- Biomedical Research (2007) Volume 18, Issue 3
Free excitatory postsynaptic densities: Morphological substrates for synapse formation and evidence of synapse elimination
Aijaz Ahmed Khan*
Department of Anatomy, J.N. Medical College, Aligarh Muslim University, Aligarh, India
- Corresponding Author:
- Aijaz Ahmed
Khan Department of Anatomy JN Medical College
Aligarh Muslim University Aligarh-202002
India
Phone: 0091-571-2720596
e-mail: aijazahmedkhan7 ( at ) hotmail.com
Accepted date: August 9 2007
Abstract
Nerve cells communicate primarily through chemical synapses. Study of brain development essentially consists of study of synapse formation. The role played by prospective pre-and postsynaptic processes in synaptic development is not fully understood. The present study was aimed at understanding the role of postsynaptic component is synaptogenesis. This study has been carried out on prenatal (13 to 35 weeks) human lateral geniculate nuclei (LGN) immersion fixed in Karnovsky?s fixative and processed for transmission electron mi-croscopy. It was observed that the period between 16 to 20 weeks of gestation is marked by occurrence of numerous, pleomorphic free postsynaptic densities (PSDs). These free sites were located on different parts of differentiating neurons. Many of them had no prospective presynaptic partners in their vicinity, while others revealed variable amount of incongru-ency with their presynaptic partners and in some occasions they were associated with degenerating profiles. This period also coincided with many other events previously reported in the developing human LGN, e.g., spurt in volumetric growth, glial cell differentiation, synaptogenesis, cytoarchitectonic lamination as well as neurochemical maturation. It was concluded that the free PSDs are heterogenous in origin, being programmed/de novo and waiting to make synaptic contact for the first time; and vacated, the left out sites after pre-synaptic terminal degeneration and waiting to establish new contact for the second time and that glial cells play important roles in the whole process of synaptogenesis
Synaptogenesis, Free postsynaptic density, growth cones, prenatal, lateral geniculate nucleus
Abstract
Nerve cells communicate primarily through chemical synapses. Study of brain development essentially consists of study of synapse formation. The role played by prospective pre-and postsynaptic processes in synaptic development is not fully understood. The present study was aimed at understanding the role of postsynaptic component is synaptogenesis. This study has been carried out on prenatal (13 to 35 weeks) human lateral geniculate nuclei (LGN) immersion fixed in Karnovsky’s fixative and processed for transmission electron mi-croscopy. It was observed that the period between 16 to 20 weeks of gestation is marked by occurrence of numerous, pleomorphic free postsynaptic densities (PSDs). These free sites were located on different parts of differentiating neurons. Many of them had no prospective presynaptic partners in their vicinity, while others revealed variable amount of incongru-ency with their presynaptic partners and in some occasions they were associated with degenerating profiles. This period also coincided with many other events previously reported in the developing human LGN, e.g., spurt in volumetric growth, glial cell differentiation, synaptogenesis, cytoarchitectonic lamination as well as neurochemical maturation. It was concluded that the free PSDs are heterogenous in origin, being programmed/de novo and waiting to make synaptic contact for the first time; and vacated, the left out sites after pre-synaptic terminal degeneration and waiting to establish new contact for the second time and that glial cells play important roles in the whole process of synaptogenesis.
Introduction
Most central synapses utilize neurotransmitters for interneuronal communication and are therefore called chemical synapses. They are of two types: 1) excitatory (type I, asymmetric, or glutamatergic) and 2) inhibitory (type II, symmetric or GABAergic). The presynaptic compartment of a chemical synapse is characterized by presence of synaptic vesicles and active zone (AZ) (an electron-dense presynaptic membrane where synaptic vesicles fuse) while the postsynaptic compartment is characterized by PSD-an electrondense thickening juxtaposed to AZ. The excitatory synapse differs from inhibitory synapse in many ways including requirement of extrinsic trophic factors during its development [1], presence of neuroligin 1 [2], and leaving free PSD after deafferentation.
