Research Article - Biomedical Research (2017) Volume 28, Issue 20
Glutathione S-transferase M1 polymorphism and the risk of pancreatitis
Minzhen Qin1, Mengbin Qin2, Zhihai Liang3 and Guodu Tang3*
1Department of Clinical Medicine, the First Clinical Medical School of Guangxi Medical University, Nanning, Guangxi, China
2Department of Gastroenterology, the Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
3Department of Gastroenterology, the First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- *Corresponding Author:
- Guodu Tang
Department of Gastroenterology
The First Affiliated Hospital of Guangxi Medical University, China
Accepted date: July 26, 2017
Abstract
Previous meta-analysis has found that no significant relationship between GSTM1 and chronic pancreatitis risk. However, we found a mistake in the abstract of the data in that meta-analysis. Additionally, new study was published. Thus, we conducted this meta-analysis. We did a systematic search of PubMed, EMBASE, Web of Science, and CNKI up to May 2017. We included 8 studies with 751 cases and 1614 controls in this meta-analysis. Subjects with GSTM1 null genotype were significantly associated with an increased risk for pancreatitis compared those carrying the GSTM1 present genotype (OR=1.17, 95% CI: 1.05-1.30). In addition, GSTM1 null genotype was significantly associated with chronic pancreatitis risk (OR=1.17, 95% CI: 1.05-1.30). Furthermore, Caucasians with GSTM1 null genotype showed an increased pancreatitis risk (OR=1.19, 95% CI: 1.06-1.32). In conclusion, this study indicated that GSTM1 null genotype were significantly associated with an increased risk for pancreatitis.
Keywords
GSTM1, Pancreatitis, PolymorphismIntroduction
Pancreatitis is a pancreas inflammatory disorder. It has high mortality and significant global socioeconomic burden [1]. Chronic pancreatitis carries a high burden of morbidity because of its long duration and recurrent attacks. Acute pancreatitis is an inflammatory condition of the pancreas with a clinical course that varies from mild to severe.
The superfamily of Glutathione S transferases (GSTs) is associated with the regulation of inflammation through modulation of prostaglandin signaling pathways and oxidative stress and through the regulation of normal cellular physiology [2,3]. The GSTM1 locus has been mapped on chromosome 1p13.3. Persons with deletion of the GSTM1 locus have noenzymatic functional activity of the enzyme [4]. Hu et al. found that the GSTM1 null genotype might be significantly associated with a reduced overall survival in breast cancer [5]. Huang et al. found that GSTM1 and GSTT1 polymorphisms contribute to renal cell carcinoma risk by a meta-analysis [6]. Lu et al. suggested that GSTM1 null polymorphism may be associated with an increased risk for esophageal cancer in Asian [7]. Sun et al. suggested that GSTM1 null genotype may contribute to the cervical cancer development in Chinese [8]. A meta-analysis has found that no significant relationship between GSTM1 and chronic pancreatitis risk [9]. However, we found a mistake in the abstract of the data in that metaanalysis. Additionally, new study was published [10]. Thus, we conducted this meta-analysis to investigate the association between GSTM1 null polymorphism and pancreatitis risk.
Materials and Methods
Publications search
We did a systematic search of PubMed, EMBASE, Web of Science, and CNKI up to May 2017 by using the combination of the following key words: “glutathione S-transferase M1”, “GSTM1”, “polymorphism”, and “pancreatitis”. Additional eligible studies were identified through references that were cited in the relevant articles.
Inclusion criteria
All selected studies complied with the following criteria: (1) The study design should be case-control study; (2) The study should investigate the association between GSTM1 null polymorphism and pancreatitis risk; and (3) The study should have genotype frequencies of cases and controls.
Data extraction
Two of the authors extracted all data independently following the selection criteria. The following data were collected from each study: the first author, year, ethnicity, age, gender, sample size, and subtype of pancreatitis.
Statistical analysis
Crude Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were used to estimate the strength of the relationship between the GSTM1 null polymorphism and pancreatitis risk. The heterogeneity was estimated using the χ2-based Q statistic, and heterogeneity was considered statistically significant when P heterogeneity ≤ 0.1. The statistical analyses were performed using Review Manager Software (v.5.0; Oxford, England) with two-sided P values and a 0.05 significance level.
