Mini Review - Journal of Diabetology (2023) Volume 7, Issue 1
General study on metformin
Nalawade Dipak D1*, Charmal Vaishnavi D2, Gaikwad Mayuri B21Department of Pharmaceutical Chemistry, Dr. Kolpe Institute of Pharmacy, Kolpewadi, Maharashtra, India
2Dr. Kolpe Institute of Pharmacy, Kolpewadi, Maharashtra, India
- *Corresponding Author:
- Nalawade Dipak D
Department of Pharmaceutical Chemistry
Dr. Kolpe Institute of Pharmacy
Kolpewadi, Maharashtra, India
E-mail: dipakn1515@gmail.com
Received: 07-Jan-2023, Manuscript No. AADY-23-84540; Editor assigned: 09-Jan-2023, Pre QC No. AADY-23-84540 (PQ); Reviewed: 23-Jan-2023, QC No. AADY-23-84540; Revised: 25-Jan-2023, Manuscript No. AADY-23-84540 (R); Published: 31-Jan-2023, DOI: 10.35841/aady-7.1.135
Citation: Dipak ND. General study on metformin. 2023;7(1):135
Abstract
Metformin was rediscovering during the hunt for an antimalarial drug. The French physician jean Sterne, who first reported the use of metformin to treat diabetes in 1957. Aim: Over View of metformin. The commonly used medication metformin has definite advantages in terms of issues related to diabetes and glucose metabolism
Keywords
Metformin, Diabetes.
Introduction
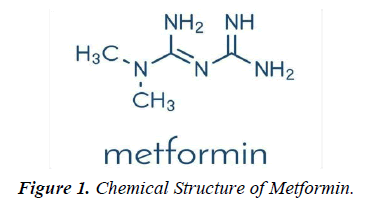
Metformin was discovered in 1922. French physician Jean Sterne began the study in humans in the 1950s. In the 1940s, metformin was rediscovered during the hunt for an antimalarial drug and proved helpful in treating influenza during clinical trials when it occasionally caused blood glucose levels to drop. The French physician Jean Sterne, who first reported the use of metformin to treat diabetes in 1957, pursued this property (figure 1).
Chemical Formula: C4H11N5
Molecular weight: 129.1
Metformin (1,1-dimethylbiguanide hydrochloride) is a relatively planar hydrophilic molecule, monoprotonated at neutral pH with several tautomeric configurations. The standard immediate-release tablet formulation taken orally in doses of 500 to 1000 mg is rapidly absorbed in the small intestine (time to maximum plasma concentration after administration (T max), 2.5h; approximately 50% bioavailability), typically producing a peak plasma concentration ( C max) of about 2g/ ml, and in some cases exceeding 4g/ml, with a steady-state concentration range of 0.3-1.5g/ml. Plasma protein binding is negligible and distribution is extensive (usual volume of distribution [Vd],100-300l). The elimination half-life (T1/2) of metformin is approximately 6-7 hours, or longer if real function is compromised [1].
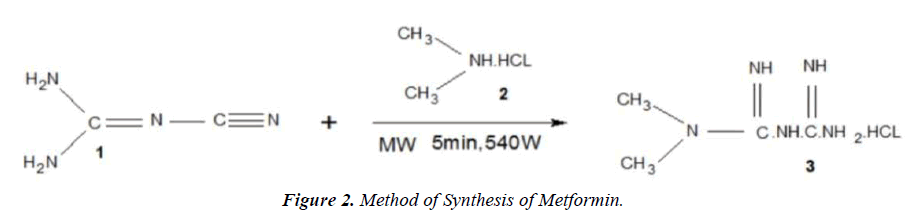
Method of synthesis of metformin
In a typical experiment, a test reaction was carried out on a 5×20cm 1.TLC platelet, a spot of solution of dicyanodiamide.
1. (0.42g) and dimethylamine hydrochloride.
2. (0.4g) in 5ml ethanol was placed on a TLC platelet and subjected ro MWI at a 540 W at an Internal of 40 Seconds intermittently for 5 min. Then, the TLC platelet was run in appropriate system. A promient spot of metformin hydrochloride (figure 2).
3. Was seen and the rf value of which was consistent with that of the standard sample. In other two get an appreciable yield of pure product, the reaction was carried out on a preparative TLC plate. An array of spot of reactants for the Synthesis of metformin hydrochloride was put on a preparative TLC plate along with a reference TLC with two spot (one of the reactants and oder of expected products). Both the plate where subjected to MWI intermittently at an interval of 40s at 540 power for 5 min. The reference TLC was viewed in an iodine chamber and accordingly that portion of silica gel containg the product was scratched from the preparative TLC platelet and the product was extracted in EtOH. Evaporation of the solvent afforded 0.82g (92% yields) of the desired product.
Melting point: 224-225°C
IR (kbrcm-1): 1580,1620,1063,1075,935,740
UV (Meyhanol,λmax): 236nm [2]
1H-NMR (DMSO-d6): δ(ppm) :2.92 (s, 6H, CH3); 6.77 (s, 4H, amide); 7.19 (s, 2H, imine)
13C-NMR(125 MHz, D2O): δ(ppm): 160.6, 158.9, 38.1
The mechanisms of action of metformin
The commonly used medication metformin has definite advantages in terms of issues related to diabetes and glucose metabolism. These advantages' complicated and incompletely understood mechanism. Although metformin has been proven physiologically to decrease the generation of hepatic glucose, not all of its effects can be accounted for by this route, and there is mounting evidence that the gut plays a significant role. Metformin has been shown to act via AMP-activated protein kinase AMPK -dependent and AMPK-independent mechanism by inhibition of mitochondrial respiration, but it may also act via inhibition of mitochondrial glycerophosphate dehydrogenase and a mechanism involving the lysosome. At the molecular level, the results vary depending on the doses of metformin used and duration of treatment, with clear differences between acute and chronic administration. Over the past ten years, our understanding of metformin's mechanisms of action has expanded from the straightforward notion that it affects the liver's AMPK activation to enhance glycemia. To fully comprehend how this medication affects the people who are its target market—those with type 2 diabetes—more research is necessary [3].
