Research Article - Journal of Parasitic Diseases: Diagnosis and Therapy (2020) Volume 5, Issue 1
Genotyping of Giardia lamblia using semi-nested PCR technique and restriction fragment length polymorphism (RFLP) in Sohag Governorate, Egypt.
Amal Mostafa Ahmed*, Hanaa Ahmed El-Hady, Hisham Ibrahim Osman and Noha Sammer Ahmed
Medical Parasitology Department, Sohag University, Egypt
- Corresponding Author:
- Amal Mostafa Ahmed
Medical Parasitology Department
Egypt
E-mail: moustafad658@yahoo.com
Accepted date: January 23, 2020
Citation: Ahmed AM, El-Hady HA, Osman HI, et al. Genotyping of Giardia lamblia using semi-nested PCR technique and restriction fragment length polymorphism (RFLP) in Sohag Governorate, Egypt. J Parasit Dis Diagn Ther 2020;5(1):1-6.
Abstract
Background: Giardia lamblia is a protozoan parasite, and it is most frequently detected as an intestinal pathogenic parasite in humans worldwide. Objective: To determine the genotype of Giardia lamblia and the sub-assemblages for each genotype using semi-nested PCR and restriction fragment length polymorphism. Methodology: DNA samples were isolated from the stools of 100 patients infected with G. lamblia (after examination by copromicroscopy) and amplified by PCR. The 93 positive samples after using PCR, were digested with NlaIV and RsaI fragment restriction fragment enzymes. The samples were taken randomly and the residence and age for every case were taken. According to the location of residence, the cases were divided into urban areas (the cities) and rural areas (the villages). Result: Genotype A represented (32.3%) {AI (5.3%), AII (26.9%)}, genotype B represented (39.8%) {BIII (22.5%), BIV (17.3%)} and mixed A, B represented (28%). Giardia genotype B was the most common genotype among cases included in this study while sub-genotype AII accounted for the majority of cases. Males were more affected 62 (66.66%) than females 31 (33.33%), (79.6%) cases were from rural areas while only (20.4%) cases were from urban; the highest incidence was between (2-6) years represented 72.1%. The variation in sex, residence and age distribution was found to be statistically significant (PV=0.05, 0.03, 0.03 respectively). Molecular analysis for glutamate dehydrogenase (gdh) gene had been shown to assemble genotypes A and B isolates into four groups: AI, AII, BIII, and BIV by using semi-nested PCR technique. Conclusion: Giardiasis is a still common parasitic infection, particularly in young children in rural areas more than urban areas, and in males more than in females in Sohag Governorate, Upper Egypt. Molecularly it was found that both genotype A (sub-genotypes AI, AII), genotype B (sub-genotypes BIIIm B IV), as well as mixed genotypes A and B, were found in examined patients.
Keywords
Giardia lamblia, Glutamate dehydrogenase (gdh) gene, NlaIV, RsaI Restriction fragment enzymes.
Introduction
Giardia lamblia is one of the most common diarrhea-related parasites in almost all vertebrates, including humans [1]. It affects all age groups; with prevalence rate varying from (2%-5%) in the industrialized world and (20%-30%) in the developing world. It is more prevalent among children [2,3].
Giardia is transmitted through the orofecal route following direct or indirect exposure to the cyst, by ingestion of contaminated food, drinking contaminated water or direct contact with the infected individuals [4].
Due to its impact on health, especially among children in developing countries, giardiasis has been included in the Neglected Diseases of the World Health Organization (WHO) since 2004 [5].
A variety of molecular techniques, including Multiplex PCR, Restriction Fragment Length Polymorphism (RFLP), real-time PCR, and sequence analysis of housekeeping genes have proved to be used as tools for discrimination of all assemblages and to provide powerful tools for understanding the molecular epidemiology, infection sources, and zoonotic potential of human giardiasis [1].
Although it is very common in Egypt; limited studies have been done on the molecular characterization of the parasite [2]. Molecularly, Giardia lamblia has been divided into several genotypes and sub-genotypes that differ in virulence and clinical outcome [6]. Hence, we performed the detection of G. lamblia and determination of its genotype simultaneously by Polymerase chain reaction using semi-nested PCR technique on isolates obtained from humans.
