCAR-3 cells at all the selected time points indicating its therapeutic potential. However, during early time points significant antiproliferative effect of stevioside was witnessed at only 10 μM of treatment dose. While, more time was required for lower doses of stevioside to show prominent antiproliferative effect. MTT assay showed approximately 70 % decrease in the viability of OVCAR-3 cells after 72 h of treatment at 10 μM dose of stevioside (Figure 1a). In a similar manner, LDH assay showed approximately 60 % enhanced cell cytotoxicity at 10 μM of stevioside treatment after 72 h which was in agreement to MTT assay (Figure 1b). Based on MTT and LDH assay 72 h was selected as an effective time point for further experiments.
Stevioside enhances the production of intracellular ROS and decreases ΔΨm level
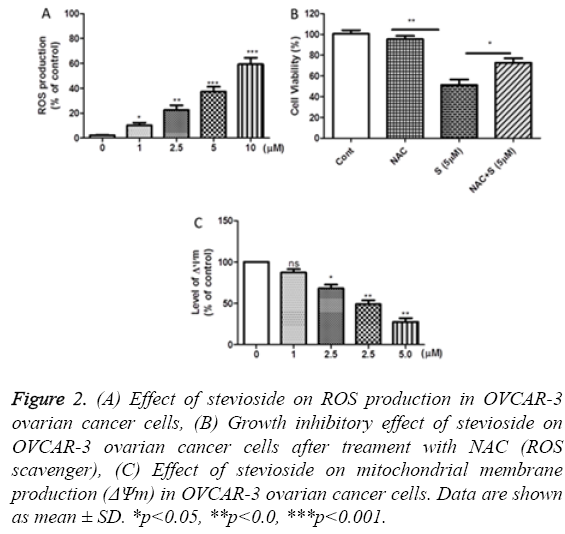
To examine the role of ROS generation in stevioside induced apoptosis in OVCAR-3 ovarian cancer cells, cells were first treated with stevioside for 72 h and then stained with DCFHDA DCF followed by fluorescence intensity measurement. Increased level of ROS was observed with an increase in the treatment dose of stevioside indicating the involvement of ROS production in inducing apoptosis in stevioside treated OVCAR-3 cell (Figure 2a). This phenomenon was confirmed by MTT assay after stevioside exposure in NAC (ROS scavenger) pretreated OVCAR-3 cells. Data showed that NAC pretreatment inhibited the antiproliferative effect of stevioside confirming the association of ROS generation and apoptosis in OVCAR-3 ovarian cancer cells (Figure 2b). According to previous studies, the correlation of apoptosis and variation in ΔΨm is well defined [27]. Therefore, we also evaluated the effect of stevioside treatment on ΔΨm in OVCAR-3 cells using DiOC6 fluorescent dye, a voltage and mitochondria specific dye. As anticipated, a dose dependent decrease in ΔΨm was observed in stevioside exposed OVCAR-3 cells signifying the stimulation of mitochondria mediated apoptotic signaling pathway in the cells (Figure 2c).
Figure 2: (A) Effect of stevioside on ROS production in OVCAR-3 ovarian cancer cells, (B) Growth inhibitory effect of stevioside on OVCAR-3 ovarian cancer cells after treament with NAC (ROS scavenger), (C) Effect of stevioside on mitochondrial membrane production (ΔΨm) in OVCAR-3 ovarian cancer cells. Data are shown as mean ± SD. *p<0.05, **p<0.0, ***p<0.001.