Synaptogenesis is a multistage dynamic process and its each stage involves specific set of molecules for its elegant execution. Many neuron-specific molecules like synapsins [3,4] and neurexin [5] are preferentially associated with the presynaptic terminal while others like PDS-95 [6], neuroligin [2,7,5]; and synapsin III [8] with the post-synaptic terminal. Recently, many studies have stressed on the role of glia and glia-secreted soluble factors in syn-aptogenesis [9,10,11,12].
During early stage of axo-dendentritic synapse development, the axonal process (prospective presynaptic terminal) comes into contact with the dendritic process (prospective postsynaptic terminal) and makes a temporary contact (nascent synapse) which subsequently either gets eliminated or stabilized into a mature and functionally active connection. A nascent synapse is most likely to be a moving target – a continuum of transitional structures rather than single entity and therefore, pose difficulty in their identification with confidence, more so because the appearance of PSD differs with the technique used for its visualization [13]. PSD is an integral part of the postsynaptic signaling machinery. It organizes diverse structural and regulatory components into an elegant macromolecular assembly and within this may lie the clues to the mechanisms of the most intriguing of brain functions, including learning and memory. Therefore, a large number of studies have focused attention to unravel its molecular structure [6,2,14,5] and its possible roles in synaptic development and plasticity [5]. Though there is rapid formation and remodeling of PSD [15], and that the as-sembly of PSD is fundamentally different from that of the AZ assembly [16], temporal order of assembly of pre- and postsynaptic compartment has drawn sparse attention [17]. Free PSDs have been reported in adults after experimental deafferentation in the visual system [18], cerebellum [19] and septal nuclei [20] and in hippocampus during estrous cycle [9]. However, their occurrence in abundance during critical period of prenatal development [21] has not drawn much attention and hence remains an unresolved issue. Therefore, the present study was aimed at examining these free PSDs for their possible nature, role and fate during synaptogenesis as well as to seek correlation with other reported relevant events including glial cell differentiation in the establishment of interneuronal contacts.
Material and Method
The study was conducted on human fetuses with prior permission from an ethics committee and consent of the parents. A total of 21 fetuses of both sexes ranged in gestational ages from 13-14 to 34-35 weeks were included in this study. The fetuses were collected from hysterotomies (13-14 to 20-21 weeks) or from autopsies (25-26 and 34-35 weeks) where foetal death occurred due to spontaneous abortion. The LGN were dissected out and immersion fixed in Karnovsky’s fixative for a period of two weeks and processed for araldite embedding. Ultrathin silver grey (70-80 nm) sections stained with uranyl acetate and lead citrate were used for transmission electron microscopic observations. Foetal data is given in our earlier publications [22,21].
Observations
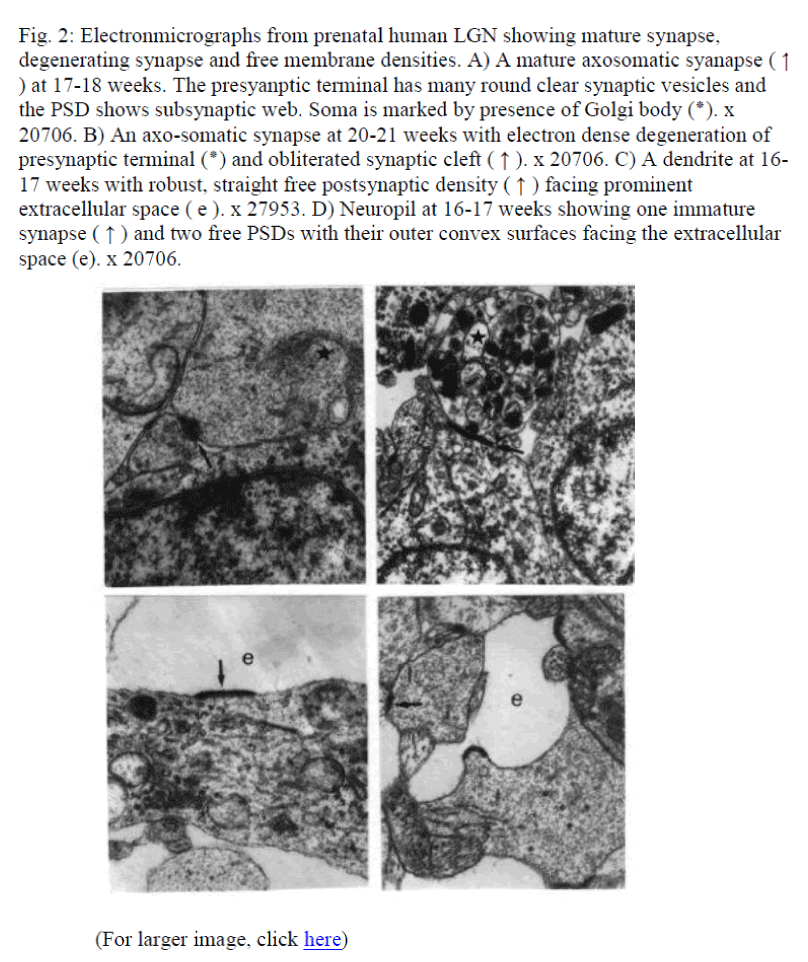
Cell and neuropil maturation. At early age of 13-14 weeks, cells are immature and both neurons and glia look alike. Only by the age of 16-18 weeks glial cells may be identified. The neuronal cell bodies during 16-21 weeks reveal tubulovesicular whirls and convolutions of membranes (Fig. 1A, 1B). Neuropil had multiple growth cones (GCs) containing pleomorphic growth vesicles, free membrane densities and frequent synaptic contacts at different stage of maturation (Fig. 1C, 1D). By 20-21 weeks the neuropil is more packed and many mature synapses (Fig. 2A) as well as some degenerating synaspes (Fig. 2B) on dendrites (Fig. 2C, 2D) and soma and synaptic conttacts are quite frequent. GCs make multiple contacts. The PSDs remained intact even in samples obtained from autopsy performed within 4 hours of death.
Fig. 1: Electronmicrographs from prenatal human LGN showing cytoplasmic organelles and growth cones making synaptic contacts. A) Neuronal soma at 17-18 weeks showing tubulovesicular convolutions ( ↑ ) suggestive of Golgi body with TGN-organelles at its centre. X 27953. B) Neuronal soma at 20-21 weeks showing two Golgi bodies placed side by side making a figure of δ or 8 ( ↑ ) and their tubulovesicular arrangements making half and complete rings with TGN-organelles at their centre. C) Neuropil at 15-16 weeks showing axonal (a) and dendritic (d) growth cones making a nascent synapse ( ↑ ). A free membrane density (*) on dendritic process and prominent extracellular space (e) are also seen x16565. D) Neuropil at 16-17 weeks showing an immature axodendritic synapse ( ↑ ) between an axonal process with few synaptic vesicles and a denditic growth cone (*).
Fig. 2: Electronmicrographs from prenatal human LGN showing mature synapse, degenerating synapse and free membrane densities. A) A mature axosomatic syanapse ( ↑ ) at 17-18 weeks. The presyanptic terminal has many round clear synaptic vesicles and the PSD shows subsynaptic web. Soma is marked by presence of Golgi body (*). x 20706. B) An axo-somatic synapse at 20-21 weeks with electron dense degeneration of presynaptic terminal (*) and obliterated synaptic cleft ( ↑ ). x 20706. C) A dendrite at 16-17 weeks with robust, straight free postsynaptic density ( ↑ ) facing prominent extracellular space ( e ). x 27953. D) Neuropil at 16-17 weeks showing one immature synapse ( ↑ ) and two free PSDs with their outer convex surfaces facing the extracellular space (e). x 20706.
Synaptic contacts: At 13-14 weeks the synapses are only few, simple, immature and exclusively asymmetric (excitatory) in nature. By the age period of 16-18 weeks growth cones and excitatory synapses are found in abundance. Some of them reveal subsynaptic web as well (Fig. 2A). By 20-21 weeks the additional features noticed are axosomatic synapses, synaptic triads and increased incidence of free PSDs. At 25-26 weeks and onwards, though the frequencies of complex synapses increased but somatic synapses as well as free PSDs are infrequent.
Free PSDs: Morphologically, these resemble the PSDs of a classical asymmetrical, (excitatory) chemical synapse but remain unaccompanied with appropriate presynaptic profiles (Fig 2C, 2D). These PSDs are focal, welldefined, electron dense membrane specializations. They vary in their forms, sizes and locations, i.e., both on the soma and dendrites as well as on GCs. Some PSDs are in apposition with one or more profiles (prospective presynaptic/ otherwise), variably incongruent with each other (Fig.2D). They are also located in isolated areas with no other profiles in their vicinity (Fig. 1C, 2C, 2D). Occasionally, PSDs are found to be associated with degenerating pre-synaptic terminals (Fig 2B). The cross sectional contours of PSDs vary from being straight (Fig. 2C), wavy, convex (Fig. 2D), concave, and concavoconvex. Some elaborate features like subsynaptic web in relation to the PSD were not observed. The presynaptic profiles (committed/ otherwise), reveal variable features with respect to its congruency with PSD, membrane specializations; number of vesicle present and their location with respect to AZ.
Discussions
The present study is a part of the work conducted on the developing human visual system dealing with its cytoarchetectural, and neurochemical maturation as well as synaptogenesis in LGN. Like other parts of nervous system, the development of LGN involves both progressive and regressive events. Synaptogenesis is a highly dynamic process. Its beginning is heralded by formation of a nascent synapse when prospective pre and postsynaptic elements come together. The retinal afferents enter the human LGN by 7-8 weeks of gestation [23]. The first retinogeniculate synapse appears at 13-14 weeks [18, 24]. Gliocytes differentiate by 16-17 weeks [22], spurt in syn aptogenesis between 15-20 weeks [21] and cytoarchitectonic lamination at 20-21 weeks [25, 22]. Like many other brain regions [26] LGN also shows critical period in synaptogensis [21], which is15-20 weeks and is marked by concurrence of spurt in its volumetric growth [21], peak afferent inputs [27,28], substance P positive fibres [29], and GABA positive neurons [30].
The peculiar membranous arrangements (Fig 1A, 1B), also noticed in the neuronal soma around 16-18 weeks [21] were thought to be associated with high protein turn over [24]. However, a fresh look on these structures in the light of current literature [31 suggests that in fact, quite few of them are trans-Golgi network (TGN) which has been shown to carry proteins both for PSD and AZ at site of development of new synapse. As the last station of the Golgi complex, the TGN plays a pivotal role in directing proteins in the secretory pathways to the appropriate cellular destination [32]. GCs are specialized sensory structures located at the tip of neurites. In the present study GCs were found in abundance between 15-21 weeks. This period is marked by spurt in synaptogenesis which requires active growth of neurites towards their prospective synaptic partners. Synapsin III concentrated in GCs plays a role in axonal elongation [8] and interaction of actin and myosin filaments [33] and actin polymerization and de-polymerization act as force generating system [34]. Studies seeking dynamic relationship between dendritic growth and synaptogenesis suggest a „synaptotropic model’ in which synapse formation can direct dendritic arborization [35]. That is, functionally viable synapses determine the branching pattern of dendrites.
Differentiation of PSD region appears to be a prerequisite for development of synapse. Neuroligin 1 is clustered in the PSD [2], can alone stimulate formation of presynaptic specializations [36, 37] and neuroliginneurexin link triggers synapse formation [7]. PSD in mature synapse is a complex organelle directing various cellular functions in order to integrate synaptic physiology. Although a major proportion of PSD may appear and disappear within few minutes [15], even in immature synapses it is highly organized [38]. In the present study it is most likely that the high frequency of free PSDs during 16- 20 weeks of gestation might have resulted by two mechanisms: 1) As a reminiscent of fully developed synapses, in which the presynaptic terminal degenerated (vacated PSDs). And, possibly due to prompt handling of degeneration debris by glia, the high incidence of free PSDs was not associated with comparable incidence of degenerating terminals. And 2) PSDs formed de novo as a part of general synaptogenic process (programmed PSDs), as they were observed even in the total absence of presynaptic profile in their vicinity. The dual nature of free PSDs has also been suggested in case of experimental deafferentation of cerebellum [19]. Though morphologically, all PSDs look almost alike as electron dense structures, structurally they may be different with respect to laminar organization of their various PSD protein constituents [39, 40, 41, 42, 43], with possibility of further change in response to neural activity to match the identities of their axonal counterparts [44]. Latest studies on the measurement of mass of PSD and enumeration of key molecules [45], suggest that meticulous morphological analysis of synapses under TEM holds promise to give insight into the evolution of receptors composition in the PSD during development.
Until very recently, the role of glia in synaptogenesis remained speculative, but now the picture is becoming clearer. In developing human LGN the glia differentiate by 16-17 weeks of gestation with spurt in synaptogenesis at 16-18 weeks [21] indicating concurrence in two events. A revisit to these observations in the light of current literature, for instance, association between astrocytic processes and estradiol levels [46], steroiddependent synapse turnover and incidence of free PSD [9] appears interesting. Observation in the present study is also in agreement with the one [11] suggesting that in vivo, most synapses are generated concurrently with development of glia and that they appear to induce and stabilize the plastic and immature synapse Now, many gliaderived molecules for example, neuroligin [10], cholesterol [47, 48], thrombospondin [12] and neurosteroids [49] are known to play important roles in synaptogenesis and neuronal circuit maturation. Thus, it underlines the significance of concurrent study on glia to make the study on various parameters of neurons more meaningful.
There is increasing evidence of roles played by neurotransmitters and neurotrophic factors in synaptogenesis. The observation of accelerated pace of synaptogenesis during 16-18 weeks is consistent with large number of substance P positive afferents [29], and GABA immunopositive neurons [30]. This assumed significance due to dual role of GABA [50] and nitric oxide [51] as synaptic transmitter and promoter of synaptogenesis. Moreover, in contrast to mature brain, where GABA is the major inhibitory neurotransmitter, in developing brain GABA can be excitatory, and glutamate may play inhibitory role in modulating the calcium elevating actions of GABA that may affect many stages of neuronal differentiation including synaptogenesis. Role of insulin [52] in promoting function of silent synapses, and those of progesterone [53] and neurosteroids [49] in neuronal circuit maturation via autocrine and /or paracrine action underline the significance of hormonal profile during development nervous system. Recent studies [54] have implicated neuroligins in mental retardation and PSD-95 in pain modulation [55], learning and addiction.
It is concluded that both pre-and post-synaptic profiles provide indispensable and interdependent contribution in the formation of mature synapse. There are at least two subsets of PSDs namely programmed and vacated but in the absence of suitable presynaptic profiles and specific immunolabelling their distinction and quantification is rather difficult. In case these PSDs fail to find suitable partners they possibly disappear after a waiting period of few weeks. Glial cells play important roles throughout the whole process of synaptogenesis.
Acknowledgement
I gratefully acknowledge the financial support and laboratory facility provided by All India Institute of Medical Science, New Delhi.
References
- Hamakawa T, Woodin MA, Bjorgum MC, Painter SD, Takasaki M, Nagle GT and Syed NI. Excitatory synaptogenesis between identified Lymnea neuron requires extrinsic trophic factors and is mediated by receptor tyrosine kinase. J Neurosci 1999; 19: 9306-9312.
- Song JY, Ichtchenko K, Sudof TC, and Brose N. Neu-roligin 1 is a postsynaptic celladhesion molecule of excitatory synapse. Proceedings of Natl Acad Sci USA 1999; 96: 1100-11055.
- Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T, Takahashi T and Hirokawa N. Synapsin I deficiency results in the structural change the presynaptic terminals in the murine nervous system. J Cell Biol 1995; 131: 1789-1800.
- Gitler D, Takagishi Y, Geng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P and Augustine GA. Differen presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci 2004; 24: 11368-11380.
- Washbourne P, Ditytev A, Scheiffle P, Briederer T, Weiner JA, Christopherson KS, El-Husseini A. Cell adhesion molecules in synapse formation. J Neurosci 2004; 24: 9244-9249.
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl T, Sudhof TC. Binding of neuroligins to PSD-95. Science 1997; 277: 1511-1515.
- Georgiev, Danko D. Binding of neuroligins to PSD-95. Science 2003; 277: 1511-1515.
- Ferreira A, Li L, Chin LS, Greengard P, Kosik KS. Postsynaptic element contributes to the delay in synaptogenesis in synapsin I-deficient neurons. Mol Cell Neurosci 1996; 8: 286-299.
- Desmond NL, Levy WB. Free postsynaptic densities in the hippocampus of the female rat. NeuroReport 1998; 9: 1975-1979.
- Gilbert MM, Smith J, Roskams AJ, Auld VJ. Neuroligin 3 is expressed in wide range of glia during development. Dev. Biol 2000; 222: 256.
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science 2001; 291: 257-258.
- Freeman MR. Glial control of synaptogenesis. Cell 2005; 120: 292-293.
- Ahmari SE, Smith SJ. Knowing a nascent synapse when you see it. Neuron 2002; 34: 333-336.
- Bolliger MF, Free K, Winterhalter KH, Gloor SM. Identification of a novel neuroligin in humans which binds to PSD-95 and has a widespread expression. Bio-chem J 2001; 356: 581-588.
- Marrs GS, Green SH, Dailey ME. Rapid formation, remodeling of postsynaptic densities in developing dendrites. Nature Neurosci 2001; 4: 1006-1013.
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. Neurosci 2004; 24: 1507-1520.
- Vardinon-Friedman H, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapse- Time course and temporal order of synaptic molecule recruitment. Neuron 2000; 27: 5779.
- Ralston HJ III, Chow KL. Synaptic reorganization in the degenerating lateral geniculate nucleus of rabbit. J Comp Neurol 1973; 147: 321-349.
- Hamori J. “De novo” formation of synapses by experimentally induced presynaptic dendrites in adult mammalian brain. Acta biol Acad Sci hung 1982; 33: 173-187.
- Raisman G, Field PM. Synapse formation in the adult brain after lesion and after transplantation of embryonic tissue. J Exp Biol 1990; 153: 277-287.
- Khan AA, Wadhwa S, Bijlani V. Development of human lateral geniculate nucleus: an electronmicroscopic study. Int J Dev Neurosci 1994; 12: 661-672.
- Khan AA, Wadhwa S, Pandey RM, Bijlani V. Prenatal human lateral geniculate nucleus: a quantitative light-microscopic study. Dev Neurosci 993; 15: 403-409.
- Cooper ERA. The development of human lateral geniculate body. Brain 945; 68: 222-237.
- Teuchert-Noodt G, Breuker K-H, Dawirs PR. Neuronal lysosome accumulation in degrading synapses of sensory motor and limbic systems in the duck Anas platyrhynchos: indication of rearrangements during avian brain development Dev Neurosci 1991; 13: 151-163.
- Wadhwa S, Bijlani V. Cytodifferentiation and develop-ing neural circuitry in the human lateral geniculate nucleus. Int J Dev. Neurosci 1988; 6: 59-75.
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci 2000; 20: 4954-4961.
- Provis JM, van Driedel D, Billson FA, Russel P. Hu-man foetal optic nerve: Over production and elimination of retinal axons during development. J Comp Neurol 1985; 238: 92-100.
- Wadhwa S, Bijlani V. Developing human optic nerve in prenatal period: changes in the number of retinal axons. Ind. J Ophthalmol 1987; 35: 11-16.
- Wadhwa S, Rizvi TA, Bijlani V (1988a): Substance P immunoreactivity in the developing human retinogeniculate pathway. Neurosci Lett. 89: 25-30.
