Research Article - Biomedical Research (2017) Health Science and Bio Convergence Technology: Edition-II
Genetically engineered nitrifier cooperated with biochar improves disease resistance of tobacco through regulation of NPIR-induced SIPK/WIPK pathway
Lin Dai1, Jinxia Fan2, Lu Feng1, Maomao Xu1, Hongtao Xu1 and Hongyan Wang1*
1College of Resources and Environment, Northeast Agricultural University, Harbin, PR China
2College of Engineering, Northeast Agricultural University, Harbin, PR China
- *Corresponding Author:
- Hongyan Wang
College of Resources and Environment
Northeast Agricultural University
PR China
Accepted date: October 26, 2016
Abstract
Nitrifier plays crucial role in improving soil ecological factors and regulates physiological function of crops. Evidences have suggested that biochar can be regarded as high quality energy to regulate the biochemical processes of crops. In this study, we investigated the effects of nitrifier cooperated with biochar on soil ecological factors and disease resistance of tobacco. A genetically engineered nitrifier delivered monoamine oxidase gene (MO) were constructed and developed to acquire the available nitrogen source in soil. The effects of biochar on activity of genetically engineered nitrifier delivered MO (gNitrifier-MO) were detected in soil. The physiological functions and disease resistance of tobacco were analyzed. Results showed that biochar enhanced the activity of gNitrifier-MO compared the soil treated by single gNitrifier-MO, resulting in the more production of nitrate nitrogen. We observed that gNitrifier-MO cooperated with biochar significantly increased the numbers of bacterium and nitrobacteria in the soil. Activity of gNitrifier-MO enhanced by biochar promoted nitrogen deposition or precipitation. In addition, gNitrifier-MO combined with biochar markedly improved the quality and the yield of tobacco. We also found that disease resistance of tobacco was improved through integrate microbial diversity and enrichment. Furthermore, gNitrifier-MO combined with biochar treatment increased expression levels of nonexpressor of PR and SR. NPIR-induced SIPK and WIPK expression and phosphorylation were up-regulated in tobacco plants infected with tobacco mosaic virus (TMV) cultured in gNitrifier-MO combined with biochar soil. SAR, PR-1α and POD in tobacco plants were increased in tobacco plants infected with tobacco mosaic virus (TMV) cultured in soil treated by gNitrifier-MO combined with biochar. In conclusion, these results indicate that gNitrifier-MO combined with biochar treatment improves disease resistance through regulation of NPIR-induced SIPK/WIPK pathway, suggesting gNitrifier-MO combined with biochar may be potential scheme for improvement of disease resistance of tobacco plant.
Keywords
gNitrifier-MO, Biochar, Disease resistance, Tobacco, NPIR, SIPK/WIPK
Introduction
Microbial community structure and functions in rhizosphere soil of crops are important for evaluation of soil ecological factors, saline and alkali, physical and chemical properties [1,2]. Fertile degree, density and soil respiration responses to changing nutrient elements are associated with the microbial community composition in the agricultural soil [3]. Activity, abundance and diversity of microbial affect the soil carbon, nitrogen, phosphorus, sulfur and kalium cycle typical ecosystem in soil [4]. Reports also have indicated that nitrifier system is a crucial part in microbial community structure, functions and balance of nitrogen source in agricultural soil, which is beneficial for the maintenance and metabolism of nutrient element regulated by biological nitrogen fixation and denitrification [5-7]. Soil nitrogen cycle processes of biological nitrogen fixation and denitrification are mainly depended on the activities of nitrifying bacteria and denitrifying bacteria in nitrifier system and the percent of nitrifier system among microbial community structure [8,9]. Particularly, Microbial Nitrifier can impact nitrogen management and enrichment cycling in soil microenvironments, which also has been used to improve the diversity of soil ecological factors in agriculture [10].
Biochar is biological organic material generated by pyrolysis using high temperature thermal in hypoxia or anaerobic environment [11,12]. In addition, biochar is an insoluble solid matter with high aromatization that has been widely applied in carbon emission reduction, water purification, adsorption of heavy metals and soil improvement due to its high specific surface area and capacity of water holding capacity [13]. Currently, Biochar also provide solutions for climate change, environmental pollution and soil degradation in the world [14]. In addition, biochar from traw has been widely used in soil improvement of moisture, nutrient, dynamics metals and microbial community structure and functions in the agricultural soil [15]. Furthermore, large numbers of studies have reported that after applying biochar to soils may improve soil fertility, increase soil pH and CEC and enhance nutrient retention and the efficiency of crop production as well as ameliorate poor soil through its special physical and chemical properties [16-18]. All these characteristics suggest that biochar may an ideal amendment for improvement of enrichment and Microbial community structure and functions in rhizosphere soil of crops.
In recent years, nitrogen and carbon is evidenced associated with the initiation and progression of disease resistance of plants. Talukder et al. have showed that plant diseases susceptibility is often increased by nitrogen application and most of plant nitrogen status alters disease severity [19]. In addition, cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response have been investigated [20]. Furthermore, SIPK pathway is associated with disease resistance and plays a role in affecting translation efficiency as one mechanism for enacting rapid genome-wide, physiological reprogramming during defence responses in tobacco [21]. Moreover, research has indicated that silencing of WIPK and SIPK genes reduces resistance to a bacterial pathogen of P. cichorii, predicting WIPK and SIPK involves in the progression of disease resistance of plants [22]. These investigations may suggest that nitrogen and carbon sources are related with disease resistance of plants by regulation of WIPK and SIPK signal pathway.