Materials and Methods
This experimental study was performed at the laboratory of Medical Parasitology Department, Faculty of Medicine, Sohag University and Molecular Biology Center at Assuit University, from October 2017 to September 2018.
The research team followed the ethical standards of confidentiality and freedom to participate. The respondents or their parents were informed that the study was voluntary and they were assured that their privacy would be protected and all of them gave written consent for taking and studying their specimens.
Stool samples had been collected from 525 patients who had diarrhea (loose watery stool more than three times per day) from different localities in Sohag Governorate. 100 samples proved to have Giardia after coproscopical examination by saline and iodine wet mount examination. The positive samples were amplified using the PCR technique. Only 93 positive samples resulted after using PCR. These 93 samples were digested with NlaIV and RsaI fragment restriction fragment enzymes.
Extraction of DNA from PCR positive samples (QiAmp® DNA stool Mini kit (Qiagen, UK) was done according to the manufacturers' technique. Amplification of the glutamate dehydrogenase gene was performed, using specific primers, in semi-nested PCR thermal cycler and restriction of the DNA fragments using two restriction enzymes (NIaIV and RsaI restriction fragment enzymes) were done.
Statistical analysis
Data was organized, tabulated, and statistically analyzed using the SPSS version, 23.00. p-value was calculated. Chi-square test (χ2) was used to compare the frequency of data. p-value <0.05 indicates significant value. p-value<0.001 indicates highly significant value.
Results
PCR amplification of glutamate dehydrogenase (gdh) gene
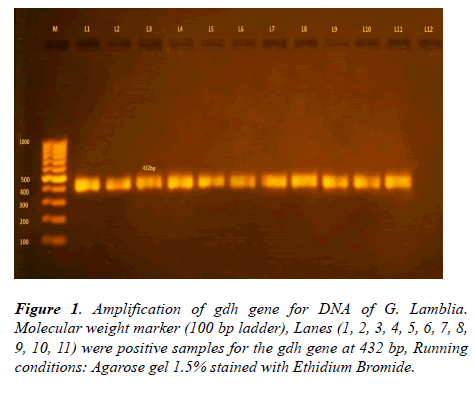
The glutamate dehydrogenase gene was successfully amplified from 100 positive samples for Giardia lamblia. Out of 100, only 93 positive cases were detected (Table 1). A 432 bp fragment of the gdh gene was amplified in a semi-nested PCR (Figure 1).
| Cases | Positive | Negative | p-value | X2 |
|---|---|---|---|---|
| Male | 62 | 5 | 0.05 (Significant) | 7.839 |
| Female | 31 | 2 | ||
| Total | 93 | 7 |
Table 1: The number of positive and negative cases and their gender.
Restriction fragment length polymorphism (RFLP) analysis
The following (Table 2) shows the sizes of the expected fragments (bp) and the diagnostic fragment sizes (bp) for each sub-genotype which we used to analyze our results.
| Sub-genotypes | Enzyme | Expected fragments sizes (bp) | Diagnostic fragments sizes (bp) |
|---|---|---|---|
| AI | NlaIV | 16, 39 ,47 ,87 ,146 | 146 |
| AII | NlaIV | 16, 39, 47, 69, 77, 87, 123 | 123 |
| BIII | RsaI | 30, 131, 298 | 298 |
| BIV | RsaI | 30, 428 | 428 |
Table 2: The diagnostic sub-genotypes.