Results
Characteristics of the studies
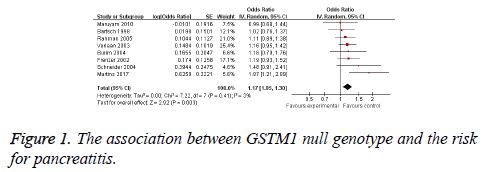
We included 8 studies with 751 cases and 1614 controls in this meta-analysis [10-17]. Seven of the studies were conducted in Caucasians, and one study was conducted in Asians, respectively. Seven studies included chronic pancreatitis patients and one study included acute pancreatitis patients. The main characteristics of these studies are presented in Table 1.
| First author | Ethnicity | Age | Gender | Type of pancreatitis | Case (n) | Control (n) |
|---|---|---|---|---|---|---|
| Bartsch [11] | Caucasian | NA | Mixed | Chronic pancreatitis | 114 | 78 |
| Frenzer [12] | Caucasian | 53 | Mixed | Chronic pancreatitis | 71 | 257 |
| Verlaan [13] | Caucasian | NA | Mixed | Chronic pancreatitis | 142 | 261 |
| Burim [14] | Caucasian | NA | NA | Chronic pancreatitis | 14 | 262 |
| Schneider [15] | Caucasian | NA | NA | Chronic pancreatitis | 103 | 183 |
| Rahman [16] | Caucasian | 51 | Mixed | Chronic pancreatitis | 121 | 245 |
| Maruyam [17] | Asian | 59 | NA | Chronic pancreatitis | 53 | 96 |
| Martins [10] | Caucasian | NA | Mixed | Acute pancreatitis | 133 | 232 |
Table 1. Characteristics of included studies [10-17].
Meta-analysis
The random-effects model (DerSimonian-Laird method) was used to calculate the pooled ORs. Subjects with GSTM1 null genotype were significantly associated with an increased risk for pancreatitis compared those carrying the GSTM1 present genotype (OR=1.17, 95% CI: 1.05-1.30, Figure 1). In addition, GSTM1 null genotype was significantly associated with chronic pancreatitis risk (OR=1.17, 95% CI: 1.05-1.30, Table 2). Furthermore, Caucasians with GSTM1 null genotype showed an increased pancreatitis risk (OR=1.19, 95% CI: 1.06-1.32, Table 2).
| No. of studies | OR (95% CI) | P value | Pheterogeneity | |
|---|---|---|---|---|
| Pancreatitis | 8 | 1.17 (1.05-1.30) | 0.003 | 0.41 |
| Chronic pancreatitis | 7 | 1.14 (1.02-1.26) | 0.02 | 0.87 |
| Caucasian | 7 | 1.19 (1.06-1.32) | 0.003 | 0.38 |
Table 2. Summary of results from meta-analysis and subgroup analysis.
Discussion
In this update meta-analysis, we included 8 studies with 751 cases and 1614 controls. Subjects with GSTM1 null genotype were significantly associated with an increased risk for pancreatitis compared those carrying the GSTM1 present genotype. In addition, GSTM1 null genotype was significantly associated with chronic pancreatitis risk. Furthermore, Caucasians with GSTM1 null genotype showed an increased pancreatitis risk.
Cho et al. found that variants of PRSS1, SPINK1, and CFTR were associated with idiopathic pancreatitis [18]. Gui et al. suggested that the IL-18-607C/A and -137G/C polymorphisms do not influence susceptibility to acute pancreatitis in the Chinese population [19]. Padureanu et al. found that the risk of developing chronic pancreatitis was not increased by the presence of the iNOS-2087A>G polymorphism [20]. Anilir et al. indicated that IL-8 AA genotype was detected with a significantly higher frequency among the patients with acute biliary pancreatitis [21]. Ma et al. found that LP-PLA2 gene polymorphisms, V279F and R92H, may be associated with susceptibility to and severity of acute pancreatitis [22].
The present study does have limitations. Firstly, we failed to make the subgroup analysis stratified by different genetic backgrounds because of insufficient data. Second, gene-gene or gene-environment interactions were not incorporated in this study. Third, only one study with Asians was included.
In conclusion, this study indicated that GSTM1 null genotype were significantly associated with an increased risk for pancreatitis.
Disclosure of Conflict of Interest
The authors have declared that no competing interests exist.
Acknowledgment
This study was supported by National Natural Science Foundation of China (No. 81260087 and 81560111).
References
- Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015; 386: 85-96.
- Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem 2003; 278: 22243-22249.
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L. Regulation of JNK signaling by GSTp. EMBO J 1999; 18: 1321-1334.
- Mo Z, Gao Y, Cao Y, Gao F, Jian L. An updating meta-analysis of the GSTM1, GSTT1, and GSTP1 polymorphisms and prostate cancer: a HuGE review. Prostate 2009; 69: 662-688.
- Hu XY, Huang XY, Ma J, Zuo Y, Luo NB, Lai SL, Su DK. GSTT1 and GSTM1 polymorphisms predict treatment outcome for breast cancer: a systematic review and meta-analysis. Tumour Biol 2016; 37: 151-162.
- Huang W, Shi H, Hou Q, Mo Z, Xie X. GSTM1 and GSTT1 polymorphisms contribute to renal cell carcinoma risk: evidence from an updated meta-analysis. Sci Rep 2015; 5: 17971.