Stevioside enhances caspase activity
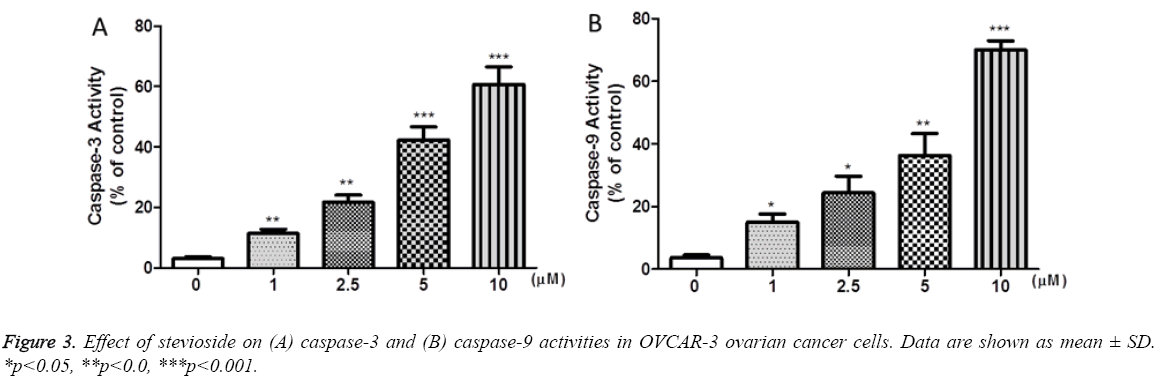
Followed by 72 h of stevioside treatment, caspase-3 and caspase-9 activities were investigated in OVCAR-3 cells to determine the potential mechanism of stevioside induced apoptosis. It was observed that treatment with stevioside lead to an increase in the levels of both caspase-3 and caspase-9 activities dose dependently in OVCAR-3 cells which were found significant (Figures 3a and 3b). As measured by caspase detection kit, caspase-3 activity was increased by 50% while caspase-9 activity was enhanced by 57%, approximately, at 10 μM dose. These results suggest that stevioside induced apoptotic pathway is caspase dependent in OVCAR-3 cells.
Stevioside induces apoptosis and affects cell cycle arrest
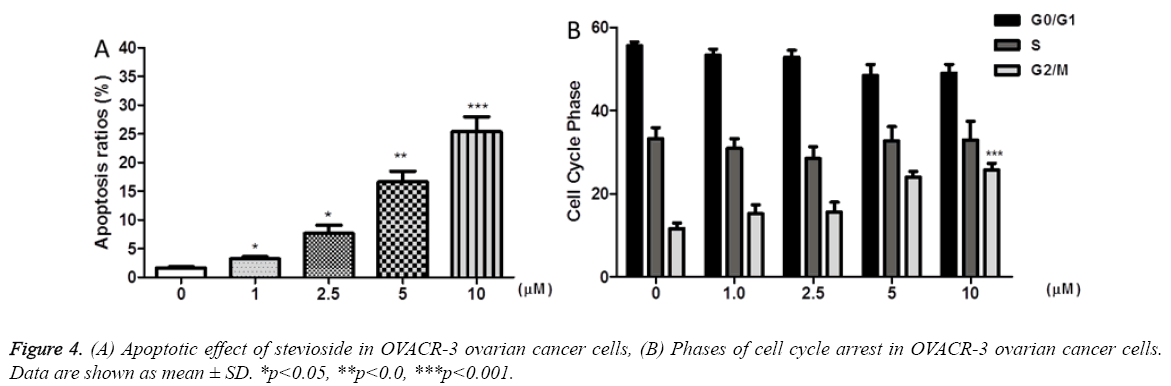
Apoptotic effect of stevioside on OVCAR-3 cells was explored using flow cytometry by staining the cells with DNA binding dye, propidium iodide, to ascertain that the antiproliferative effect of stevioside was due to cell apoptosis. OVCAR-3 cells exhibited increased apoptosis rate after 72 h of stevioside treatment, in a dose dependent manner (Figure 4a). The results revealed increase in apoptosis rate from 1.63% to 25.35 % at 0 μM to 10 μM of treated stevioside, respectively. Corresponding to apoptosis rate, it was observed that stevioside also promoted dose dependent cell cycle arrest at G2/M phase in OVCAR-3 cells (Figure 4b).