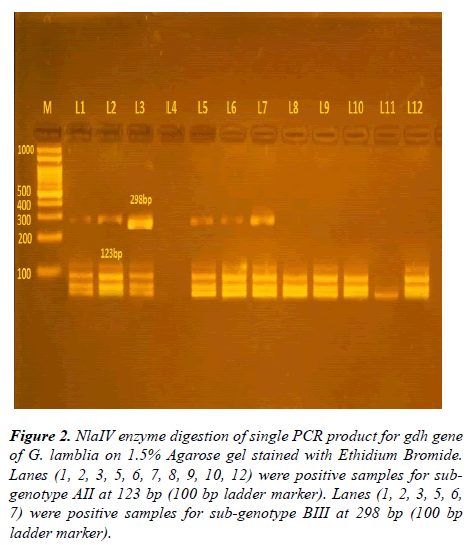
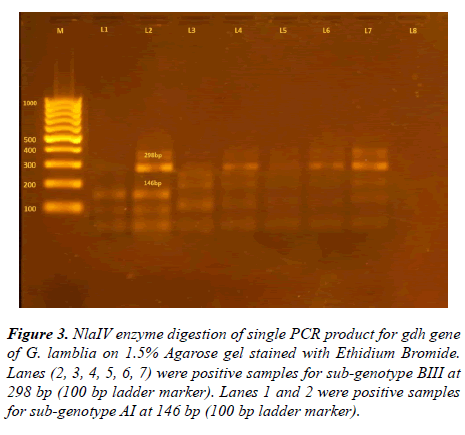
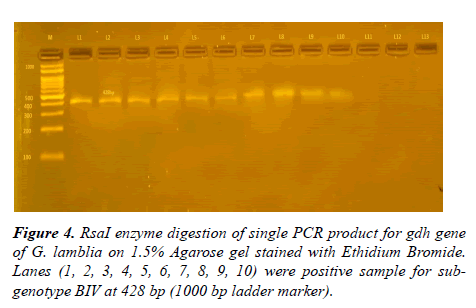
After using NlaIV and RsaI restriction fragment enzymes on 93 positive samples resulted from amplification of 432 bp glutamate dehydrogenase gene, four sub-genotypes were detected, shown in Table 3 and Figures 2-4.
| Genotype A | Genotype B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Sub-genotype AI | Sub-genotype AII | Sub-genotype BIII | sub-genotype BIV | Mixed A and B | Total | p-value | χ2 test | |||
| Female | 2 | 8 | 7 | 4 | 12 | 33 | 0.05 (Significant) | 7.839 | |||
| 2.10% | 8.60% | 7.50% | 4.40% | 12.90% | 35.50% | ||||||
| Male | 3 | 17 | 14 | 12 | 14 | 60 | |||||
| 3.20% | 18.30% | 15.00% | 12.90% | 15.10% | 64.50% | ||||||
| p-value | 0.03 (Significant) | ||||||||||
| χ2test | 15.763 | ||||||||||
| Total | 30 (32.20%) | 37 (39.8%) | 26 (28%) | 93 (100%) | |||||||
Table 3: The frequency of each Giardia lamblia genotype and sub-genotype.
Figure 2: NlaIV enzyme digestion of single PCR product for gdh gene of G. lamblia on 1.5% Agarose gel stained with Ethidium Bromide. Lanes (1, 2, 3, 5, 6, 7, 8, 9, 10, 12) were positive samples for subgenotype AII at 123 bp (100 bp ladder marker). Lanes (1, 2, 3, 5, 6, 7) were positive samples for sub-genotype BIII at 298 bp (100 bp ladder marker).
Figure 3: NlaIV enzyme digestion of single PCR product for gdh gene of G. lamblia on 1.5% Agarose gel stained with Ethidium Bromide. Lanes (2, 3, 4, 5, 6, 7) were positive samples for sub-genotype BIII at 298 bp (100 bp ladder marker). Lanes 1 and 2 were positive samples for sub-genotype AI at 146 bp (100 bp ladder marker).
• Frequency of each Giardia lamblia genotype and subgenotype (Table 3)
• The relation between locality and sub-genotypes AI, AII, BIII, BIV and mixed A, B genotypes (Table 4)
| Sub-genotype | AI | AII | BIII | BIV | Mixed (A, B) | Total |
|---|---|---|---|---|---|---|
| Urban | 1 (1.1%) | 7 (7.5%) | 2 (2.2%) | 3 (3.2%) | 6 (6.4%) | 19 (20.4%) |
| Rural | 4 (4.3%) | 18 (19.4%) | 19 (20.4%) | 13 (14.0%) | 20 (21.5%) | 74 (79.6%) |
Table 4: The relation between locality and sub-genotypes AI, AII, BIII, BIV and mixed A, B.
• The relation between age groups and sub-genotypes AI, AII, BIII, BIV and mixed genotypes A, B (Table 5)
| Sub-genotype | AI | AII | BIII | BIV | Mixed A, B | Total |
|---|---|---|---|---|---|---|
| Age <2 | 0 (0.0%) | 0 (0.0%) | 3 (3.2%) | 1 (1.1%) | 1 (1.1%) | 5 (5.4%) |
| Age 2-6 | 3 (3.2%) | 22 (23.7%) | 14 (15.1%) | 9 (9.7%) | 19 (20.4%) | 67 (72.1%) |
| Age 6-12 | 2 (2.2%) | 3 (3.2%) | 3 (3.2%) | 6 (6.4%) | 5 (5.3%) | 19 (20.3%) |
| Age 12-18 | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 1 (1.1%) | 2 (2.2%) |
Table 5: The relation between age groups and sub-genotypes AI, AII, BIII, BIV and mixed A, B.