In this study, we investigated the effects of Nitrifier cooperated with biochar on soil ecological factors and disease resistance of tobacco. The improvements of soil ecological factors, microbial community structure and functions, density and activity of nitrifier system were analyzed in the soil after treatment with genetically engineered Nitrifier cooperated with biochar. The mechanism of disease resistance of tobacco cultured in the experimental soil was explored through analysis of NPIR-induced SIPK/WIPK pathway. Based on our knowledge, we hypothesized that disease resistance of tobacco would improve by regulation of NPIR-induced SIPK/WIPK pathway derive from genetically engineered Nitrifier cooperated with biochar treatment
Materials and Methods
Ethics statement
This study was approved by the Ministry of Agriculture of the China and the safety of genetically engineered nitrifier was identified by Northeast Agricultural University. The study was carried out at Shuguang farm (46°13′N, 130°17′E), Sanjiang plain, where was not privately owned or protected in any ways, and the field studies did not involve any endangered or protected species.
Construction of genetically engineered nitrifier
A higher efficient nitrifier was isolated from fertile soil and identified as Bacillus subtilis. Function gene MO was cloned into pET-27b and transformed into bacillus subtilis. The purpose clone expressed MO was selected and identified by gene sequencing. The genetically engineered nitrifier expression MO gene was named gNitrifier-MO.
Microbial community structure and functions
Microbial community structure and functions in the experimental soil were analyzed according to previous study [23].
Quantitative real time PCR (qRT-PCR) analysis
Total RNA was obtained from tobacco leaf by using RNAeasy Mini Kit (QIAGEN, Gaithersburg, MD). Expressions of NPR-3, PR-1α and POD in cells were measured by applying a qRT-PCR as an endogenous control [24] (Invitrogen, CA, USA). All the forward and reverse primers were synthesized by Invitrogen. Relative mRNA expression changes were calculated by 2-ΔΔCt. The results are expressed as the n-fold of β-actin manner compared to control.
Western blot
Tobacco leaf were obtained from experimental soil and homogenized in lysate buffer containing protease-inhibitor and were centrifuged at 8000 rpm at 4°C for 10 min. The supernatant of mixture were used for analysis of purpose protein. For detection of purpose protein, transmembrane protein were extracted by using Transmembrane Protein Extraction Kit (QIAGEN, Gaithersburg, MD) according to the manufacturer’s instructions. SDS assays were performed as previous descript [25]. For western blotting, primary antibodies were added after blocking (5% skimmed milk) for 1 hours at 37°C and then incubating with second antibodies 24 hours at 4°C. The results were visualized by using chemi-luminescence detection system.
Experiment design
A randomized complete block design with three replicates was performed from April to October in 2014. Each treatment plot was 200 m2 (10 m × 20 m) in area. The experimental soil was pretreated by biochar (50 kg), nitrifier (50 kg) or both with non-treated soil as control. All soil was planted the same variety of tobacco. The tobacco was infected with TMV when plant height reached 20 centimeter in each experimental soil. Disease resistance and biochemical indexes of tobacco were analyzed according to previous study [26].
Analysis of changes of soil ecological factors
All soil ecological factors in this study were performed according to previous work [27]. The number of nitrifier activity of nitrifier was analyzed by previous report [28].
Statistical methods
All presented data were reported as means and SEM. Unpaired data was analyzed by Student’s t test. Comparisons of data between multiple groups were analyzed by variance (ANOVA). *P<0.05 and **P<0.01 was considered statistically significant.
Results
Effects of nitrifier cooperated with biochar on integrate microbial diversity and community structure
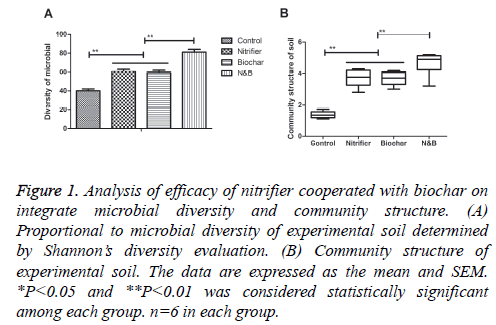
In order to analyze the efficacy of Nitrifier cooperated with biochar on agricultural soil, we first examined integrate microbial diversity and community structure. As shown in Figure 1A, integrate microbial diversity was increased by nitrifier cooperated with biochar (N&B) compared to either nitrifier or biochar. Community structure also was enriched in the soil treated by nitrifier cooperated with biochar compared to either nitrifier or biochar (Figure 1B). These results suggest that nitrifier cooperated with biochar increased microbial diversity and community structure in the experimental soil.
Figure 1: Analysis of efficacy of nitrifier cooperated with biochar on integrate microbial diversity and community structure. (A) Proportional to microbial diversity of experimental soil determined by Shannon’s diversity evaluation. (B) Community structure of experimental soil. The data are expressed as the mean and SEM. *P<0.05 and **P<0.01 was considered statistically significant among each group. n=6 in each group.