- Lu QJ, Bo YC, Zhao Y, Zhao EJ, Sapa WB. Glutathione S-transferase M1 polymorphism and esophageal cancer risk: An updated meta-analysis based on 37 studies. World J Gastroenterol 2016; 22: 1911-1918.
- Sun P, Song WQ. GSTM1 null genotype and susceptibility to cervical cancer in the Chinese population: An updated meta-analysis. J Cancer Res Ther 2016; 12: 712-715.
- Zhong Y, Zou R, Cao J, Peng M. Glutathione S-transferase M1 and glutathione S-transferase T1 genotype in chronic pancreatitis: a meta-analysis. J Int Med Res 2015; 43: 9-16.
- D Oliveira Martins F, Gomes BC, Rodrigues AS, Rueff J. Genetic Susceptibility in Acute Pancreatitis: Genotyping of GSTM1, GSTT1, GSTP1, CASP7, CASP8, CASP9, CASP10, LTA, TNFRSF1B, and TP53 Gene Variants. Pancreas 2017; 46: 71-76.
- Bartsch H, Malaveille C, Lowenfels AB, Maisonneuve P, Hautefeuille A, Boyle P. Genetic polymorphism of N-acetyltransferases, glutathione S-transferase M1 and NAD(P)H: quinone oxidoreductase in relation to malignant and benign pancreatic disease risk. The International Pancreatic Disease Study Group. Eur J Cancer Prev 1998; 7: 215-223.
- Frenzer A, Butler WJ, Norton ID, Wilson JS, Apte MV, Pirola RC, Ryan P, Roberts-Thomson IC. Polymorphism in alcohol-metabolizing enzymes, glutathione S-transferases and apolipoprotein E and susceptibility to alcohol-induced cirrhosis and chronic pancreatitis. J Gastroenterol Hepatol 2002; 17: 177-182.
- Verlaan M, te Morsche RH, Roelofs HM, Laheij RJ, Jansen JB, Peters WH, Drenth JP. Glutathione S-transferase Mu null genotype affords protection against alcohol induced chronic pancreatitis. Am J Med Genet A 2003; 120A: 34-39.
- Burim RV, Canalle R, Martinelli Ade L, Takahashi CS. Polymorphisms in glutathione S-transferases GSTM1, GSTT1 and GSTP1 and cytochromes P450 CYP2E1 and CYP1A1 and susceptibility to cirrhosis or pancreatitis in alcoholics. Mutagenesis 2004; 19: 291-298.
- Schneider A, Togel S, Barmada MM, Whitcomb DC. Genetic analysis of the glutathione s-transferase genes MGST1, GSTM3, GSTT1, and GSTM1 in patients with hereditary pancreatitis. J Gastroenterol 2004; 39: 783-787.
- Rahman SH, Nanny C, Ibrahim K, OReilly D, Larvin M. Genetic polymorphisms of GSTT1, GSTM1, GSTP1, MnSOD, and catalase in nonhereditary chronic pancreatitis: evidence of xenobiotic stress and impaired antioxidant capacity. Dig Dis Sci 2005; 50: 1376-1383.
- Maruyama K, Harada S, Yokoyama A, Mizukami S, Naruse S, Hirota M, Nishimori I, Otsuki M. Association analyses of genetic polymorphisms of GSTM1, GSTT1, NQO1, NAT2, LPL, PRSS1, PSTI, and CFTR with chronic alcoholic pancreatitis in Japan. Alcohol Clin Exp Res 2010; 34: 34-38.
- Cho SM, Shin S, Lee KA. PRSS1, SPINK1, CFTR, and CTRC pathogenic variants in Korean patients with idiopathic pancreatitis. Ann Lab Med 2016; 36: 555-560.
- Gui HB, Du XG, Fu ZH, Chen XM. Influence of interleukin-18 gene polymorphisms on acute pancreatitis susceptibility in a Chinese population. Genet Mol Res 2016; 15.
- Padureanu V, Enescu AS, Silosi I, Fortofoiu M, Enescu A, Bogdan M, Fortofoiu MC, Dumitrescu AG, Tudorascu DR, Mita A, Streata I, Ioana M, Petrescu F, Saftoiu A. The association between chronic pancreatitis and the iNOS-2087A>G polymorphism. Rom J Intern Med 2017; 55: 89-95.
- Anilir E, Ozen F, Yildirim IH, Ozemir IA, Ozlu C, Alimoglu O. IL-8 gene polymorphism in acute biliary and non-biliary pancreatitis: probable cause of high level parameters? Ann Hepatobiliary Pancreat Surg 2017; 21: 30-38.
- Ma M, Zhai CX, Sun CX. Correlations between LP-PLA2 gene polymorphisms and susceptibility and severity of acute pancreatitis in a Chinese population. Genet Test Mol Biomarkers 2017; 21: 206-212.