Discussion
The intestinal protozoan Giardia lamblia was frequently found in diarrheal disease throughout the world affecting humans and other mammalian species [7].
In the current study, 100 positive stool samples for Giardia lamblia were detected by microscopy. After the amplification of the glutamate dehydrogenase (gdh) gene, only 93 samples showed positive results. The negative fecal samples could be explained by low DNA levels, the presence of a solid wall that inhibit the release of DNA from the cysts or the presence of PCR inhibitors in some of the fecal samples such as lipids, bile salts, polysaccharides from mucus, bacteria, and food degradation product or dissolution of parasite during storage [8].
The gdh gene is one of the most common and advantageous genetic markers for genotyping of G. lamblia. Molecular analysis for this gene had been shown to assemble genotypes A and B isolates into four groups: AI, AII, BIII, and BIV by using PCR-RFLP techniques [9,10].
The results of Giardia genotyping in the present study, revealed that out of 93 positive cases after amplification of (gdh) gene and after using NlaIV and RsaI restriction fragment enzymes, there were genotype A, 30 cases (32.3%) {subgenotypes AI, 5 (5.3%), AII, 25 (26.9%)}, genotype B, 37 cases (39.8%) {sub-genotype BIII, 21 (22.5%) BIV, 16 (17.3%)} and mixed A, B 26 (28%). So Giardia genotype B was the most common genotype among cases included in this study while sub-genotype AII accounted for the majority of cases with Giardia.
In Egypt, some studies found that Giardia assemblage B was the predominant assemblage [2,11-13] while other studies done in Egypt found Giardia assemblage A was the predominant assemblage in human infections [3,14-16].
Outside Egypt, assemblage B was the predominant genotype but with different ratios, [17] in Australia, [18] in Saudi Arabia, [19] in Rwanda, [20] in Spain, and [21] in Cuba, assumed that assemblage B ratios were 70%, 52.78%, 86%, 66.1%, and 52.78% respectively.
In other studies assemblage A was the predominant genotype as in the studies of [22], in Italy, [23]in Mexico, [24]in New Zealand and [3] from Egypt who assumed that assemblage A ratios were 80%, 100%, 100%, and 90.9%respectively.
In the present study, the ratio of assemblages A, B, and mixed A and B are near to each other and this can be explained by the high grade of pollution, poor sanitary conditions and the great role of zoonosis in infection transmission in our locality.
The difference in these studies may be explained by the difference in localities, the difference in population, some studies were limited to symptomatic individuals only, others for symptomatic and asymptomatic, and also amplifications were done on different Giardia lamblia genes, using different methods of amplification and restriction enzymes.
In regard to the relation of sex to the prevalence of giardiasis, out of the 93 positive samples, there were 60 (64.5%) males while 33 (35.5%) females. The variation in sex distribution was found to be statistically significant (PV=0.03). This agreed with [25] in Egypt, [26] in Portugal, [2] in Egypt and [20] in Spain, who stated that the incidence of giardiasis is higher in males than in females. These results disagreed with [27] in Malaysia, [28] in Zambia and [29] in Slovakia, who stated that the prevalence in females was higher than males. They assumed that this was due to these females taking care of their own children or from working in nursing homes or care-day centers. The results in this study agreed with the behavioral and familial habits in our locality. Male children are highly active with great freedom than females and this makes them more subjected to infection with Giardia cysts from the polluted environment around them.
In regard to the relation of residence to the prevalence of giardiasis in the present study, out of the 93 positive cases, 74 (79.6%) cases were from rural areas and 19 (20.4%) cases were from urban areas. The variation in resident distribution was found to be statistically significant (PV=0.03). These results agreed with [25] in Egypt, [30] in Nigeria, [2] in Egypt and [31] in Iran, who reported that the prevalence of giardiasis was more in rural than urban areas. These results agreed with the nature of our locality which is mostly rural areas. The infection in rural areas is more likely to occur than urban areas because of many social, behavioral, environmental, familial and zoonotic factors. However, the present results disagreed with the results reported by [32] in Saudi Arabia as they reported that the infection rate was higher in urban than in rural areas. These changes in the distribution might be due to the differences in the nature of rural and urban areas.