Effects of nitrifier cooperated with biochar on nitrification system and activity of nitrifier
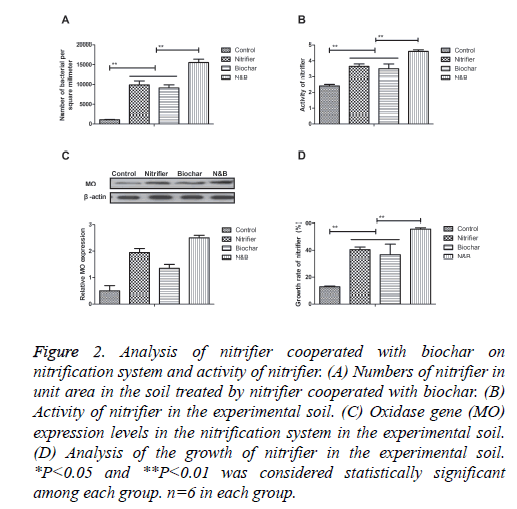
Nitrification system is crucial for the community structure in the soil ecosystem. Therefore, we analyzed the nitrification system and activity of nitrifier in the soil treated by nitrifier cooperated with biochar. The results in Figure 2A showed that the numbers of nitrifier in unit area were significantly increased in the soil treated by nitrifier cooperated with biochar compared to single treatment. Activity of nitrifier was also promoted in the soil treated by nitrifier cooperated with biochar compared to single treatment (Figure 2B). In addition, we found that oxidase gene (MO) expression levels were increased in the nitrification system in the soil treated by nitrifier and nitrifier cooperated with biochar compared to biochar treatment (Figure 2C). Furthermore, results showed that biochar increased the growth of nitrifier compared to single nitrifier treatment (Figure 2D). These results suggest that nitrifier cooperated with biochar promotes the enrichment of nitrification system and increases the activity of nitrifier compared to single agent.
Figure 2: Analysis of nitrifier cooperated with biochar on nitrification system and activity of nitrifier. (A) Numbers of nitrifier in unit area in the soil treated by nitrifier cooperated with biochar. (B) Activity of nitrifier in the experimental soil. (C) Oxidase gene (MO) expression levels in the nitrification system in the experimental soil. (D) Analysis of the growth of nitrifier in the experimental soil. *P<0.05 and **P<0.01 was considered statistically significant among each group. n=6 in each group.
Effects of nitrifier cooperated with biochar on soil ecological factors
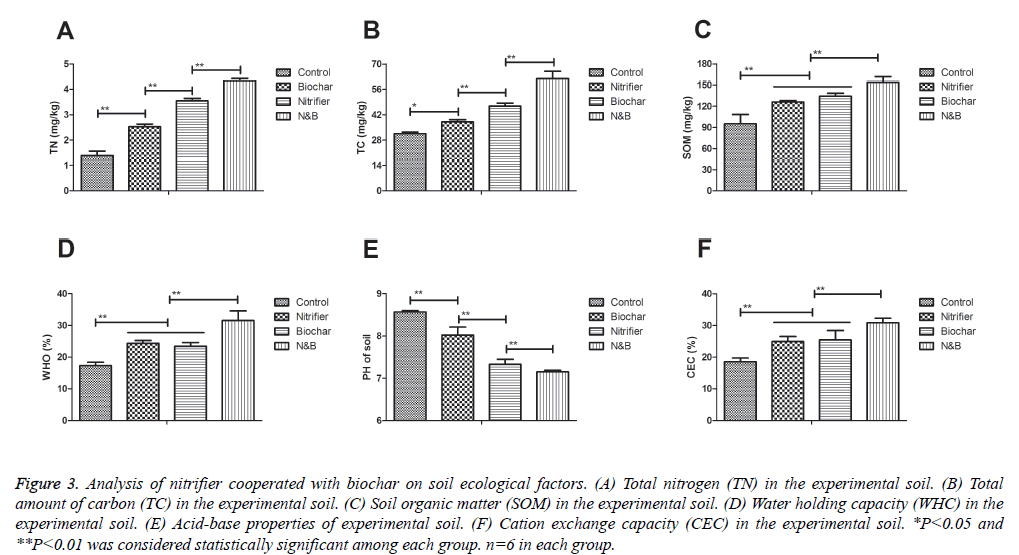
We next analyzed the benefits of nitrifier cooperated with biochar on soil ecological factors and changes of physical features. As shown in Figure 3A, total nitrogen (TN) was increased in soil treated by nitrifier cooperated with biochar compared to single treatment. Total amount of carbon (TC) was also increased in soil treated by nitrifier cooperated with biochar compared to single treatment (Figure 3B). In addition, content of soil organic matter (SOM) presented an increasing trend compared to single agent (Figure 3C). Furthermore, water holding capacity (WHC) of soil treated by nitrifier cooperated with biochar was also enhaced compared to single agent (Figure 3D). Moreover, acid-base properties and cation exchange capacity (CEC) were improved by nitrifier cooperated with biochar (Figures 3E-F). Collectively, these results showed that nitrifier cooperated with biochar markedly improved soil ecological factors in the experimental soil.
Figure 3: Analysis of nitrifier cooperated with biochar on soil ecological factors. (A) Total nitrogen (TN) in the experimental soil. (B) Total amount of carbon (TC) in the experimental soil. (C) Soil organic matter (SOM) in the experimental soil. (D) Water holding capacity (WHC) in the experimental soil. (E) Acid-base properties of experimental soil. (F) Cation exchange capacity (CEC) in the experimental soil. *P<0.05 and **P<0.01 was considered statistically significant among each group. n=6 in each group.
Effects of nitrifier cooperated with biochar on physiological function and yield in tobacco
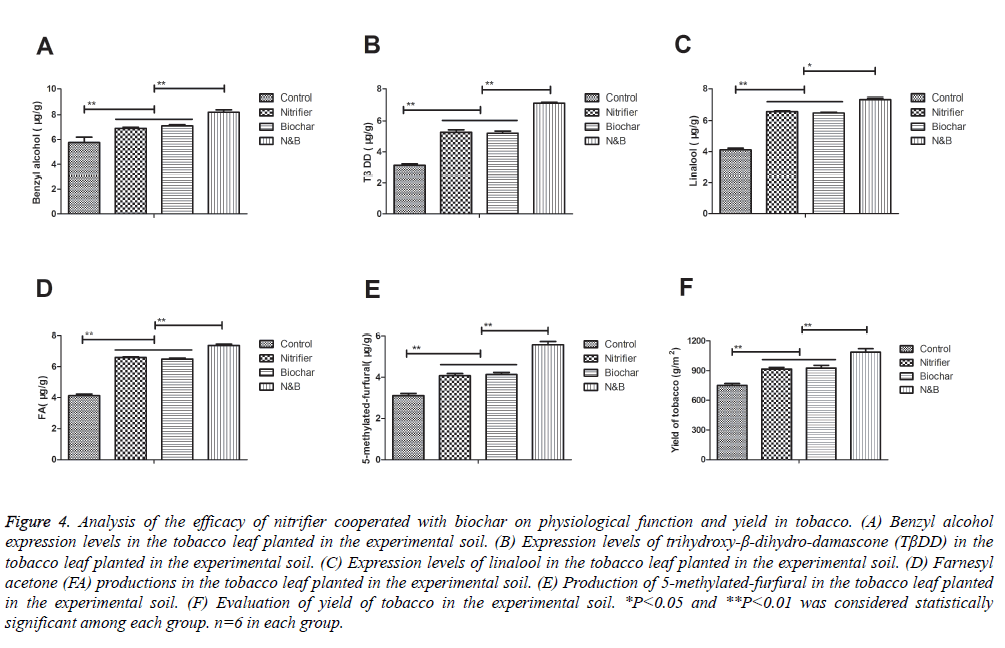
Improvement of physiological function and yield in tobacco plant are the essential indictors for evaluating the efficacy of nitrifier cooperated with biochar. As shown in Figure 4A, benzyl alcohol expression levels were up-regulated in tobacco plant cultured in the soil treated by nitrifier cooperated with biochar. Levels of trihydroxy-β-dihydro-damascone (TβDD) was increased in the tobacco leaf planted in nitrifier cooperated with biochar compared to single agent (Figure 4B).
Figure 4: Analysis of the efficacy of nitrifier cooperated with biochar on physiological function and yield in tobacco. (A) Benzyl alcohol expression levels in the tobacco leaf planted in the experimental soil. (B) Expression levels of trihydroxy-β-dihydro-damascone (TβDD) in the tobacco leaf planted in the experimental soil. (C) Expression levels of linalool in the tobacco leaf planted in the experimental soil. (D) Farnesyl acetone (FA) productions in the tobacco leaf planted in the experimental soil. (E) Production of 5-methylated-furfural in the tobacco leaf planted in the experimental soil. (F) Evaluation of yield of tobacco in the experimental soil. *P<0.05 and **P<0.01 was considered statistically significant among each group. n=6 in each group.
Linalool expression levels were also increased in the tobacco leaf planted in nitrifier cooperated with biochar compared to single agent (Figure 4C). In addition, farnesyl acetone (FA) productions were up-regulated in the tobacco leaf planted in nitrifier cooperated with biochar compared to single agent (Figure 4D). We also observed that levels of 5-methylated-furfural were increased in the tobacco leaf planted in nitrifier cooperated with biochar compared to single agent (Figure 4E). Furthermore, yield of tobacco was marked increased in the soil treated by nitrifier cooperated with biochar (Figure 4F). Taken together, these results suggest that nitrifier cooperated with biochar can significantly improve physiological function and yield in tobacco.
