Research Article - Biomedical Research (2016) Volume 27, Issue 4
Functional analysis of auditory pathway in type 2 diabetes by brainstem auditory evoked response-a pilot study
Rajesh Paluru1*, Yogananda Reddy Indla2, Ramaswamy C3 and Rajani Santhakumari1
1Department of Physiology, MediCiti Institute of Medical Sciences, Telangana, India
2Department of Physiology, SVS Medical College, Telangana, India
3Department of Physiology, Saveetha Medical College, Tamilnadu, India
- *Corresponding Author:
- Rajesh P
Department of Physiology
MediCiti Institute of Medical Sciences
India
Accepted date: April 13, 2016
Abstract
Background: Diabetic neuropathy is one of the major complications reported widely in type 2 diabetes. It affects all the sensory, autonomic and motor systems including auditory pathway.
Objectives: Present study is focused on functional analysis of auditory pathway by Brainstem Auditory Evoked Response (BAER) and with Pure Tone Audiometry (PTA) in type 2 diabetes. To compare the same in healthy individuals with euglycaemia.
Materials and methods: This is a cross sectional study, ten type 2 diabetic subjects were recruited as test group, age and sex matched ten healthy subjects were selected as control group. Inter wave peak latencies with BAER and pure tone averages with PTA for both the ears were recorded in test and control groups. Unpaired “t” test was carried out to compare the data, p value <0.05 is considered as significant.
Results: Right ear stimulus threshold in controls and test groups are 14.9 ± 2.04 & 30.9 ± 11.43 (p=0.01) respectively and for left ear, 18.9 ± 7.48 & 30.6 ± 4.0 (p=0.01) respectively. Wave I-III inter peak latencies of BAER for right ear in control and test groups are 1.79 ± 0.15 & 2.20 ± 0.30 (p=0.02) respectively and for left ear, 1.79 ± 0.12 & 2.25 ± 0.40 (p=0.04) respectively.
Conclusion: The present study concludes that in type 2 diabetes inter wave peak latencies and pure tone averages were higher when compared with controls.
h2 class="post-title">KeywordsType 2 diabetes, Glycosylated haemoglobin, Pure tone audiometry, Brainstem auditory evoked response.
Introduction
Diabetes affects the sensory, motor and autonomic nervous systems, including the auditory pathway. Uncontrolled diabetes causes difficulty in hearing [1] and Jardao first ever reported hearing loss in a diabetic patient [2]. Sensory neuronal deafness is observed in diabetes, particularly for high frequency sounds [3]. Globally 382 million people have diabetes and the number is set to rise beyond 592 million in less than 25 years. Currently 175 million undiagnosed diabetic cases are present across the globe and the number is increasing at a robust rate, a vast amount of people with diabetes are progressing towards complications unawares [4]. It is an established fact that diabetes effects the functional status of the endothelium and studies shows that there is sudden sensory neuronal hearing loss with endothelial dysfunctioning [5,6]. Type 2 diabetics with a familial history of diabetes are having low levels of nitric oxide, and this can damage the endothelium [7]. The glycosilated haemoglobin (HbA1c) and Pure Tone Audiometry (PTA) tests are gold standard [8,9] in monitoring the diabetic control and assessing the hearing threshold respectively. In the present study HbA1c concentration is measured to assess the glycaemic status. PTA and Brainstem Auditory Evoked Response (BAER) are used for the functional analysis of auditory pathway.
Hypothesis
Functional status of auditory pathway is affected in type 2 diabetes.
Objectives of the study
1. To observe the effect of type 2 diabetes on inter wave peak latencies in BAER.
2. To illustrate the auditory threshold in type 2 diabetics with PTA.
3. To observe the glycaemic status by measuring the HbA1c levels.
Materials and Methods
Study design
It is a cross sectional study. Study was approved by the institutional ethical committee (FWA00002084 dated 16/03/2015).
Inclusion criteria
Ten type 2 diabetic subjects of both the sex, aged between 30-55 years were included in the study as cases. Age and sex matched 10 healthy individuals were also included in the study as controls. Written informed consent was obtained from both the groups after making them to understand the objectives of the study.
Exclusion criteria
Subjects were excluded from the study if they have present or past history of using ototoxic drugs, noise exposure, ear surgeries, chronic middle ear diseases, cranial trauma, metabolic disorders except for diabetes mellitus, underwent recent surgeries, any type of chronic infections, congenital hearing problems, type 1 diabetes, smokers and alcoholics.
Glycosylated haemoglobin (HbA1c)
Glycosylated haemoglobin was estimated on the basis of latex agglutination inhibition assay by using Rx imola automated analyser. Here the haemoglobin is hydrolysed by the enzyme protease in the haemoglobin denaturant reagent. The reported HbA1c result is calculated as a percentage of the total haemoglobin concentration (Randox, UK).
Pure Tone Audiometry (PTA)
Hearing threshold was measured with PTA and is performed in a sound attenuated chamber. Pure tone audiometry was recorded with Elkon3N3 Multi Diagnostic Audiometer, Bombay, India.
Brainstem Auditory Evoked Response (BAER)
Inter wave peak latencies in auditory pathway were recorded with Biologic Navigator Pro system, AEP Software version 6.3 (Natus Hearing, USA). Considering the test particulars, click stimulus with an alternating polarity was used at intensity levels of 80, 70, 60, 50, 40, 30 dB nHL. The filter setting ranges from 150Hz-1500Hz, with an epoch time of 10.26 ms and stimulus rate of 11.1/sec and 1024 sweeps of stimulus.
Statistical analysis
Statistical analysis of the data was conducted by using Med Calc Statistical Software version 12.7.8 (Med Calc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). Unpaired t-test was performed to compare the mean differences between test and control groups, male and female subjects. P value<0.05 is considered as statistically significant.
Results
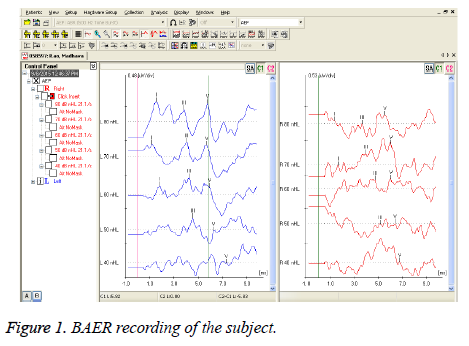
HbA1c percentage among test group subjects (7.82 ± 1.57) and controls (5.18 ± 0.27), is significant (0.006). HbA1c percentage among diabetic males (7.30 ± 1.52) and diabetic females (7.96 ± 1.45) is not significant (0.53). Pure tone averages and inter wave peak latencies were compared between the test group subjects and controls, mentioned in the Tables 1 and 2 respectively. Tables 3 and 4 shows the PTA and BAER values for diabetic male and diabetic female subjects. Figure 1 reflects the recording of BAER in test group subject.
| Parameter | C Right Ear | T Right Ear | p value | C Left Ear | T Left Ear | p value |
| Pure tone average | 14.9 ± 2.04 | 30.9 ± 11.43 | 0.01 | 18.9 ± 7.48 | 30.6 ± 4.00 | 0.01 |
Table 1. PTA thresholds in dB in control(C) and test (T) groups with mean and SD.
| IPL | C Right Ear | T Right Ear | p value | C Left Ear | T Left Ear | p value |
|---|---|---|---|---|---|---|
| I-III | 1.79 ± 0.15 | 2.20 ± 0.30 | 0.02 | 1.79 ± 0.12 | 2.25 ± 0.40 | 0.04 |
| III-V | 1.53 ± 0.26 | 1.60 ± 0.41 | 0.74 | 1.64 ± 0.13 | 1.92 ± 0.17 | 0.02 |
| I-V | 3.46 ± 0.32 | 3.98 ± 0.33 | 0.03 | 3.71 ± 0.15 | 4.14 ± 0.33 | 0.03 |
Table 2. BAER results in milliseconds in control(C) and test (T) groups with mean and SD.
| Parameter | M Right Ear | FM Right Ear | p value | M Left Ear | FM Left Ear | p value |
| Pure tone average | 27.00 ± 12.8 | 17.00 ± 2.6 | 0.23 | 25.43 ± 8.22 | 20.67 ± 5.13 | 0.38 |
Table 3. PTA Thresholds in dB in diabetic males (M) and females (FM) with mean and SD.
| IPL | M Right Ear | FM Right Ear | p value | M Left Ear | FM Left Ear | p value |
|---|---|---|---|---|---|---|
| I - III | 1.79 ± 0.32 | 1.82 ± 0.35 | 0.92 | 2.39 ± 0.44 | 2.18 ± 0.23 | 0.45 |
| III - V | 1.82 ± 0.47 | 2.05 ± 0.61 | 0.53 | 1.56 ± 0.33 | 1.65 ± 0.42 | 0.71 |
| I - V | 3.57 ± 0.54 | 3.87 ± 0.36 | 0.42 | 3.95 ± 0.53 | 3.83 ± 0.46 | 0.75 |
Table 4. BAER Results in milliseconds in diabetic males and females with mean and SD.
Discussion
In the present study it was observed that the stimulus threshold was increased in both the ears for diabetic patients when compared with control group. Test group subjects are having mild hearing loss for both the ears. These findings were in line with earlier studies where the auditory thresholds were increased in type 2 diabetes [10-13]. The threshold is much higher in right ear than the left ear for diabetic males when compared with the diabetic females though both the values were not significant. Inter wave peak latencies are more dependable than the absolute latencies in assessing the functional status of various nuclei or ganglion in the auditory pathway, and that is why, in the present study inter wave peak latencies were recorded. The obtained BAER results have shown that in test group, except for right ear inter wave peak latency of wave III-V, all the other inter wave peak latencies were increased when compared with controls. Though the inter wave peak latencies were increased in test group subjects they were within the physiological limit that is the inter wave peak latencies of wave I-III and wave III-V are not more than 2.5 ms which is the upper limit and the inter wave peak latency of wave I-V is also less than 4.5ms, which is also the critical value [14]. In the present study it was observed that increased inter wave peak latency of wave I-V for both the ears in test group subjects, which is also in line with the earlier research findings [13,15-20].
These increased inter wave peak latencies along with increased strength of the threshold stimulus in both the ears of the test group subjects clearly indicates the adverse effects of hyperglycaemia on auditory pathway in type 2 diabetes. Further studies are needed in exploring why the inter wave peak latency of right ear wave III-V is not increased significantly when the rest of the inter wave peak latencies were increased in test group subjects when compared with the controls. Normally in females the inter wave peak latencies are less when compared with the males and this is attributed to their higher core temperature and lesser head size [21,22]. But in the present study there was no significance of inter wave peak latencies between diabetic male and diabetic female subjects in both the ears. This finding is in contradiction with the earlier research reported, where inter wave peak latencies were prolonged in diabetic males than in diabetic females [23].
Mild hearing loss in both ears in test group is observed and earlier studies attributed this hearing loss to the effect of hyperglycaemia on the inner row of hair cells through altering the endolymph concentration by changing the secretion of striavascularis [24]. Some other studies have explained that in type 2 diabetes, hyperglycaemia causes thickening of the basilar membrane, demyelination of the cochlear nerve, and atrophy of the spiral ganglion [25] resulting in hearing loss. Increased intra neuronal glucose levels in the auditory pathway structures results in damaging them by enhancing the enzymatic activity of poly ADP ribose and that will result in alteration in the functioning of the auditory structures [26] and disrupt cochlear functions as well [27]. Diabetes also enhances the aging of auditory system [28,29]. At this juncture the present study is unable to explain the exact pathophysiological basis for the deafness but, all above mentioned changes might have attributable to the mild hearing loss. Cohort and prospective studies with larger sample size are helpful in further analysis of the effect of diabetes on auditory pathway.
Conclusion
Hyperglycaemia in type 2 diabetes affects the functional status of structures along the auditory pathway. Early diagnosis of increased strength of acoustic threshold stimulus and functional delay in auditory conduction that is increased inter peak latencies, definitely helps the patients in taking the prompt prophylactic and therapeutic measures. These precautionary measures will help in enhancing the quality of life and also delaying the further complications.
Acknowledgements
Research reported in this publication was conducted by scholars at the Fogarty International Center of the NIH training program under Award Number D43 TW 009078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
References
- Said G. Diabetic neuropathy-a review. Nat Clin Pract Neurol 2007; 3: 331-340.
- Jardao AMD. Consideration sur un cas du diabete. Union medicale du paris 1875; 11: 446.
- Bainbridge K, Hoffman H, Cowie C. Diabetes and hearing impairment in the United States: Audiometric evidence from the National Health and Nutrition Examination Survey,1999 to2004. Annals of Internal Medicine 2008; 149: 1-10.