In regard to the relation of age to the prevalence of giardiasis in the present study, the highest incidence was between (2-6) years represented 72.1% then (6-12) years represented 20.3% then (<2) years represented 5.4% lastly (12-18) years represented 2.2%. The variation in age distribution was found to be statistically significant (PV=0.03). The most affected age was (2-6) years, this can be explained by a highly active attitude of these children who play outside their home and this makes them more vulnerable to the highly polluted surrounding by Giardia cyst not only from humans as a source of infection but also from animals as another source of infection.
These results agreed with [33] in Ghana, [2] in Egypt, [34] in Iraq, and [29] in Slovakia who reported that the highest prevalence was between 3-5, 1-10, 2-4 and (<7) years respectively. However, the present results disagreed with [35], in Mongolia, and [13] in Egypt who stated that giardiasis was highest in children aged (6-12) years. They attributed these results to a greater risk of exposure, lack of immunity and those infections in adults are mostly asymptomatic and thus are less likely to be diagnosed. On the other hand, [26] Portugal reported that there was no significant association between Giardia prevalence and age.
Conclusion
Giardiasis is a still common parasitic infection, particularly in young children in rural areas in Sohag Governorate, Upper Egypt. Genetically, it was found that both genotype A (subgenotypes AI, AII), genotype B (sub-genotypes BIIIm B IV), as well as mixed genotypes A, B, were present in the examined patients.
References
- Lebbad M, Petersson I, Karlsson L, et al. Multilocus genotyping of human Giardia isolates suggests limited zoonotic transmission and association between assemblage B and flatulence in children. PLoS Negl Trop Dis. 2013;5(8):1-10.
- Mohran AF, Rashed SM, Nasr ME, et al. Genotyping of Giardia lamblia in human and animal feces in Qaliopia governate. Egypt J Med Sci. 2013;34(2):639-55.
- Hussein EM, Zaki WM, Ahmed SA, et al. Predominance of Giardia lamblia assemblage A among iron deficiency anemic pre-school Egyptian children. Parasitol Res. 2016;115(4):1537-45.
- Garcia LS. Intestinal protozoa: flagellates and ciliates-Giardia lamblia, Diagnostic medical parasitology. 2016;6th edition. 22:584-98
- Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the Neglected diseases initiative. Trends Parasitol. 2006;22(5):203-08.
- Caccio SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. 2008;160(2):75-80.
- Ryan U, Caccio SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43(12-13):943-56.
- Mbae C, Mulinge E, Guleid F, et al. Molecular characterization of Giardia duodenalis in children in Kenya. BMC Infect Dis. 2016;16(135):1-7.
- Amar CFL, Dear PH, Pedraza-Diaze S, et al. Sensitivity of PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human faces. J Clin Microbiol. 2002;40(2):446-52.
- Nahavandi KH, Fallah E, Asgharzadeh M, et al. Glutamate dehydrogenase and triose-phosphate isomerase coding genes for detection and genetic characterization of Giardia lamblia in human feces by PCR and PCR-RFLP. Turk J Med Sci. 2011;41(2):283-89.
- Mohammadi SS, Singer SM. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J Immunol. 2011;187(7):3769-75.
- Amer SE. Genotyping and phylogenetic characterization of Giardia intestinalis from human and dairy cattle in Kafr El Sheikh Governorate, Egypt. J Egypt Soc Parasitol. 2013;43(1):133-46.
- El-Badry AA, Mohammed AF, Gawad EA. Predominance of Giardia intestinalis assemblage B in diarrhoeic children in Sharkia, Egypt. PUJ. 2017;10(1-2):39-43.
- EL-Shazly AM, Mowafy N, Soliman M, et al. Egyptian genotyping of G. lamblia J Egypt Soc Parasitol. 2004;34(1):265-80.
- Sabry MA, Taher ES, Meabed EMH. Prevalence and genotyping of zoonotic Giardia from Fayoum Governorate, Egypt. Res J Parasitol. 2009;4(4):105-14.