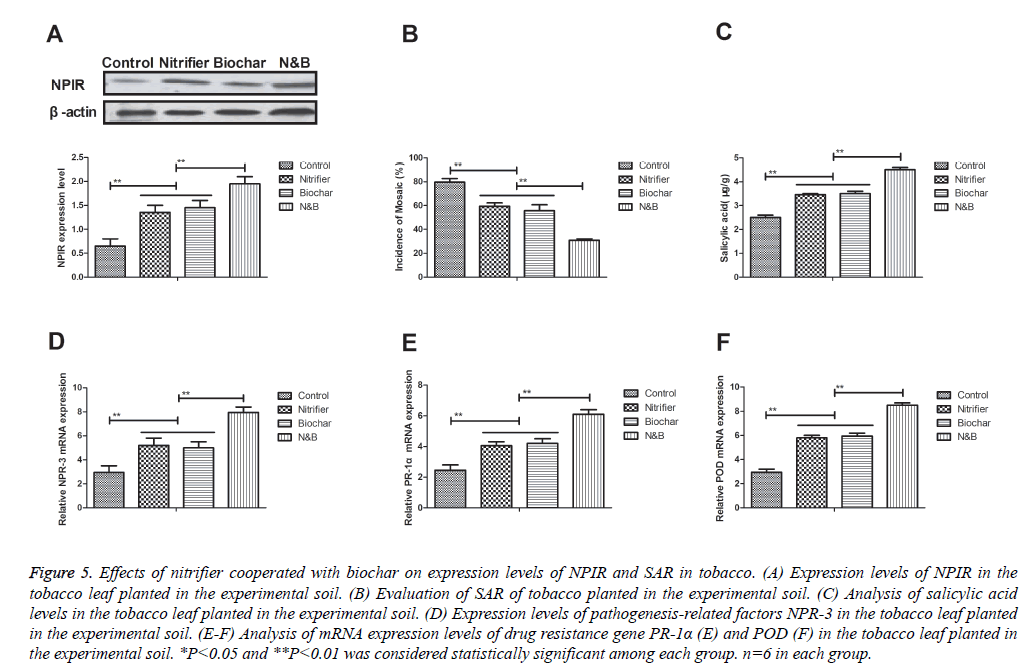
Effects of nitrifier cooperated with biochar on expression levels of nonexpressor of PR (NPIR) and systemic acquired resistance (SAR) in tobacco
Previous study has showed that NPIR plays important role in the progression of disease resistance of plant [29]. Therefore, we examined NPIR and SAR in the experimental tobacco in soil infected with TMV. As shown in Figure 5A, NPIR expression levels were up-regulated in the tobacco planted in the soil treated by nitrifier cooperated with biochar. We observed that SAR of tobacco was increased after soil treatment with nitrifier cooperated with biochar (Figure 5B). In addition, we found that salicylic acid and pathogenesis-related factors NPR-3 were up-regulated in obacco leaf planted in nitrifier cooperated with biochar compared to single agent (Figures 5C and 5D). Furthermore, results also indicated that mRNA expression levels of resistance gene PR-1α and POD were up-regulated in tobacco leaf planted in soil treated by nitrifier cooperated with biochar (Figures 5E and 5F). These data indicate that nitrifier cooperated with biochar up-regulate expression levels of nonexpressor of PR (NPIR) and systemic acquired resistance (SAR)-related genes in tobacco cultured in the experimental soil.
Figure 5: Effects of nitrifier cooperated with biochar on expression levels of NPIR and SAR in tobacco. (A) Expression levels of NPIR in the tobacco leaf planted in the experimental soil. (B) Evaluation of SAR of tobacco planted in the experimental soil. (C) Analysis of salicylic acid levels in the tobacco leaf planted in the experimental soil. (D) Expression levels of pathogenesis-related factors NPR-3 in the tobacco leaf planted in the experimental soil. (E-F) Analysis of mRNA expression levels of drug resistance gene PR-1α (E) and POD (F) in the tobacco leaf planted in the experimental soil. *P<0.05 and **P<0.01 was considered statistically significant among each group. n=6 in each group.