- Leonor Guariguata. International Diabetes Federation Diabetes Atlas. 6th Ed, 2013.
- Ciccone MM. Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss. Atherosclerosis 2012; 225: 511-516.
- Berjis N, Moeinimehr M, Hashemi SM, Hashemi SM, Bakhtiari EK, Nasiri S. Endothelial dysfunction in patients with sudden sensorineural hearing loss. Advanced Biomedical Res 2016; 5: 5.
- Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab 2014; 5: 364.
- Dobie RA, Van-Hemel SB. Assessment of the auditory system and its functions. Hearing loss: determining eligibility for social security benefits. The National Academies Press, Washington D.C. 2004, 75.
- Mosa LAA, Fedele D. The general use of glycated haemoglobin for the diagnosis of diabetes and other categories of glucose intolerance: Still a long way to go. Nutri Metabol Cardiovascular Dis 2011; 21: 467-475.
- Dalton DS, Cruickshanks KJ, Klein R, Klein BE, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care 1998; 21: 1540-1544.
- Ren J, Zhao P, Chen L, Xu A, Brown SN, Xiao X. Hearing loss in middle aged subjects with type 2diabetes mellitus. Arch Med Res 2009; 40: 1823.
- Sunkum AJ, Pingile S. A clinical study of audiological profile in diabetes mellitus patients. Eur ArchOtorhinolaryngol 2013; 270: 8759.
- Konrad-Martin D. Hearing Impairment in Relation to Severity of Diabetes in a Veteran Cohort. Ear Hearing 2015; 36: 381-394.
- Biswas A. Clinical Audio-Vestibulometry for Otologists and Neurologists.4th ed., Bhalanipublishing house, India, 2009, 115.
- Martini A, Comacchio F, Fedele D, Crepaldi G, Sala O. Auditory brainstem evoked responses in clinical evaluation and follow-up of insulin-dependent diabetic subjects. Acta Oto-laryngologica (Stockh) 1987; 103: 620-627.
- Toth F. Investigation of auditory brainstem functions in diabetes patients. Int Tinnitus J 2003; 9: 84-86.
- Al-Azzawi LM, Mirza KB. The usefulness of brainstem auditory evoked potential in early diagnosis of cranial neuropathy associated with diabetes mellitus. Electromyogr Clin Neurophysiol 2004; 44: 387-394.
- Diaz de Leon MLV, Jauregui RK, Garay SME, Hernandez PJ, Malacara HJM. Auditory impairment in patients with type 2 diabetes mellitus. Arch Med Res 2005; 36: 507-510.
- Gupta R, Mohd A, Hasan SA, Siddiqi SS. Type 2 diabetes mellitus and auditory brainstem responses- a hospital based study. Indian J Endocrinol Metab 2010; 14: 9-11.
- Habib SS, Husain A, Omar SA, Al Drees AM. Brainstem auditory evoked potentials and electrocochleographic findings in patients with idiopathic sudden sensorineural hearing loss. J Coll Physicians Surg Pak 2011; 21: 415-419.
- Dehan CP, Jerger J. Analysis of gender differences in the auditory brainstem response. Laryngoscope 1990; 100: 18-24.
- Ponton CW, Eggermont JJ, Coupland SG, Winkelaar R. The relation between head size and auditory brainstem response interpeak latency maturation. J Acoust Soc Am 1993; 94: 2149-2158.
- Alexander M, Thomas SV, Mohan PK, Narendranathan M. Prolonged brainstem auditory evoked potential latencies in tropical pancreatic diabetics with normal hearing. Electromyogr Clin Neurophysiol 1995 ; 35: 958.
- Hirose K. Hearing loss and diabetes: You might not know what you’re missing. Ann Intern Med 2008; 149: 54-55.
- Makishima K, Tanaka K. Pathological changes of the inner ear and central auditory pathway indiabetics. Annal Otolo Rhinol Laryngol 1971; 80: 218-228.
- Drel VR. New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine and tumor necrosis factor-α. Endocrinology 2010; 151: 2547-2555.
- Frisina ST. Characterization of hearing loss in aged type II diabetics. Hearing Res 2006; 211: 103-113.
- Kent S. Is diabetes a form of accelerated aging? Geriatrics 1976; 31: 140-151.
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol 2002; 441: 1-14.