- Helmy MFH, Abddel-Fattah HS, Rashed L. Real-time PCR∕RFLP assay to detect Giardia intestinalis genotypes in human isolates with diarrhea in Egypt. J Parasitol. 2009;95(4):1000-4.
- Read C, Walters J, Robertson ID, et al. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. 2002;32(2):229-31.
- Al-Mohammed HI. Genotypes of Giardia intestinalis clinical isolates of gastrointestinal symptomatic and asymptomatic Saudi children. Parasitol Res. 2011;108(6):1375-81.
- Ignatius R, Gahutu JB, Klotz C, et al. High prevalence of Giardia duodenalis assemblage B infection and association with underweight in Rwandan children. PLoS Negl Trop Dis. 2012;6(6):1-9.
- Lucio AD, Martínez-Ruiz R, Merino FJ, et al. Molecular genotyping of Giardia duodenalis isolates from symptomatic individuals attending two major public hospitals in Madrid, Spain. PLoS One. 2015;10(12):1-21.
- Puebla JL, Nunez FA, García AB, et al. Prevalence of Giardia duodenalis among children from a central region of Cuba: molecular characterization and associated risk factors. J Parasit Dis. 2017;41(2):405-13.
- Caccio SM, Giacomo MD, Pozio E. Sequence analysis of the beta-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia intestinalis cysts from human fecal samples. Int J Parasitol. 2002;32(8):1023-30.
- Ponce-Macotela M, Martinez-Gordillo MN, Bermudez-Cruz RM, et al. Unusual prevalence of the Giardia intestinalis AII subtype amongst isolates from humans and domestic animals in Mexico. Int J Parasitol. 2002;32(9):1201-2.
- Learmonth JJ, Ionas G, Pita AB, et al. Identification and genetic characterization of Giardia and Cryptosporidium strains in humans and dairy cattle in the Waikato Region of New Zealand. Water Sci Technol. 2003;47(3):21-6.
- Abd-El Hafed WM. Polymerase chain reaction (PCR) and direct stool examination techniques, in diagnosis of Giardia lamblia Infection.2010. M.Sc. Thesis. Faculty of Medicine, Benha University.
- Julio C, Vilares A, Oleastro M, et al. Prevalence and risk factors for Giardia duodenalis infection among children: A case study in Portugal. Parasit Vectors. 2012;5(22):1-8.
- Mahdy AKM, Surin J, Wan KL, et al. Giardia intestinalis genotypes: Risk factors and correlation with clinical symptoms. Acta Trop. 2009;112(1):67-70.
- Siwila J, Phiri IGK, Enemark HL, et al. Intestinal helminthes and protozoan in children in preschool in Kafue district, Zambia. Trans R Soc Trop Med Hyg. 2010;104(2):122-8.
- Duldova A, Juris P, Jurisova S, et al. Epidemiology and geographical distribution of gastrointestinal parasitic infection in humans in Slovakia. Helminthologia. 2016;53(4):309-17.
- Opara KN, Udoidung NI, Opara DC, et al. The impact of intestinal parasitic infections on the nutritional status of rural and urban school-aged children in Nigeria. Int J MCH AIDS. 2012;1(1):73-82.
- Faraji R, Ahmadian F, Javadi GR, et al. Prevalence of Giardiasis among children in childcare centers in Kermanshah, Iran. Int J Res Med Sci. 2015;3(7):1717-20.
- AL-Shammari S, Khoja T, EL-Khwasky F, et al. Intestinal parasitic diseases in Riyadh, Saudi Arabia: prevalence, sociodemographic and environmental associates. Trop Med Int Health. 2001;6(3):184-9.
- Nkrumah B, Nguah SB. Giardia lamblia: a major parasitic cause of childhood diarrhea in patients attending a district hospital in Ghana. Parasit Vectors. 2011;4(163):1-7.
- Al-Difaie SR. Molecular Study to Detect Genotyping of Giardia lamblia from human and cattle feces in Al-Qadisiya Governorate, Iraq. Ibn Al-Haitham J for Pure and Appl Sci. 2016;29(3):1-13.
- Hong SH, Davaasuren A, Jeong Y, et al. Molecular characterization of Giardia duodenalis and Cryptosporidium parvum in fecal samples of individuals in Monogolia. Am J Trop Med Hyg. 2014;90(1):43-7.