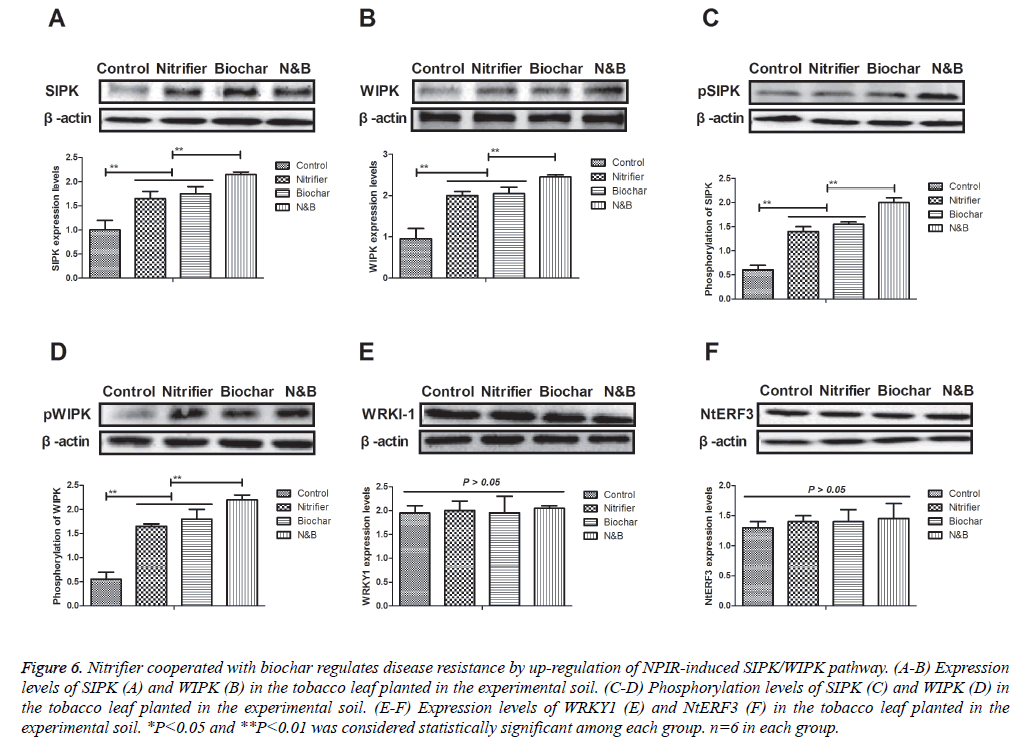
Nitrifier cooperated with biochar improves disease resistance through regulation of NPIR-induced SIPK/ WIPK pathway
To investigated the molecular mechanism of SAR of tobacco, we analyzed the SIPK/WIPK signal pathway in tobacco leaf planted in nitrifier cooperated with biochar. As shown in Figures 6A and 6B, expression levels of SIPK and WIPK were increased in the tobacco cultured in the soil treated by nitrifier cooperated with biochar. Also, phosphorylation levels of SIPK and WIPK were also up-regulated in the tobacco cultured in the soil treated by nitrifier cooperated with biochar (Figures 6C and 6D). Expression levels of WRKY1 and NtERF3 has not been affected by nitrifier cooperated with biochar (Figures 6E and 6F). These results indicate that nitrifier cooperated with biochar may improve disease resistance through regulation of NPIR-induced SIPK/WIPK pathway.
Figure 6: Nitrifier cooperated with biochar regulates disease resistance by up-regulation of NPIR-induced SIPK/WIPK pathway. (A-B) Expression levels of SIPK (A) and WIPK (B) in the tobacco leaf planted in the experimental soil. (C-D) Phosphorylation levels of SIPK (C) and WIPK (D) in the tobacco leaf planted in the experimental soil. (E-F) Expression levels of WRKY1 (E) and NtERF3 (F) in the tobacco leaf planted in the experimental soil. *P<0.05 and **P<0.01 was considered statistically significant among each group. n=6 in each group.
Discussion
Nitrifier system is a crucial part of microbial community structure and functions in soil, which participates in nitrogen cycle processes and regulates the dynamic equilibrium of nitrogen source in the soil. The species and quantities of microbes in nitrifier system play essential roles in progression of using of the nitrogen source for crops. Previous study has showed that nitrifier microbes can impact on nitrifier, denitrifier communities and N cycling in soil microenvironments [10]. Molecular biology analyzes have indicated that the diversity of microorganism community in natural environment can be identied by molecular biotechnology [30]. In addition, Griepentrog et al. have showed that nitrogen deposition could regulate by nitrifier system and promotes the production of new fungal residues decomposition of old residues in forest soil fractions [31].
Furthermore, Tsiknia et al. have suggest that total Kjeldahl nitrogen content on soil also is regulated by nitrifier system and further acts on carbon and nitrogen cycling [32]. In this study, we constructed a genetically engineered nitrifier delivered monoamine oxidase gene and investigated the potential role in acquiring the available nitrogen source in soil. Our results indicate that genetically engineered nitrifier delivered monoamine oxidase gene not only promotes the microbial community structure and functions, but also improves the soil ecological factors and disease resistance of tobacco. The nutrition in soil treated by genetically engineered nitrifier delivered monoamine oxidase gene has been increased, which further regulates the physical and chemical properties of soil. Further, physiological function and yield in tobacco have been identified associated with nitrifier system. We also found that biochar combined with nitrifier enhances the improvement of soil ecological factors and further improves the disease resistance of tobacco.
Effects of biochar on crop growth and yield have been identified relevant with soil type, soil fertility status and biochar content [33]. In this study, we investigated the function of biochar derived from maize straw on the soil ecological factors, growth and yield of tobacco, as well as the disease resistance to CMV infection and molecular mechanism of the processes. Previous study has provided a novel perspective for the efficacy of biochar on soil microbial ecology, which elaborated the benefits of biochar on the recovery of ecological environment [34]. Our research have confirmed these results and further indicated that biochar promotes the diversity and functions of nitrifier system. Although Jassal et al. have indicated that biochar can be used to remove excess nitrogen from poultry and dairy manure, which may be a good strategy to improve the nitrogen balance [35]. Therefore, we assumed that biochar may play regulatory effects in the soil ecosystem. Our outcomes have confirmed the assumption and also indicated that biochar combined with nitrifier significantly regulates microbial community structure and functions of soil and further improves the disease resistance of tobacco through up-regulation of NPIR-induced SIPK/WIPK pathway.