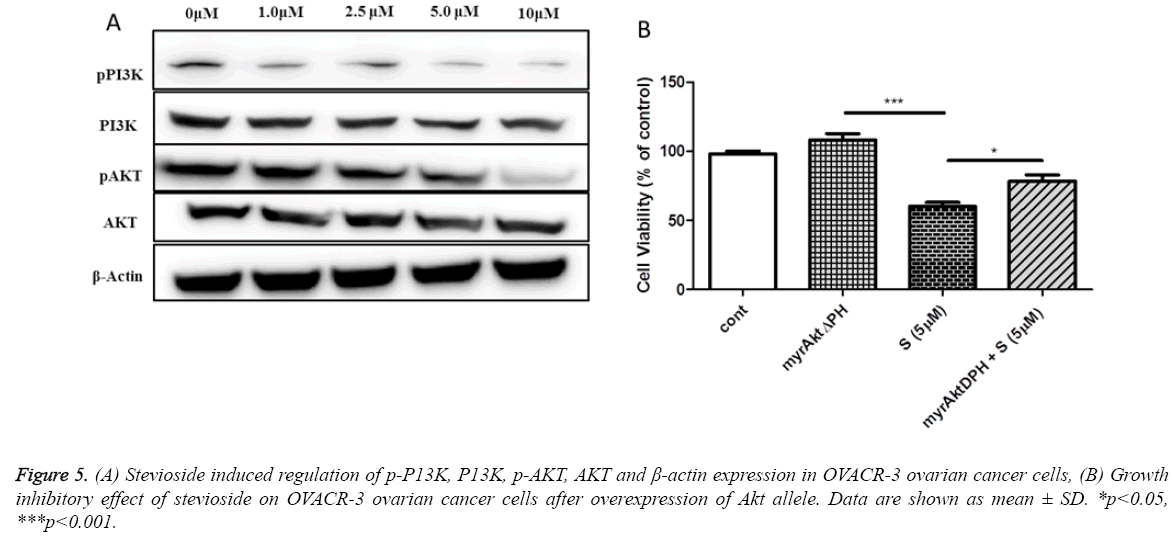
Inhibitory effect of stevioside on PI3K/AKT phosphorylation
Previous studies have reported that PI3K/AKT signaling pathway is associated with cell growth and survival [28]. Thus, in an attempt to examine whether the apoptotic effect of stevioside on OVCAR-3 cells is due to the involvement of PI3K/AKT signaling pathway western blotting was done. It is well documented that the activation of AKT phosphorylation protects the cells from apoptosis [29]. In this study it was found that stevioside inhibited the levels of pPI3K and pAKT in a dose dependent manner (Figure 5a). These results were further confirmed by overexpressing AKT through myrAktdeltaPH plasmid DNA transfection. Results demonstrated that the viability of OVCAR-3 cell was dose dependently increased after AKT overexpression (Figure 5b), suggesting that stevioside performs apoptosis via deactivating PI3K/Akt pathway in OVCAR-3 cells.
Discussion
The ability of cancer cells to develop resistance against chemotherapeutic agents is creating a need to develop novel anticancer molecules with a reduced level of toxicity and upgraded effectiveness. Nowadays, cancer suppression via dietary components is attaining scientific as well as clinical benefits. Therefore, the anticancer potential of natural compounds, mainly phytochemicals, has been explored in various studies. Phytochemicals may obstruct various pathways responsible for carcinogenesis. In addition, phytochemicals are well known to act as anticancer agents by persuading apoptosis. Stevioside, a phytochemical, showed anticancer effect on 12-O-tetradecanoylphorbol-13-acetate (TPA) induced skin cancer in mice [30]. In MCF-7 human breast cancer cells stevioside reduced cell viability by inhibiting DNA synthesis and inducing cell apoptosis [19]. Also, a hydrolysis product of stevioside (isosteviol) performed cell growth inhibitory activity by targeting DNA polymerases and DNA topoisomerase II [31]. In accordance to above studies, our MTT and LDH assay results also demonstrated dose dependent (0 μM to 10 μM) and time dependent (0 h to 72 h) growth inhibitory effect of stevioside on OVCAR-3 ovarian cancer cells (Figure 1).
The relationship between anticancer phytochemicals and ROS production is explained in various studies. ROS acts as a secondary messenger and is usually associated with cell proliferation and apoptosis [32]. ROS is mainly produced in mitochondria due to which it is also associated with destabilization of ΔΨm [33,34]. Loss of ΔΨm reflects mitochondrial damage resulting in early stage apoptosis [35]. In our study, stevioside displayed increase in ROS production along with declined ΔΨm in OVCAR-3 cells indicating the commencement of intrinsic apoptotic pathway. As ROS is able to perform dual function by inducing both apoptosis and cell proliferation, we examined the apoptotic behavior of stevioside in this study by pretreating the cells with NAC (ROS scavenger). Here, NAC tends to neutralize the apoptotic effect of stevioside. The increased cell viability of OVCAR-3 cells ascertains that the elevated ROS generation is directly related to the induction of apoptosis (Figure 2). ROS generation causes cell death by interfering with many physiological processes resulting in damage to DNA, membranes and proteins [36].
Moreover, ROS generation reduces ΔΨm which leads to activation of mitochondrial factors responsible for apoptosis. Caspase-9 and cytochrome-c are among such mitochondrial factors which are involved in apoptotic phenomenon [37]. Upon receiving stress signals, the cell secretes cytochrome c which attaches to apoptotic protease-activating factor1 (Apaf-1) finally forming a complex. This complex activates the inactive caspae-9 (the initiator caspase) and forms apoptosome [38]. Here, significant increase in both caspase-9 and caspase-3 was also observed which showed implication of mitochondrial intrinsic apoptotic pathway (Figure 3).
The check points in a cell cycle are of great importance as they are essential to make sure that the upstream processes are well accomplished before downstream processes proceeds, such as completion of DNA replication and chromosome attachment to spindle apparatus. If the checkpoints find any improper functioning in the upstream processes, the cell undergoes apoptosis [39]. However, in case of any impairment in the checkpoints, the normal cells show uncontrolled growth and may get converted into tumor cells. Studies showed that the growth inhibitory effect of phytochemicals is also associated with cell cycle arrest and apoptosis [40]. Flow cytometeric studies performed here also demonstrated significant increase in apoptosis rate and cell population in G2/M phase indicating cell cycle arrest (Figure 4).
Furthermore, we investigated the specific regulatory mechanism of stevioside at the molecular level which may be responsible for the cell cycle arrest. In cell physiology, phosphatidylinositol acts as a secondary messenger that regulates various cellular processes that are accountable for cell survival and apoptosis. Phosphatidylinositol gets phosphorylated after the activation of a dimer enzyme i.e. PI3K. Regulatory unit (p85) and catalytic unit (p110) are the two components of PI3K which are responsible for Akt phosphorylation [41]. AKT is well identified to inhibit apoptosis and promote cell survival; therefore, the phosphorylation of AKT is a critical factor in the progression of various cancers including ovarian cancer [11]. Previous studies showed that AKT phosphorylation inhibits caspase-9 mediated apoptosis by inactivating procaspase-9 [42]. As shown in the current study, stevioside inactivate PI3K/AKT by inhibiting the phosphorylation of both PI3K and AKT in a dose dependent manner (Figure 5). Western results were further confirmed by analyzing cell viability after transfecting the cells with plasmid DNA that overexpress AKT. The decrease in pPI3K and pAKT level and an increase in caspase-9 level can be associated together, suggesting that stevioside may induce intrinsic apoptotic pathway in OVCAR-3 ovarian cancer cells.
Conclusion
In summary, our study showed the antiproliferative effect of stevioside on OVCAR-3 ovarian cancer cells by inducing apoptosis and cell cycle arrest. Furthermore, it was observed that stevioside exhibit apoptotic effect on OVCAR-3 ovarian cancer cells by promoting ROS generation and stimulating caspase-3 and caspase-9 activities. The current study also showed that stevioside deactivates PI3K/AKT signaling pathway by inhibiting phosphorylation of PI3K and AKT.
References
- Memarzadeh S, Berek JS. Advances in the management of epithelial ovarian cancer. J Reprod Med 2001; 46: 621-629.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E. Global cancer statistics.CA Cancer J Clin 2011; 61: 69-90.
- Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer.Nat Rev Cancer 2009; 9: 167-181.
- Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation.Nat Rev Cancer 2009; 9: 415-428.
- Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer.Semin Oncol 2006; 33: S12-16.
- Blackledge G, Lawton F, Redman C, Kelly K. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials.Br J Cancer 1989; 59: 650-653.
- Surh YJ. Cancer chemoprevention with dietary phytochemicals.Nat Rev Cancer 2003; 3: 768-780.
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer.Biochem Pharmacol 2006; 71: 1397-1421.
- Vergara D, Simeone P, Toraldo D, Del Boccio P, Vergaro V. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells.Mol Biosyst 2012; 8: 1078-1087.
- Ren MX, Deng XH, Ai F, Yuan GY, Song HY. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro.Exp Ther Med 2015; 10: 579-583.
- Chen J, Chen AY, Huang H, Ye X, Rollyson WD. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway.Int J Oncol 2015; 46: 2629-2638.