- Wadhwa S, Takacs J, Bijlani V, Hamori J. Numerical estimates of GABA immunoreactive neurons in the human lateral geniculate nucleus in the prenatal period. Human Neurobiol 1988b; 6: 261-262.
- Sytnyk V, Leshchyns’ka I, Delling M, Dityatev A, Schachner M. Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. Cell Biol 2002; 159: 649-661.
- Young B, Lowe JS, Stevens A, Heath JW. Cell structure and function. In Wheater’s Functional Histologya text and colour atlas, 5th Ed.Churchill Living stone. 2005; P2-32.
- Bridgman PC. Growth cones contain myosin II bipolar filament arrays. Cell motility and Cytoskeleton 2002; 52: 91-96.
- Ribak CE, Korn MJ, Shan Z, Obenaus A. Dendritic growth cones and recurrent basal dendrites are typical features of newly generated dendate granule cells in adult rat hippocampus. Brain Res 2004; 12: 195-199.
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nature Neurosci 2004; 7: 254-260.
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers pre-syanptic development in contacting axons. Cell 2000; 101: 657-669.
- Girod R, Jereb M, Moss J, Role W. Mapping of pre-synaptic acetylcholine receptors using fluorescence imaging of neuritic calcium. J Neurosci Methods 2003; 122: 109-122.
- Petralia RS, Zhao HM, Wang YX, Wenthold RJ. Varia-tion in the tangential distribution of postsynaptic glutamate receptors in Purkinje cell parallel and climbing fibres synapses during development. Neuropharmacol 1998; 37: 1321-1334.
- Kennedy MB, Bennett MK, Erondu NE. Biochemical and immunochemical evidence that the “major postsynaptic density protein” is a subunit of a calmodulin-dependent protein kinase. PNAS 1983; 80: 7357-7361.
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci 2001; 21: 1211-1217.
- Bockers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gunderfinger ED, Kreienkamp HJ. Synaptic scaffolding proteins in rat brain. J Biol Chem 2001; 276: 40104-40112.
- Bockmann J, Kreutz MR, Gundelfinger ED, Bockers TM. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem 2002; 83: 1013-1017.
- Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF, Bockaert J, Fagni L. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci 2005; 25: 3560-3570.
- Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat Neurosci 2001; 4: 569-578.
- Chen X, Vinade L, Leapman RD, Petersen JD, Naga-kawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Published online 2005; 10.1073/ pnas .050- 5359102.
- Klintsova A, Llevy WB, Desmond NL. Astrocytic volume fluctuates in the hippocampal region CA1 across the estrous cycle. Barain Res 1995; 690: 269-274.
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001; 294: 1354-1357.
- Goritz C, Mauch DH, Nagler K, Pfrieger FW. Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse-glia affair. J Physiol Paris 2002; 96:257-263.
- Mameli M, Carta M, Patridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci 2005; 25: 2285-2294.
- Wolff JR. Dual role of GABA as synaptic transmitter and promoter of synaptogenesis. In Aminoacid Neuro-transmitters (eds De Feudis VA, Mandell P), Raven Press, New York 1981; pp 459-466.
- Ogilvie P, Schilling K Billingsley ML, Schmidt HH. Induction and variants of neuronal nitric oxide synthase type I during synaptogenesis. The FASEB Jouran 1995; 9: 799-806.
- Plitzko D, Rumpel S, Gottmann K. Insulin promotes functional induction of silent synapses in differentiating rat neocortical neurons. Eur J Neruosci 2001; 14: 1412-1415.
- Sakamoto H, Ukena K, Tsutsui K. Effects of progester-one synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. Neurosci 2001; 21: 6221-6232.
- Philbert RA, Winfield SL, Sandhu HK, Martin BM, Ginns EL The structure and expression of the human neuroligin-3 gene. Gene 2000; 246: 303-310.
- Tao HW, Poo MM. Retrograde signaling at central synapses. Proced Natl Acad Sci 2001; 98: 11009-110-15.