Currently, reports have indicated that systemic signals of nitrogen status can control root nitrogen acquisition and regulate gene networks by targeting signal pathway and processes of functional response of the nitrogen acquisition systems, which are predominantly determined by the nature of the nitrogen source and structure of community in the soil [36]. Our results presented the beneficial effects of biochar combined with nitrifier on the disease resistance of tobacco. In addition, effects of nitrifier system on exudation of proteases by plant roots have been investigated between functional proteins and disease resistance for plants [37]. Furthermore, Ogata et al. have suggested that expression elves of NtERF3 involves in the cell death signalling pathway mediated by SIPK/WIPK and WRKY1 in tobacco plants [38]. Moreover, NPIR involves in many biochemical reactions in plants and associated with the physiological disease resistance and hypersensitive necrosis reaction [29]. A large numbers of anti-disease gene expression levels, such as PR1 and PR5 are also regulated by NPIR. We found that salicylic acid, pathogenesis-related factors NPR-3 PR-1α and POD were up-regulated in obacco leaf planted in nitrifier cooperated with biochar compared to single agent. These investigations showed that biochar combined with nitrifier have increased the disease resistance of tobacco through up-regulation of NPIR-induced SIPK/WIPK pathway.
In conclusion, this study not only studied the potentially large impact of nitrifier cooperated with biochar on soil microbial community structure, functions processes and composition, but also further emphasize nitrifier cooperated with biochar on molecular mechanism of disease resistance of tobacco. Either microbial or abiotic in the soil has been improved by nitrifier cooperated with biochar. The physiological characteristics, disease resistance, tobacco yield and effective constituent were improved in the tobacco planted in soil treated by nitrifier cooperated with biochar. These findings suggest that nitrifier cooperated with biochar may better strategy to improve the biodiversity and soil organic matter to lead to increasing yield and disease resistance of tobacco through up-regulation of NPIR-induced SIPK/WIPK pathway.
Acknowledgments
This work was financially supported by the [Scientific Research Fund of Heilongjiang Provincial Science and Technology Department]; under Grant [NO. GA10B502]; Doctoral Starting Funds Program of Northeast Agricultural University under Grant [NO. 2012RCB95].
References
- Yarwood S, Wick A, Williams M, Daniels WL. Parent material and vegetation influence soil microbial community structure following 30-years of rock weathering and pedogenesis. Microbial Ecol 2015; 69: 383-394.
- Zhen Z, Liu H, Wang N, Guo L, Meng J. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS One 2014; 9: e108555.
- Whitaker J, Ostle N, Nottingham AT, Ccahuana A, Salinas N. Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. J Ecol 2014; 102: 1058-1071.
- Jin ZJ, Tang HF, Li M. Microbial community abundance and diversity in typical karst ecosystem to indicate soil carbon cycle. Huan jing ke xue= Huanjing kexue/[bian ji, Zhongguo ke xue yuan huan jing ke xue wei yuan hui "Huan jing ke xue" bian ji wei yuan hui.] 2014; 35: 4284-4290.
- Wu BB, Lu DN, Liu Z. Dynamic changes in functional genes for nitrogen bioremediation of petroleum-contaminated soil cycle during. Huan jing ke xue= Huanjing kexue / [bian ji, Zhongguo ke xue yuan huan jing ke xue wei yuan hui "Huan jing ke xue" bian ji wei yuan hui.] 2012; 33: 2068-2074.
- Pan YF, Yang M, Dong D, Wu WX. Effects of biochar on soil nitrogen cycle and related mechanisms: a review. Ying Yong Sheng Tai Xue Bao 2013; 24: 2666-2673.
- Behie SW, Bidochka MJ. Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: an additional branch of the soil nitrogen cycle. Appl Environ Microbiol 2014; 80: 1553-1560.
- Groffman PM, Boulware NJ, Zipperer WC, Pouyat RV, Band LE, Colosimo MF. Soil nitrogen cycle processes in urban riparian zones. Environ Sci Technol 2002; 36: 4547-4552.
- Du Y, Guo P, Liu J, Wang C, Yang N, Jiao Z. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Global Change Biol 20: 3222-3228, 2014.
- Kong AY, Hristova K, Scow KM, Six J. Impacts of different N management regimes on nitrifier and denitrifier communities and N cycling in soil microenvironments. Soil Biol Biochem 2010; 42: 1523-1533.
- Bolster CH, Abit SM. Biochar pyrolyzed at two temperatures affects Escherichia coli transport through a sandy soil. J Environ Quality 2012; 41: 124-133.
- Xu T, Lou L, Luo L, Cao R, Duan D. Effect of bamboo biochar on pentachlorophenol leachability and bioavailability in agricultural soil. Sci Total Environ 2012; 414: 727-731.
- Ahmad M, Soo Lee S, Yang JE, Ro HM, Han Lee Y. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol Environ Saf 2012; 79: 225-231.
- Uchimiya M, Wartelle LH, Boddu VM. Sorption of triazine and organophosphorus pesticides on soil and biochar. J Agric Food Chem 2012; 60: 2989-2997.
- Jindo K, Sanchez-Monedero MA, Hernandez T. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci Total Environ 2012; 416: 476-481.
- Hernandez-Soriano MC, Kerré B, Kopittke PM, Horemans B. Biochar affects carbon composition and stability in soil: a combined spectroscopy-microscopy study. Sci Rep 2016; 6: 25127.
- Wu M, Feng Q, Sun X, Wang H, Gielen G. Rice (Oryza sativa L) plantation affects the stability of biochar in paddy soil. Sci Rep 2015; 5: 10001.
- Singh BP, Cowie AL, Smernik RJ. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ Sci Technol 2012; 46: 11770-11778.
- Talukder ZI, McDonald AJ, Price AH. Loci controlling partial resistance to rice blast do not show marked QTL x environment interaction when plant nitrogen status alters disease severity. New Phytol 2005; 168: 455-464.
- Zaninotto F, La Camera S, Polverari A, Delledonne M. Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol 2006; 141: 379-383.
- Hall HC, Samuel MA, Ellis BE. SIPK conditions transcriptional responses unique to either bacterial or oomycete elicitation in tobacco. Mol Plant Pathol 2007; 8: 581-594.
- Sharma PC, Ito A, Shimizu T, Terauchi R, Kamoun S, Saitoh H. Virus-induced silencing of WIPK and SIPK genes reduces resistance to a bacterial pathogen, but has no effect on the INF1-induced hypersensitive response (HR) in Nicotiana benthamiana. Mol Genetics Genomics (MGG) 2003; 269: 583-591.
- Goberna M, Garcia C, Insam H, Hernandez MT, Verdu M. Burning fire-prone Mediterranean shrublands: immediate changes in soil microbial community structure and ecosystem functions. Microbial Ecol 2012; 64: 242-255.
- Xiao S, Wang J, Xiao N. MicroRNAs as noninvasive biomarkers in bladder cancer detection: a diagnostic meta-analysis based on qRT-PCR data. Int J Biol Markers 2016; 31: e276-285.
- Wai-Hoe L, Wing-Seng L, Ismail Z, Lay-Harn G. SDS-PAGE-Based Quantitative Assay for Screening of Kidney Stone Disease. Biol Procedures Online 2009; 11: 145-160.
- Kumar D, Kirti PB. Pathogen-induced SGT1 of Arachis diogoi induces cell death and enhanced disease resistance in tobacco and peanut. Plant Biotechnol J 2015; 13: 73-84.
- Liu Y, Cao T, Wang J, Cao Y. Relationships between distribution of soil-born bryophytes in urban area of Hangzhou and related ecological factors. Ying yong sheng tai xue bao = The journal of applied ecology / Zhongguo sheng tai xue xue hui, Zhongguo ke xue yuan Shenyang ying yong sheng tai yan jiu suo zhu ban 2008; 19: 775-781.
- Le Roux X, Poly F, Currey P, Commeaux C, Hai B. Effects of aboveground grazing on coupling among nitrifier activity, abundance and community structure. ISME J 2008; 2: 221-232.
- Watanabe E, Shimada T, Tamura K. An ER-localized form of PV72, a seed-specific vacuolar sorting receptor, interferes the transport of an NPIR-containing proteinase in Arabidopsis leaves. Plant Cell Physiol 2004; 45: 9-17.
- Zhang YG, Wang HM, Li DQ, Xiao QM, Liu XD. [The community and structure of nitrogen-fixing microorganism in Sanjiangyuan natural reserve]. Wei Sheng Wu Xue Bao 2005; 45: 420-425.
- Griepentrog M, Bode S, Boeckx P, Hagedorn F, Heim A, Schmidt MW. Nitrogen deposition promotes the production of new fungal residues but retards the decomposition of old residues in forest soil fractions. Global Change Biology 2014; 20: 327-340.
- Tsiknia M, Tzanakakis VA, Oikonomidis D, Paranychianakis NV, Nikolaidis NP. Effects of olive mill wastewater on soil carbon and nitrogen cycling. Appl Microbiol Biotechnol 2014; 98: 2739-2749.
- Sparrevik M, Field JL, Martinsen V, Breedveld GD, Cornelissen G. Life cycle assessment to evaluate the environmental impact of biochar implementation in conservation agriculture in Zambia. Environ Sci Technol 2013; 47: 1206-1215.
- Ding YL, Liu J, Wang YY. Effects of biochar on microbial ecology in agriculture soil: a review. Ying Yong Sheng Tai Xue Bao 2013; 24: 3311-3317.
- Jassal RS, Johnson MS, Molodovskaya M, Black TA, Jollymore A, Sveinson K: Nitrogen enrichment potential of biochar in relation to pyrolysis temperature and feedstock quality. J Environ Manage 2015; 152: 140-144.
- Ruffel S, Freixes S, Balzergue S. Systemic signaling of the plant nitrogen status triggers specific transcriptome responses depending on the nitrogen source in Medicago truncatula. Plant Physiol 2008; 146: 2020-2035.
- Adamczyk B, Smolander A, Kitunen V, Godlewski M. Proteins as nitrogen source for plants: a short story about exudation of proteases by plant roots. Plant Signaling Behavior 2010; 5: 817-819.
- Ogata T, Okada H, Kawaide H, Takahashi H, Seo S. Involvement of NtERF3 in the cell death signalling pathway mediated by SIPK/WIPK and WRKY1 in tobacco plants. Plant Biol (Stuttg) 2015; 17: 962-972.