- Huh SW, Bae SM, Kim YW, Lee JM, Namkoong SE. Anticancer effects of (-)-epigallocatechin-3-gallate on ovarian carcinoma cell lines.Gynecol Oncol 2004; 94: 760-768.
- Luo H, Rankin GO, Li Z, Depriest L, Chen YC. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway.Food Chem 2011; 128: 513-519.
- Tang AQ, Cao XC, Tian L, He L, Liu F. Apigenin inhibits the self-renewal capacity of human ovarian cancer SKOV3derived sphere-forming cells. Mol Med Rep 2015; 11:2221-2226.
- Hanson JR, De Oliveira BH. Stevioside and related sweet diterpenoid glycosides.Nat Prod Rep 1993; 10: 301-309.
- Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects.Metabolism 2004; 53: 73-76.
- Chatsudthipong V, Muanprasat C. Stevioside and related compounds: therapeutic benefits beyond sweetness.Pharmacol Ther 2009; 121: 41-54.
- Gasmalla MAA, Yang R, Musa A, Hua X, Zhang W. Physico-chemical Assessment and Rebauidioside A. Productively of Natural Sweeteners (Stevia Rebaudiana Bertoni). J Food Nutr Res 2014; 2:209-214.
- Paul S, Sengupta S, Bandyopadhyay TK, Bhattacharyya A. Stevioside induced ROS-mediated apoptosis through mitochondrial pathway in human breast cancer cell line MCF-7. Nutr Cancer 2012; 64:1087-1094.
- Park HR, Furihata K, Hayakawa Y, Shin-ya K. Versipelostatin, a novel GRP78/Bip molecular chaperone down-regulator of microbial origin. Tetrahedron Letters 2002; 43:6941-6945.
- Chang J, Hsu Y, Kuo P, Kuo Y, Chiang L, Lin C. Increase of Bax/ Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sciences 2005; 76:1883-1893.
- Vijayababu MR, Kanagaraj P, Arunkumar A, Ilangovan R, Aruldhas MM, Arunakaran J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J Cancer Res Clin Oncol 2005; 131:765-771.
- Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis.J Immunol Methods 2002; 265: 49-72.
- Rizzo B, Zambonin L, Angeloni C, Leoncini E, Vieceli Dalla Sega F, Prata C, Fiorentini D, Hrelia S. Steviol Glycosides Modulate Glucose Transport in Different Cell Types. Oxidative Medicine and Cellular Longevity 2013; 2013:11.
- Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP. Cancer cells metabolically "fertilize" the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle 2011; 10:2504-2520.
- Lin JP, Yang JS, Chang NW, Chiu TH, Su CC. GADD153 mediates berberine-induced apoptosis in human cervical cancer Ca ski cells.Anticancer Res 2007; 27: 3379-3386.
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003; 8:115-128.
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci 2001; 26:657-664.
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 2002; 14:381-395.
- Yasukawa K, Kitanaka S, Seo S. Inhibitory effect of stevioside on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Biol Pharm Bull 2002; 25:1488-1490.
- Mizushina Y, Akihisa T, Ukiya M, Hamasaki Y, Murakami-Nakai C, Kuriyama I, Takeuchi T, Sugawara F, Yoshida H. Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors. Life Sci 2005; 77: 2127-2140.
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 1995; 182:367-377.
- Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant.Cell Death Differ 2009; 16: 1303-1314.
- O'Rourke TW, Doudican NA, Mackereth MD, Doetsch PW, Shadel GS. Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol Cell Biol 2002; 22:4086-4093.
- Green DR, Reed JC. Mitochondria and apoptosis.Science 1998; 281: 1309-1312.
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life.Plant Physiol 2006; 141: 312-322.
- Gollapudi S, McCormick MJ, Gupta S. Changes in mitochondrial membrane potential and mitochondrial mass occur independent of the activation of caspase-8 and caspase-3 during CD95-mediated apoptosis in peripheral blood T cells. Int J Oncol 2003; 22:597-600.
- Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie 2002; 84:203-214.
- Hartwell LH, Kastan MB. Cell cycle control and cancer.Science 1994; 266: 1821-1828.
- Sun J, Hai Liu R. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells.Cancer Lett 2006; 241: 124-134.
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem 1999; 68:965-1014.
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF. Regulation of cell death protease caspase-9 by phosphorylation.Science 1998; 282: 1318-1321.