Indications
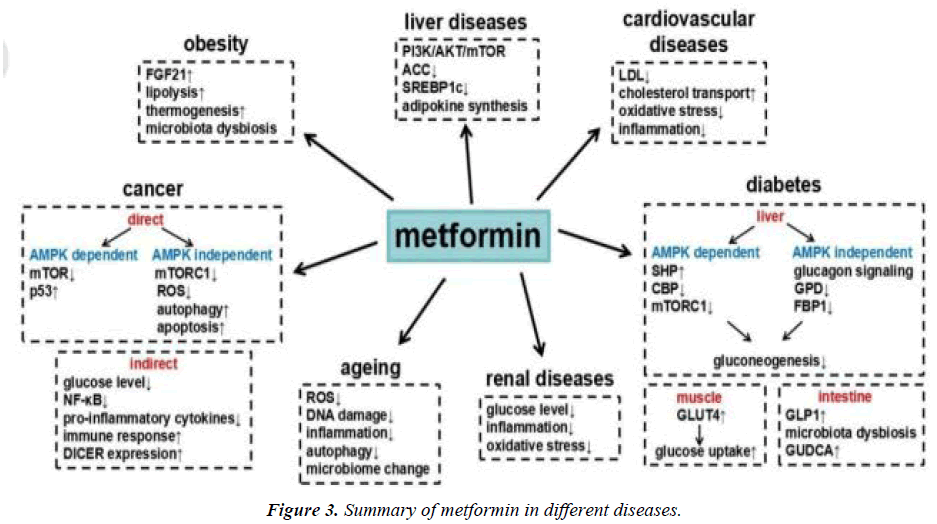
An oral diabetes medication called metformin (dimethylbiguanide) increases gluconeogenesis and peripheral glucose uptake by muscle. Metformin is a first-line medication used to treat type 2 diabetic patients who are obese and overweight. By affecting insulin resistance, it offers a selective pathophysiological approach. Numerous studies have demonstrated that it enhances clinical results in type 2 diabetes patients. Numerous studies have shown that metformin has a variety of biological impacts (figure 3). The drug's antioxidant activities, which may substantially account for its vascuar protective effects, have been demonstrated to have platelet antiaggregating effects, to lower the rate of production of advanced glycation and products (AGEs), and to diminish cellular oxidative responses. Metformin has been shown to have favourable effects on body weight, insulin resistance, hyperinsulinaemia, lipid parameters (total cholesterol, HDL cholesterol, LDL cholesterol, fibrinolysis, endothelial dysfunction), and endothelial dysfunction in a number of studies. Thus, it appears that metformin has a unique set of pharmacological properties that could make it useful in conditions other than diabetes, such as obesity, severe insulin resistance associated with acanthosis nigricans, polycystic ovary syndrome, etc. Metformin appears to be a drug with multiple therapeutic effects for beyond its effect on lowering blood glucose in diabetes mellitus, as it has been shown in the diabetes prevention programme to be a drug with great potential in preventing the conversion of IGT to type 2 diabetes [4].
Uses
Metformin: clinical use in type 2 diabetes
1. Effective glucose lowering as monotherapy and in combination with other agents, including insulin.
2. Has a weight-neutral effect and does not raise the risk of hypoglycemia.
3. Possible cardiovascular benefits.
4. The maximum daily dose is often 2000 mg.
5. GI disruption is a serious side effect.
6. The risk of lactic acidosis is limited; it mostly affects people with advanced chronic renal disease [5].
Guidelines
Most treatment guidelines recommend metformin as a first-line treatment for type 2 diabetes, a recommendation supported by the drug's effectiveness, affordability, relative safety, and the UKPDS's findings on cardiovascular benefits. The European Association for Study of Diabetes (EASD) and the American Diabetes Association (ADA) (EASD).
Side effects of metformin
1. Feeling slick (nausea).
2. Being slick (vomiting).
3. Diarrhoea.
4. Stomach ache.
5. Loss of appetite.
6. A metallic taste in the mouth.
7. Vitamin B12 deficiency.
8. Low blood sugar.
9. Gas.
10. Weight loss.
Risk factors for experiencing side effect
1. Kidney problem.
2. Heart problem.
3. Liver problem.
Conclusion
Metformin is a widely used clinical drug with numerous benefits. An oral diabetes medication called metformin increases gluconeogenesis and peripheral glucose uptake by muscle. There are different side effects of metformin including a metallic taste in the mouth and vitamin B12 deficiency etc. These compiled data may of use for research for further studies in analysis of metformin.
References
- Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566-76.
- Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577-85.
- Tankova T. Current indications for metformin therapy. Romanian Journal of Internal Medicine= Revue Roumaine de Medecine Interne. 2003;41(3):215-25.
- Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586-93.
- Lv Z, Guo Y. Metformin and its benefits for various diseases. Frontiers in endocrinology. 2020;11:191.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref