Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2024) Volume 14, Issue 103
Formulation and invitro evaluation of nabumetonepulsatile drug delivery
Chaitanya Kamanla1*, A.V Jithan21Department of pharmaceutics, Osmania University, Hydreabad Telangana, India
2Department of Technology Consultants, Hyderabad, Telangana, India
- *Corresponding Author:
- Chaitanya Kamanla
Department of pharmaceutics
Osmania University
Hydreabad Telangana, India
E-mail: chaitanyachaitu2149@gmail.com
Received: 27-Dec-2023, Manuscript No. AABPS-23-123909; Editor assigned: 01-Jan-2024, PreQC No. AABPS-23-123909(PQ); Reviewed: 15-Jan-2024, QC No. AABPS-23-123909;
Revised: 12-Jan-2024, Manuscript No. AABPS-23-123909(R); Published: 29-Jan-2024, DOI:10.35841/aabps-14.103.217
Citation: Kamanla C. Formulation and invitro evaluation of nabumetonepulsatile drug delivery. Asian J Biomed Pharm Sci. 2024;14(103):217
Abstract
The researchers have been honing an oral, cytoclear, pulsatile drug delivery system using nabumetone as a model medicine. Nabumetone is a nonsteroidal anti-inflammatory medication used to treat rheumatoid and osteoarthritis-related inflammation. The key ingredient is a hydrogel plug secured to the insoluble hard gelatin compartment before the powder mixture is added. Powder mixtures including nabumetone, croscarmellose sodium, Ludiflash, Lycoat, MCC, and powder were analyzed for explosive properties and FTIR spectrums. Based on the results, we decided to ramp up production of the F12 powder mixing method-based pulsing holders. Acceptable margins are maintained by immersing hydrogel plugs in a 1:1, 1:2, or 2:1 mixture of hydrophobic polymers like ethylcellulose and hydrophilic polymers like HPMC K15M. The dependability of the guaranteed discharge was directly proportional to the amount of polymer utilized. We investigated the impact of varying formulation weights, active component concentrations, and in vitro release studies. Increases in the concentration of hydrophilic polymer lead the pulsation to increase over the recommended withdrawal period of 9 hours, as evidenced by FTIR analysis and in vitro release trials of pulsating devices. Nabumetone took longer than planned to release from the capsule. In vitro tests demonstrated the high clarity of P5F12.
Keywords
Time course medicine, In vitro release investigations, Pulsatile mechanism, Sub-delivery nabumetone.
Aim and Objective
The place of the ongoing survey is to design and evaluate pulsincap portion structure containing Nabumetone a NSAID used to treat osteoarthritis and rheumatoid joint irritation
Objective
A controlled pulsatile temporal drug delivery schedule for nabumetone was created to postpone the beginning of prescription and ease the medicine's eventual introduction.
To prevent him from abusing his prescription, have him take the reduced dose of his normal treatment only once during the downtime.
Patients may be treated with kindness and consistency.
Wise polymer selection for connection definition to account for stop time; centralized control of super-explosives for coordinating Parsincap medication distribution.
Casing body formaldehyde treatment timing is critical for subsequent monitoring since it renders the casing body water insoluble.
Materials and Methods
Materials
Displays the many excipients and chemicals used to improve the list, including polymers, programming connection points, and more, (Table 1).
| Sl. no. | Materialsused | Grade | Manufacturer |
|---|---|---|---|
| 1. | Nabumetone | PharmaGrade | Kreysun Pharmaceutical Private Limited, Hyderabad |
| 2. | Ludiflash | LR | SdfinechemicalLtd, Mumbai |
| 3. | Lycoat | LR | SdFinechemicalLtd, Mumbai |
| 4. | Microcrystalline cellulose | LR | Lobachemiepvt.ltd |
| 5. | Talc | LR | Lobachemiepvt.ltd |
| 6. | Ethyl cellulose | LR | Otto Chemicals, Mumbai |
| 7. | HPMC K15M | LR | Otto Chemicals, Mumbai |
| 8. | Magnesium sterate | LR | Lobachemiepvt.ltd, Mumbai |
| 9. | Formaldehyde | LR | Qualigensfinechemicals,Mumbai |
| 10. | Potassium permanganate | LR | Qualigensfinechemicals,Mumbai |
| 11. | Hydrochloricacid | LR | SdfinechemicalLtd,Mumbai |
| 12. | Potassium Dihydrogen Phosphate | LR | Qualigensfinechemicals,Mumbai |
| 13. | Methanol | LR | SdfinechemicalLtd, Mumbai |
| 14. | Sodiumhydroxidepellets | LR | Qualigensfinechemicals,Mumbai |
Table 1: Listofchemicalsusedwithgradeandsupplier
Equipment’s
lists the many pieces of machinery involved in formula creation, (Table 2).
| S.No. | Instrument | Manufacturer |
|---|---|---|
| 1. | U.V.visiblespectrophotometer | T-60 UV-Visible Spectrophotometer. |
| 2. | FTIRspectrophotometer | IR-Affinity-1,Shimadzu,Japan. |
| 3. | Dessicator | Remimotors,Ahmedabad. |
| 4. | Mechanicalstirrer | Remimotors,Ahmedabad |
| 5. | Electronicbalance | CitizenscalesPvt.Ltd. |
| 6. | DigitalpHmeter | DigisunElectronics, Hyderabad. |
| 7. | USPdissolutionapparatus | LABINDIA DS 8000 |
| 8. | Disintegrationapparatus | Elecrtolab |
Table 2: ListofInstrumentsused
Methodology
Preformulationstudies
Methods
When developing novel methods of dispensing medicine, this is an important consideration. Prior to production, the formulation was subjected to solubility, melting point, and compatibility testing.
Solubility
From a pH of 1.2 to a pH of 7.4, Nabumet remains completely soluble. To test the compound's solubility, large quantities of Navmet One were dissolved in several solvents. For 24 hours, components were shaken together at a normal pace. Class No. 41 What Man's Channel paper was used to map out the team's game plan. Spectrophotometric analysis at 332 nm was used to evaluate the different approaches.
Spectroscopic study
Determination of absorption maximum (λmax)
Radiation exposure is most obvious at its peak frequency, marked by the symbol max. Each chemical has its own maximum that complements its properties. Precise smart work requires choosing the material's maintenance maxima. Most drugs absorb light in the excellent area (190-390nm) because of their attractive smells or the presence of double bonds.
Extraction of Nabumetone in 7.4pH buffer in a sterile 10 mL volumetric cup, weighed to the milligram. Filling a 10 ml volumetric flask with 7.4pH buffer yielded stock plan I with a central concentration of 1000 g/ml.
Transferring 1 ml of Stock Plan I vial content using a pipette to a 10 ml volumetric carafe. By adding 7.4pH buffer to 10 ml of stock plan II, we were able to achieve a concentration of 100 g/ml at the core. Taking a 1 ml stock from stock plan II and pipetting it into a 10 ml volumetric container. After diluting the sample to a final amount of 10 ml with 7.4 pH buffer, the concentration was determined. Analysis of the process from 200 to 400 nm using a UV-Observable double-shaft spectrophotometer allowed us to determine the osmosis maximum (max).
The freezing point of nabumet one was determined using just a few, very specific methods. Fine powder of Nabumet one was filled in glass capillary tube (previously sealed at one end).The thermometer was put in the Thais tube, and the capillary tube was lit fire. The powder at what temperature it melted was observed.
Calibration curve
Preparationof StandardCalibrationCurveofNabumetone in 0.1 N HCL
A 10 mL volumetric vial was carefully filled with 10 mg of nabumetone after weighing. The 0.1 N HCL separation and concentration procedure produced a 1000 g/ml stock solution.
By serially diluting the normal stock solutions of nabumetone with 0.1 N HCL vehicle, concentrations from 5 to 30 g/mL were achieved. Using a UV spectrophotometer set at 332 nm and a blank of 0.1 N HCL, the absorbance of the solution was calculated. Absorbance readings were graphed against concentration (g/mL) to ensure consistent array rotation.
After precisely transferring 10 mg of nabumetone into a 10 ml volumetric flask, we used this solution to generate a standard calibration curve for nabumetone on a phosphate carrier at pH 7.4. Phosphate buffer (pH 7.4) was used to dilute the substance and create a stock solution of 1000 g/ml by crushing the material.
The standard stock solution of nabumetone was diluted serially in a phosphate buffer with a pH of 7.4 to obtain a concentration range of 5-30 g/mL. The absorbance of the solution was determined at 332 nm using a UV-detectable spectrophotometer and compared to a blank of pH 7.4 phosphate buffer. A sequence twist was determined by plotting concentration (in g/mL) against absorbance value [1-3].
Compatibility Studies
FTIR analysis
A Shimadzu 8400 S FTIR spectrometer was used to concentrate the molecules of the medicinal polymer. To the KBr dry material, 2% potassium bromide (SD Fine Chem. Ltd., Mumbai, India) was added. After grinding the components into a fine powder in a mortar, he filled KBr discs with the mixture and pressed them at around 10,000 PSI in a hydraulic press. Individual 2 cm1 Happ-Genzel apodization screenings were performed on each KBr circle. T-shirts with the company logo are also provided [4-6].
Flow properties of API
Bulk Density (Db)
It is found by dividing the entire mass of the powder by its total volume. A measured amount of powder (after passing through a standard No. 20 screen) was buried in the evaluation chamber and its weight was recorded. This basic volume is what we mean when we talk about mass. This explains why the mass was off when I followed the instructions below.
Db = m/Vo
Where,
m = mass of the powder
Vo = bulk volume of powder
Tapped density (Dt): It's the extracted powder volume divided by the total powder mass. The volume of the powder was measured by tapping it for different lengths of time. We then took measurements of the tapped volume at various times (within a 2% margin of error). If the figure is more than 2%, then the button has been tapped several times. It's spread in g/cc by means of:
Dt = m/Vi
Where,
m = mass of the powder
Vi = tapped volume of powder
Angle of Repose (θ)
This is the optimum height above the horizontal plane for a stack of powder or grains. Powders were permitted to freely move by placing a channel in a stand at eye level (h). The ultimate resting place is not set in stone but rather determined by the height and breadth of the granule pile [7-9].
Tan θ= h/r
(Or)
θ= tan-1 (h/r)
Where, θ = angle of repose
h = height of the heap
r = radius of the heap
Compressibility Index: Mass thickness (Db), tapped thickness (Dt), and packing down rate may all be used to evaluate the powder's flowability. The reasons why compressibility is still a matter of debate are:
Compressibility index (%) = Dt – Db/Dt x 100
Unlike the previously described formulae, which identify the fixed characteristics of a stream, the following standard criteria may be used to quantify the kind of stream produced by a programming connection point alone or its surrounding excipients, i.e., powder,(Table 3).
| S.No | Flow property | Angle of repose | Carr’s Index | Hausner’s ratio |
|---|---|---|---|---|
| 1. | Excellent | 25-30 | <10 | 1.00-1.11 |
| 2. | Good | 31-35 | 11-15 | 1.12-1.18 |
| 3. | Fair | 36-40 | 16-20 | 1.19-1.25 |
| 4. | Passable | 41-45 | 21-25 | 1.26-1.36 |
| 5. | Poor | 46-55 | 26-31 | 1.35-1.45 |
| 6. | Very poor | 56-65 | 32-37 | 1.46-1.59 |
| 7. | Very very poor | >66 | >38 | >1.6 |
Table 3: Standard specifications for comparison of flow properties
Preparation of Cross-Linked Gelatin Capsule
Formaldehyde treatment
About100 hard gelatin capsules size '0' were taken. Their bodies were separated from the capsand placed on a wire mesh. On a network of wires, the corpses were spread out in a single layer. A formaldehyde strategy of 15% v/vo was prepared and stored in a desiccator, yielding 25 ml. To make it better, 5 grams of potassium permanganate were added. A mixture of formaldehyde liquid and smoke was poured into the desiccator, and the wire mesh lid covering the box's interior was then securely fastened [10-12] The dwellings were subjected to formaldehyde exhaust fumes during varying amounts of time, from 2 hours to 10 hours. Casings were treated in an open environment to remove residual formaldehyde after being dried on Keepona channel paper for 24 hours to guarantee the creation of a vapor bond between gelatin and formaldehyde. These suitcase frames were preserved in polythene bags, despite having untreated lids.
Use of Formaldehyde treatment
Formaldehyde treatment was first developed to facilitate the softening of hard gelatin cases. Gelatin molecule cross-association was triggered by the injection of formalin vapor. A "Schiff's base development" is evoked, whereby the amino groups for the sub-nuclear chain of gelatin connect with aldehyde social occasions, rendering the gelatin impermeable in water. In the presence of formaldehyde, gelatin forms a compound that cannot be broken down [13]. The essential amine bundle in gelatin reacts with formaldehyde, fusing the two substances together irreversibly. Potassium permanganate was added to the formaldehyde recipe for this purpose. Several kinds of hard gelatin case were insoluble in water after being exposed to formaldehyde exhaust in a sealed dessicator for varied durations of time.
Preparation of NabumetoneTablet for Filling Into Capsules
Before being included, each component was screened using a #60 lattice screen. The medication and MCC were combined gradually until a uniform mixture was achieved, and then stored away.
Next, the remaining pieces were logically assembled, sieved through a coarse lattice (#44), and compressed in a water-powered press to form tablets [14]. The machine's pressure force was adjusted such that the hardness of all clumps fell between 3 and 4 kg/cm2. All eight formulations (F1–F8) had the same 100mg tablet weight, (Table 4).
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nabumetone (Equivalent to 500 mg) | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Ludiflash | 4 | 8 | 12 | 16 | -- | -- | -- | -- | -- | -- | -- | -- |
| Lycoat | -- | -- | -- | -- | 2 | 4 | 6 | 8 | -- | -- | -- | -- |
| Croscarmellose sodium | -- | -- | -- | -- | -- | -- | -- | -- | 2 | 4 | 6 | 8 |
| MCC | 115 | 111 | 107 | 103 | 115 | 111 | 107 | 103 | 115 | 111 | 107 | 103 |
| Mg. sterate | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Talc | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
Table 4: Formulation tablefor preparation of blend for filling ofNabumetonepulsincap
Formulation of Pulsincapof Nabumetone
The improved delivery method employs 4mg Nabumetone pulsincaps, which were formulated using different excipients and polymers in varied quantities. Disintegration time had a large role in the selection of formaldehyde-treated case bodies implanted at 6 hours for the pulsincap definition. Nabumetonetablet's new meaning was laser etched into the outside of the bottle. In order to define the hydrogel plug, several concentrations of Ethyl cellulose and HPMC K15M were used. Initial fitting parameters were a constant 100 mg weight and 1:1, 1:2, and 2:1 ratios of hydrophobic to hydrophilic polymer.
Method of preparation of Pulsincap dosage form
Preparation of powder blend
Hard gelatin'size 0' cases, selected for their attention to detail, were hardened by being treated with formaldehyde for 6 hours. Thebodiesandcapsseparatedmanually. The base of the case now has upgraded F4 details.
Preparation of Hydrogel plug
The opening of the bottle was sealed with a tablet-shaped plug. The contents of the case were kept safe by a cover.
Polymers like ethyl cellulose and hydroxypropyl methyl cellulose were combined at varying quantities to create a hydrogel stopper.
Hydrophilic hydroxypropyl methyl cellulose (HPMC) was blended with the hydrophobic ethyl cellulose.
Water might get into the container, diluting the medications within, before the fitting material completely deteriorated and let the water in. If all went according to plan, the delivery medium would just need to dissolve the fitting at the point of contact.
Multiple mechanisms, such as the plug's expansion in water, movement in response to increasing inner tension, disintegration, or protein breakdown, should allow for its release.
Capsule filling
A filling process was used to inject a homogeneous combination of prescription and excipients into a case body that had been treated with formaldehyde for 6 hours.
The hydrogel stopper is inserted into the case like a tablet and laid on top of the contents.
The top of the case served as a closure.
Evaluation of tablets
Tablet Dimensions
A vernier caliper that had been properly calibrated was used to confirm the thickness and the approximation. The thickness of three randomly selected tablets from each formulation was recorded.
Hardness
The hardness of a tablet indicates how well it can withstand mechanical shocks, such as being dropped. The tablets' hardness was tested using a Monsanto hardness tester. The unit of measurement is kg/cm2.The difficulty of swallowing was tested with three pills chosen at random.
The tablets' friability was measured using an electrolabfriabilator. A rate is used to represent the value. A weight pointer (WI) was used to load 10 pills into a riabilator. The ice chest was shaken at a rate of 25 times per second for a whole four minutes. The drug contributed an additional weight (WF). Using the formula % F = 100 (1-WI/WF%), we can see that there is still some pliability present. Tablets with a friability of less than 1% were deemed suitable for usage [15-17].
Weight Variation Test
To determine whether there was a statistically significant variation in total weight, ten pills were chosen at random from each batch and weighed. The US Pharmacopeia examined alterations to a tablet's weight. It was OK to make such drastic changes to one's weight, (Table 5).
| Average weight of a tablet | Percentage deviation |
|---|---|
| 130 mg or less | ±10 |
| >130mg and <324mg | ±7.5 |
| 324 mg or more | ±5 |
Table 5: Weight variation
Assuming a tablet weight of 100 mg across all formulations, a maximum variation of 10% is permitted.
Test for Content Uniformity
We utilized a volumetric container to disseminate 10 milligrams of medication from a tablet into 50 milliliters of 6.8-pH support. The drug was blended so that it would disperse in the liquid. After isolating the blueprint, two milliliters were diluted to the engraving concentration using distilled water in a ten milliliter volumetric container before being analyzed using spectrophotometry at 253 nm. A conventional drug arrangement twist was used in the selection of nabumetone compulsion. Three distinct overlay approaches were used to assess the prescription reliance of each strategy bundle [18].
In vitro Disintegration Time
The tablet was dissolved in 37°C 0.5°C, pure water (900 ml).A study was conducted to determine how long it takes for a pill to dissolve.
In vitro Dissolution Study
Nabumetone was used in an in vitro tablet crushing study using USP XXIV material. Degradation medium was 900 mL of phosphate pad 7.4 (replica fluid).Thestirrerwasadjustedtorotateat100RPM. The test was conducted with the medium degrading at a constant 37 0.5oC. One pill was used in each study. Using a needle fitted with a pre-channel, 5 ml samples of the dissolving solution were taken at regular intervals, and the absorbance at 332 nm was analyzed to assess the rate of drug release. At regular intervals, the disposal quantities were swapped out with new supplies of the same volume of dissolving media [19-21]. We showed time communicated versus concentration joined for Nabumetone.
Evaluation Of Pulsincap Dosage Form
In-vitrorelease studies
Based on the pulsincap definition, studies were done to calculate how rapidly the prescription might be provided. The in-vitro dissolution profile of each formulation was evaluated using the rotating basket method of the USP I device. The use of two different dissolving media, one with a pH of 1.2 and the other with a pH of 7.4, or both, was suggested as the "Successive pH change method" to promote the pH changes throughout the GI tract. The temperature of the dissolving medium was maintained at 37 0.5°C and the rotating speed of the basket was maintained at 100 rpm during the whole assessment. Each time 900 ml of dissolving media was utilized. Each coffin was loaded with a box of Nabumetone Pulsin caps to weigh it down and keep it from floating away [22-25].
Following 2 hours of testing with a pH 1.2 buffered stimulated gastric fluid (SGF), which mimics the average stomach emptying interval of 2 hours, the SGF was removed and replaced with a pH 7.4 buffered stimulated intestinal fluid (SIF). Needle-drawn dilutions of the degradation fluid, measuring 5 ml each, were taken at regular intervals. At each time point, 5 ml of new degradation media was added to maintain the initial volume. Absorbance at 332 nm was used to identify nabumetone in the isolated models after a variable-duration UV ingestion strategy, (Table 6,7).
| Vessel temperature | 37 ± 0.5oC |
| Bath temperature | 38± 0.5oC |
| Dissolution media | pH1.2, pH7.4 |
| Volume of dissolution media | 900 ml |
| Aliquot withdrawn | 5 ml |
| Aliquot replaced | 5 ml of the fresh solution |
| Dissolution apparatus | USP type I (Basket) |
| Speed | 100RPM |
Table 6:Dissolution specifications of Nabumetone
| Diffusion (n) | Overall solute diffusion mechanism |
|---|---|
| 0.45 | Fickian diffusion |
| 4 | Anomalous (non fickian) diffusion |
| 0.89 | Case- transport |
| n>0.89 | Super case-II transport |
Table-7: Diffusion exponents and solute release mechanism
Results and Discussion
Preformulationstudies
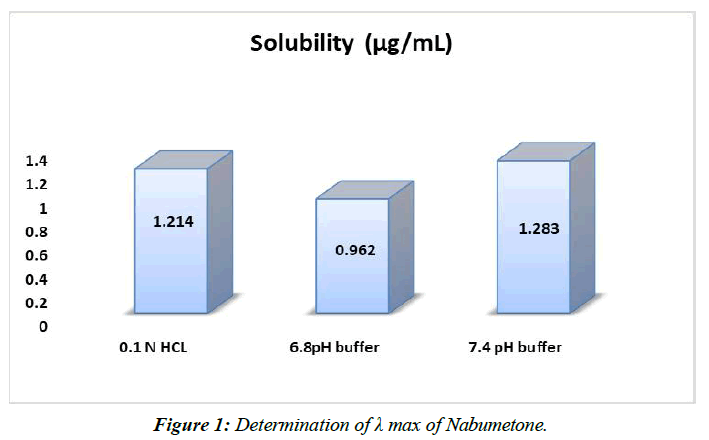
Solubility: It was decided in accordance with established protocol. Table displays the obtained outcomes, (Table 8, Figure 1).
| Solvent | Solubility (µg/mL) |
|---|---|
| 0.1 N HCL | 1.214±0.027 |
| 6.8pH buffer | 0.962±0.054 |
| 7.4 pH buffer | 1.283±0.036 |
Table 8: Solubility studies of Nabumetone in various solvents.
A 7.4 pH phosphate pad is preferable to a 6.8 pH phosphate pad or 0.1 N HCl plan for solubilizing nabumetone.
Melting point
Nabumetone has a melting point between 80 and 83 degrees Celsius.
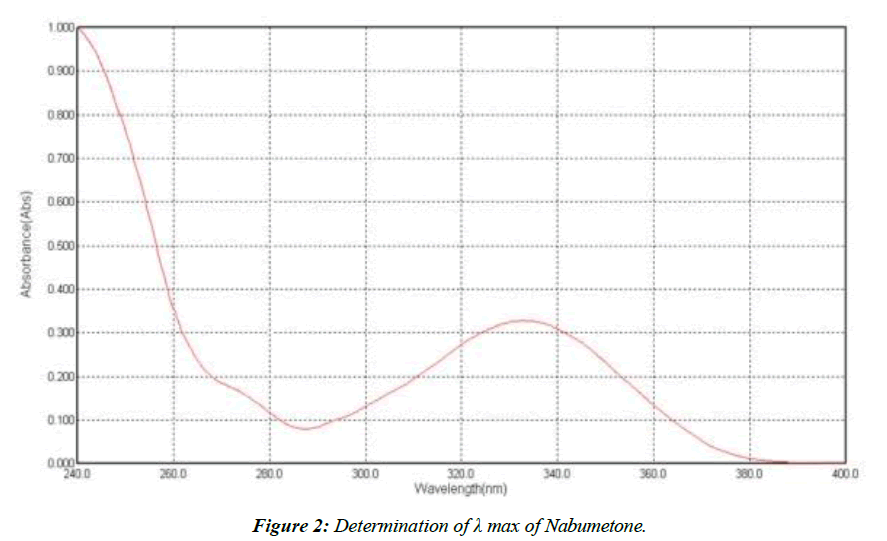
Determination of λ max of Nabumetone
An UV channel was carried out between 200 and 400 nm, yielding a maximum peak at 332 nm; this value was used to determine the best UV channel for Nabumetone, (Figure 2).
At 332 nm, we see the highest absorption.
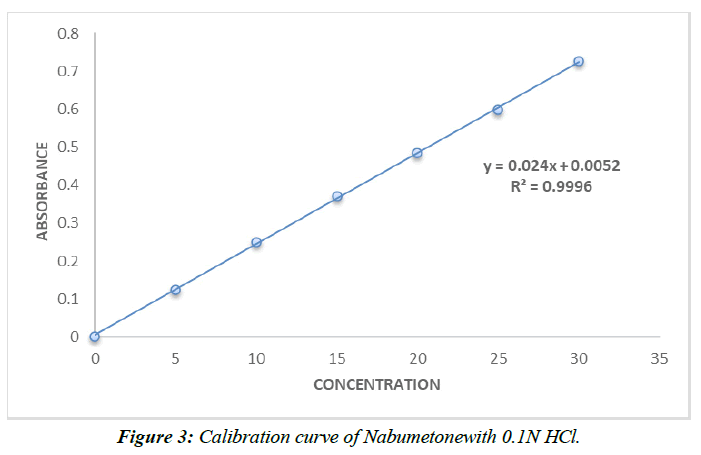
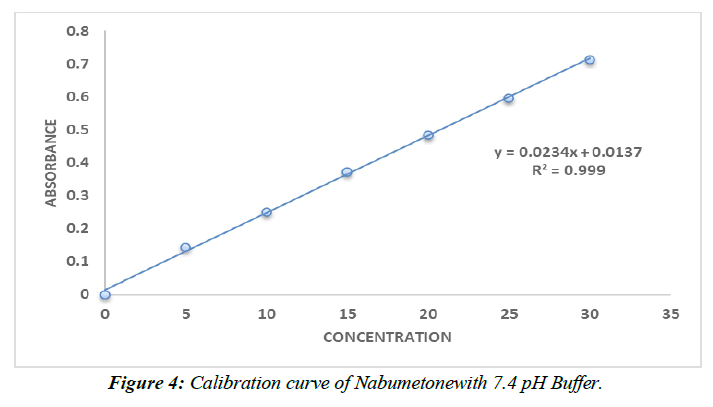
Calibration curve of Nabumetone (Table 9,10, Figure 3,4).
| Conc (µg/ml) | Absorbance |
|---|---|
| 0 | 0 |
| 5 | 0.124±0.021 |
| 10 | 0.251±0.021 |
| 15 | 0.372±0.021 |
| 20 | 0.486±0.021 |
| 25 | 0.598±0.021 |
| 30 | 0.726±0.021 |
Table 9 : Calibration data of Nabumetonein 0.1N HCl.
| Conc (µg/ml) | Absorbance |
|---|---|
| 0 | 0 |
| 5 | 0.142±0.026 |
| 10 | 0.249±0.026 |
| 15 | 0.372±0.026 |
| 20 | 0.484±0.026 |
| 25 | 0.596±0.026 |
| 30 | 0.712±0.026 |
Table 10 : Calibration data of Nabumetonein pH 7.4 phosphate buffer.
The range of the still hanging out there in a pad plan of pH 7.4 and 0.1N HCl is 5–30 g/ml. Ale lambert's rule of thumb was obviously being followed as the reversal result trended toward 1.
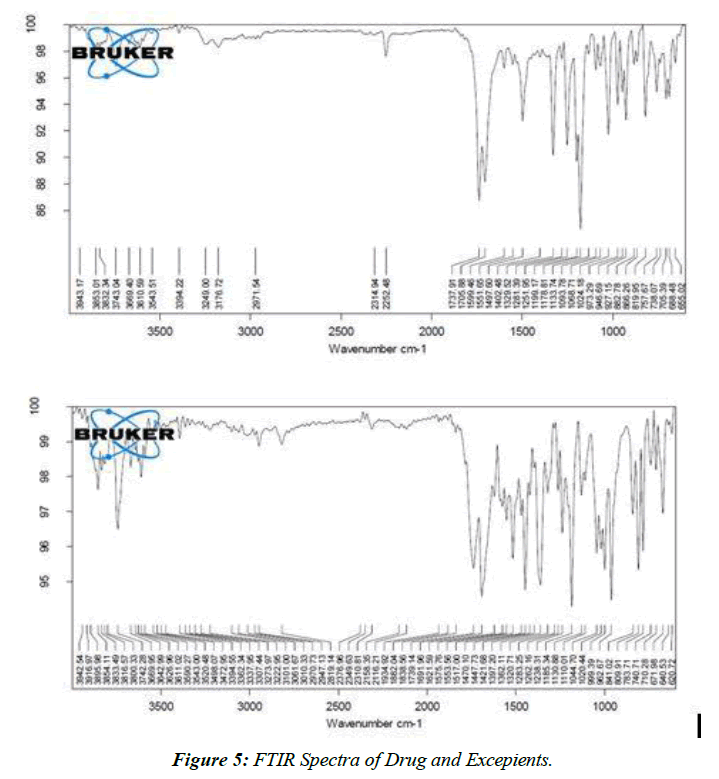
FTIR
Drug-Excipient compatibility studies
The improved medication had an IR spectrum that was identical to Nabumetone's.
The spectra of nabumetone reveal that the drug's recognized peaks have not been altered by the addition of polymers, and that all of the peaks may be seen as before [26]. Both the original nabumetone and the modified version of the compound's FTIR spectra are shown in the figure.
FTIR Spectra of Pure drug (Figure 5)
The results of the similarity tests between the medication and its excipients show that there are no discernible differences between the two formulations.
Flow properties of powder blend (Table 11)
| Formulation Code | Angle of Repose±SD | Bulk Density (g/ml)±SD | Tapped Density (g/ml)±SD | Carr’s Index. (%)±SD | Hausner’s ratio±SD |
|---|---|---|---|---|---|
| F1 | 25.29±0.24 | 0.365±0.41 | 0.421±0.41 | 16.21±0.02 | 1.11±0.24 |
| F2 | 26.78±0.17 | 0.348±0.28 | 0.446±0.28 | 17.74±0.52 | 1.28±0.39 |
| F3 | 27.24±0.38 | 0.352±0.974 | 0.472±0.32 | 15.56±0.36 | 1.36±0.15 |
| F4 | 25.28±0.74 | 0.334±0.65 | 0.465±0.47 | 18.78±0.98 | 1.27±0.25 |
| F5 | 28.21±0.32 | 0.374±0.81 | 0.432±0.12 | 14.92±0.42 | 1.32±0.36 |
| F6 | 25.78±0.48 | 0.354±0.25 | 0.489±0.96 | 17.17±0.15 | 1.26±0.48 |
| F7 | 29.28±0.67 | 0.365±0.48 | 0.421±0.32 | 16.32±0.15 | 1.18±0.26 |
| F8 | 27.39±0.28 | 0.352±0.26 | 0.436±0.28 | 14.75±0.15 | 1.20±0.45 |
| F9 | 26.84±0.25 | 0.357±0.19 | 0.475±0.42 | 15.78±0.21 | 1.26±0.32 |
| F10 | 25.41±0.19 | 0.358±0.26 | 0.428±0.47 | 16.21±0.19 | 1.15±0.38 |
| F11 | 29.57±0.26 | 0.359±0.37 | 0.438±0.36 | 17.68±0.14 | 1.21±0.42 |
| F12 | 28.78±0.24 | 0.374±0.36 | 0.475±0.84 | 14.95±0.16 | 1.19±0.28 |
Table 11: Flow properties of powder blend
Resting-place of many definitions was 29.570.26, indicating the exceptional stream feature of the material. So, it was said that mixes included the ability to stream for free. The calculated mass density of the blend was found to be between 0.3340.65 and 0.3740.81g/cm3.The range of tapped thicknesses between 0.428 and 0.47 g/cm3 was examined.These values prove that the mixtures exhibit superior stream characteristics [27-30]. Both the Carr and Hausner ranges at 14.7501518.780.98 and 1.110241.360.15, respectively, imply that the blends have remarkable stream character.
Characterization of Tablets
Post Compression parameters
The range of tablet weights, tablet hardness, friability, tablet thickness, and drug content were all meticulously analyzed and depicted. (Table 12)
| Formulation code | %Weight variation (mg) | Thickness (mm) | Diameter (mm) | Hardness | Friability (%) | Disintegrating time(sec) | Drug content (%) |
|---|---|---|---|---|---|---|---|
| F1 | 150.21 | 3.94 | 9.34 | 5.7 | 0.57 | 14 | 95.28 |
| F2 | 149.25 | 3.51 | 8.48 | 5.9 | 0.46 | 15 | 97.14 |
| F3 | 151.26 | 3.48 | 9.25 | 6.2 | 0.75 | 20 | 96.39 |
| F4 | 148.21 | 3.57 | 9.62 | 4.5 | 0.85 | 13 | 99.42 |
| F5 | 152.78 | 3.68 | 9.47 | 4.6 | 0.54 | 21 | 98.34 |
| F6 | 150.18 | 3.75 | 8.38 | 4.8 | 0.68 | 16 | 96.81 |
| F7 | 152.19 | 3.68 | 9.42 | 5.1 | 0.61 | 19 | 97.32 |
| F9 | 148.26 | 3.82 | 8.19 | 5.2 | 0.78 | 17 | 98.45 |
| F10 | 151.47 | 3.84 | 9.57 | 5.7 | 0.65 | 16 | 98.56 |
| F11 | 148.21 | 3.85 | 8.41 | 5.6 | 0.74 | 14 | 97.58 |
| F12 | 150.62 | 3.74 | 9.68 | 5.8 | 0.84 | 11 | 99.45 |
Table 12: CharacterizationNabumetoneTablets
The tablet's hardness, which ranged from 4.5 to 6.5 kg/cm2, was suitable and constant between batches.The % weight fluctuation was well within the absolute limitations for tablet weight, hence the investigation of the weight variation was declared complete. Since the friability ratings for all of the designs F1–F12 were less than 1%, it was determined that they were all suitable. The medical aspects of all of the designs (F1-F12) were judged to be 95-100% complete [31].
Dissolution studies of the tablets
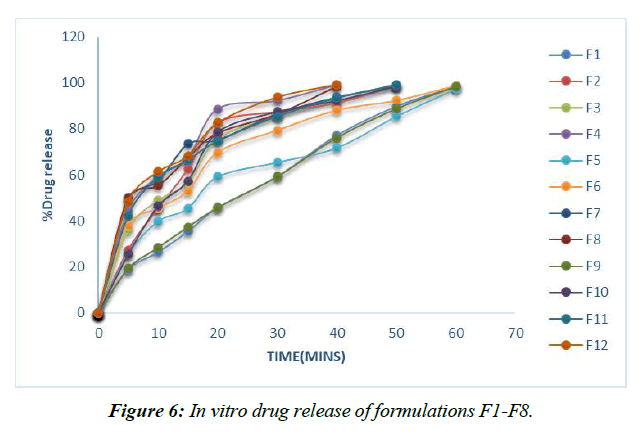
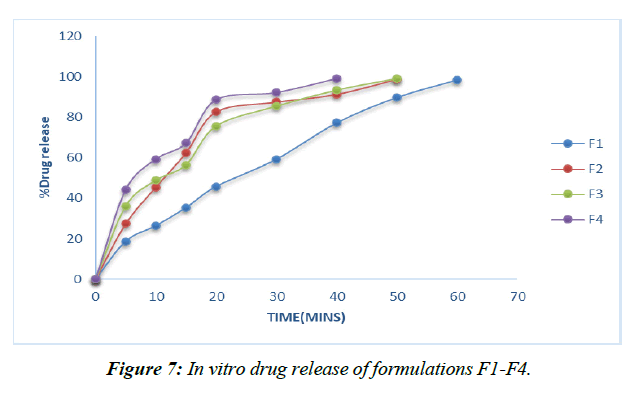
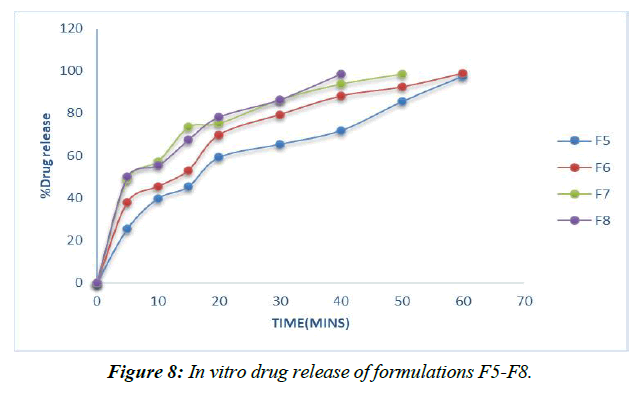
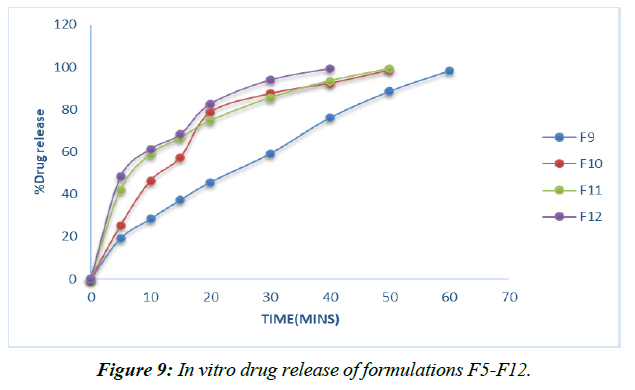
Dissolution experiments were performed on the finished tablets to determine the drug release rate. (Table 13, Figure 6-9)
| Time (mins) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 18.74 | 27.36 | 36.18 | 44.28 | 25.48 | 38.08 | 48.89 | 49.89 | 19.28 | 25.36 | 42.18 | 48.28 |
| 10 | 26.41 | 45.42 | 48.83 | 59.34 | 39.82 | 45.48 | 57.24 | 55.24 | 28.41 | 46.42 | 58.83 | 61.34 |
| 15 | 35.36 | 62.35 | 56.49 | 67.25 | 45.45 | 53.05 | 73.42 | 67.42 | 37.36 | 57.35 | 66.49 | 68.25 |
| 20 | 45.74 | 82.63 | 75.65 | 88.66 | 59.21 | 69.78 | 75.19 | 78.19 | 45.74 | 78.63 | 74.65 | 82.66 |
| 30 | 59.25 | 87.49 | 85.48 | 92.25 | 65.48 | 79.36 | 86.46 | 86.46 | 59.25 | 87.49 | 85.48 | 93.84 |
| 40 | 77.17 | 91.31 | 93.41 | 99.15 | 71.82 | 88.09 | 93.78 | 98.53 | 76.17 | 92.31 | 93.41 | 99.26 |
| 50 | 89.75 | 98.83 | 99.14 | 85.49 | 92.31 | 98.53 | 88.75 | 98.53 | 99.14 | |||
| 60 | 98.41 | 97.46 | 98.75 | 98.41 |
Table 13: % Cumulative drug release of formulations F1-F8
Plans containing ludiflash as a super disintegrant in different centers, like 2,4, 6, and 8%, have been evaluated for their drug release in vitro, and the results show that an increase in the super disintegrant obsession shortens the release time of the prescription, with the F4 definition containing ludiflash8% center showing the most notable proportion of medicine release (99.15%) close to the completion of 40mins [32-34].
In contrast, the super disintegrant core's prolonged nature speeds up drug release, with the F8 definition containing 8% lycoat showing the most prominent effect of prescription release (98.53%) at the end of 40 minutes.
However, the drug enters the bloodstream faster in formulations with 2, 4, 6, and 8% Croscarmellose sodium thanks to the extended super disintegrant core, and the highest percentage of medicine release (99.26%) is seen in formulation F8 with lycoat with 8% obsession just before the end of 40 minutes.
Given that maximal release may be attained within 40 minutes with the F12 formulation including an 8% Croscarmellose sodium intercalation, this option is selected as the superior definition.
Optimization of formaldehyde treated capsule bodies exposed at various time intervals viz., 2, 4, 6, 8, 10hrs (Table 14)
| Code | Disintegration Time (hrs) | |
|---|---|---|
| 1.2 pH (2hrs) | 7.4.8 pH (upto 24hrs) | |
| F13 (2rdhr) | 2 | _ |
| F14 (4thhr) | 2 | 1 |
| F15 (6thhr) | 2 | 7 |
| F16 (8thhr) | 2 | 9 |
| F17 (10thhr) | 2 | 12 |
Table 14: Disintegration test for Treated Capsules
Case F13, which was treated for 6 hours, remained stable for 7 hours, according to independent tests, highlighting the critical window of opportunity. Since the needed lag time for the arrangement was 6 hours, F15 and F16, which stay flawless for 9, 12 hours separately, were not picked. Due to the need for a delay, it was considered that 6 hours after formaldehyde (F13) treatment of the compartment would be the best time for further assessment [35-38].
Invitro release studies
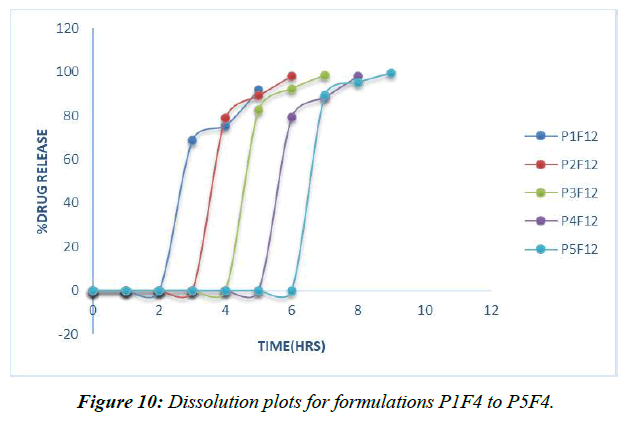
To simulate in vivo GIT circumstances, a degradation investigation was conducted at 370C utilizing a USP I deterioration apparatus and two distinct pH 1.2, pH7.4 phosphate supports as the breaking down media. Deterioration occurred initially in a pH1.2 medium for 2 hours, then in a pH7.4 phosphate medium for 10 hours. (Table 15, Figure 10)
| Time (hrs) | P1F12 | P2F12 | P3F12 | P4F12 | P5F12 |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 68.47 | 0 | 0 | 0 | 0 |
| 4 | 75.21 | 78.85 | 0 | 0 | 0 |
| 5 | 91.63 | 89.12 | 82.45 | 0 | 0 |
| 6 | 98.24 | 92.26 | 79.28 | 0 | |
| 7 | 98.37 | 88.49 | 89.21 | ||
| 8 | 98.21 | 95.28 | |||
| 9 | 99.62 | ||||
| 10 |
Table 15: Invitro dissolution data of formulations P1F12 to P5F12
Nabumetonepulsincaps's 5 nuances have all been the subject of dissection research. Hydrogel plugs P1F4, P2F4, P3F4, P4F4, and P5F4 are each composed of either 100 mg of Ethyl cellulose, 100 mg of HPMC, or a 1:1, 1:2, or 2:1 blend of the two [39].
Hydrogel plugs made from a 2:1 mixture of Ethyl cellulose and HPMC K15M have been found to keep drugs in the upper GIT for 6 hours. Hydrophilic polymer collection time was positively correlated with drug delivery delay; data revealed that drug arrived in a concentrated burst after a period of inactivity. In light of these findings, we chose the P5F4 defining, which includes a hydrogel connection of ethyl cellulose and HPMC K15M in a 2:1 extent, as a refined pulsincap method [40].
Release kinetics
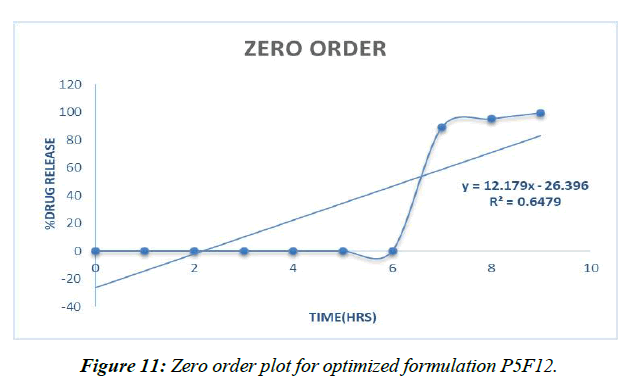
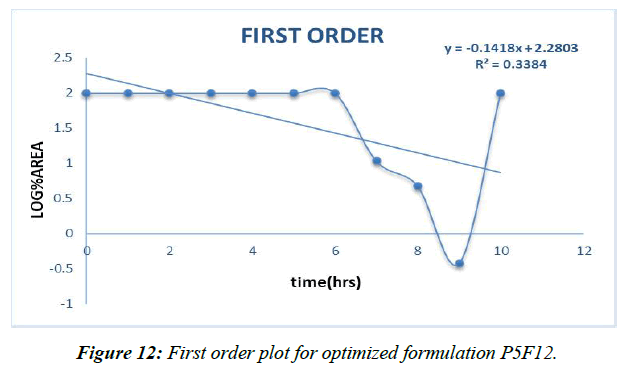
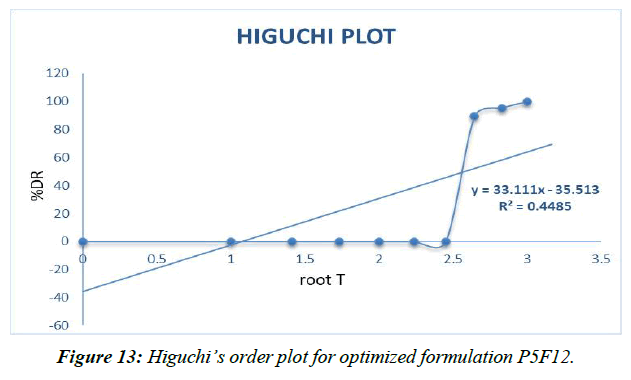
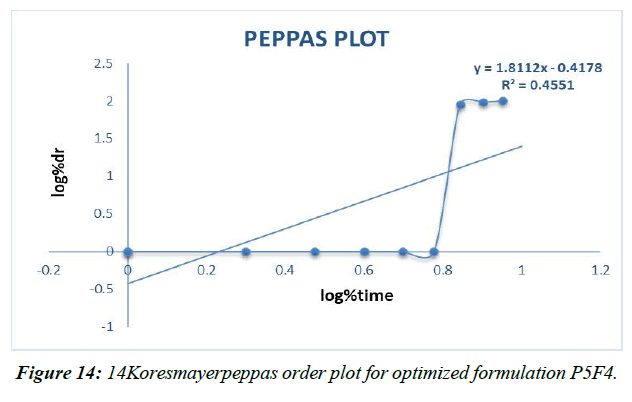
Different decay fitting equations, such as those developed by Higuchi and koresmayerpeppa, were used to analyze the data. Plan P5F4's backslide coefficient "R" values were calculated to be 0.966, 0.835, 0.982, and 0.606, respectively, for zero solicitation, beginning solicitation, higuchi's, and peppas.
A vehicle with a revised P5F4 definition 'n' respect of 1.302 is classified as a super case II vehicle.
Zero order (Figure 11)
First order (Figure 12)
Higuchi plot (Figure 13)
Peppas plot (Figure 14, Table 16)
| Models | R values |
|---|---|
| Zero order | 0.647 |
| First order | 0.338 |
| Higuchi | 0.448 |
| Koresmayerpeppas | 0.455 |
| Peppas “n” | 1.811 |
Table 16: Correlation coefficient “R” values of P5F4 optimized formulation
The prescription release instrument was untangled from the updated P5F12pulsincap definition with the use of data collected from the drug release audit. The correlation coefficient (R2) was used to compare all of the models. Super case II vehicle instrument came after zero solicitation energy and prescription release energy [41].
Summary and Conclusion
Summary
Over the past, well, forever, there has been rising confirmation of the influence of the prescription link on the limits of pharmacodynamics and pharmacokinetics, and increased knowledge of the role of circadian rhythms on GIT physiology and on disease states. Experts in the area of medicine transportation have recognized the relevance of these changes and have been rather ingenious in developing time-delayed medication transport techniques to address new Chronotherapeutic requirements. The Pulsincap technique facilitates medication delivery to the colon, which in turn facilitates the treatment of chronotherapeutic.
If it were feasible to assign drugs in the colon, where distribution and absorption may occur, treatment results would improve. down order to zero down on particular medicine, this research included prescription maintenance based on the drug's efficacy in treating mild to moderate depression.
Nabumetone and the excipients were analyzed using FTIR spectroscopy for homogeneity prior to the enumeration process. The results suggest that the medication and polymers are compatible with one another, with little impact on the intended properties of Nabumetone.
The best-treated holders were chosen after subjecting the case bodies to formaldehyde for varying amounts of time (two, four, six, eight, and ten hours), then to a high-level treatment with a disintegration survey, and finally to a battery of physical and manufactured tests (ID cards, visual deformations, layered assessments, and an emotional test assuming formaldehyde-free conditions).
The features of the stream and the results of an FTIR investig
References
- Priyanka PS, Hemalatha V, Kumar PP, et al. Formulation and evaluation of pulsatile drug delivery system of celecoxib.
- Patel RJ. Formulation and evaluation of pulsatile drug delivery system of hypolipidemic agent.
- Grundy SM. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin Cornerstone. 2006;8:21-7.
- Youan BB. Chronopharmaceutics: gimmick or clinically relevant approach to drug delivery? J Control Release. 2004;98(3):337-53.
- Anal AK. Time-controlled pulsatile delivery systems for bioactive compounds. Recent Patents on Drug Delivery and Formulatio. 2007;1(1):73-9.
- LEMMER B. The clinical relevance of chronopharmacology in therapeutics. Pharmacol Res. 1996;33(2):107-15.
- Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235(1-2):1-5.
- Krishna GR, Neelima K, Rao DS, et al. Formulation and evaluation of pulsatile drug delivery system of flurbiprofen. Int J Pharma Bio Sci. 2015;5(4):817-28.
- Zhang Y, Zhang Z, Wu F. A novel pulsed-release system based on swelling and osmotic pumping mechanism. J Control Release. 2003;89(1):47-55.
- PRIYA PV, Sharma JV, Archana G, et al. THE Formulation and In-vitro evaluation of press coated tablets of pravastatin for pulsatile drug delivery. J Drug Deliv. Ther. 2019;9(2):437-44.
- Krogel I, Bodmeier R. Floating or pulsatile drug delivery systems based on coated effervescent cores. Int. J. Pharm. 1999;187(2):175-84.
- Ross AC, Macrae RJ, Walther M, et al. Chronopharmaceutical drug delivery from a pulsatile capsule device based on programmable erosion. J Pharm Pharmacol. 2000;52(8):903-9.
- Chhabra VS, Tilloo SK, Walde SR, et al. The essentials of chronopharmacotherapeutics. Int. J. Pharm. Sci. 2012;4(3):1-8.
- Mandal AS, Biswas N, Karim KM, et al. Drug delivery system based on chronobiology—A review. J Control Release. 2010;147(3):314-25.
- Moturi V, Khan AB. Chronotherapeutics in development of pulsatile delivery systems. Int J Pharm Pharm Res. 2012;3(11):4086.
- Chandra Nandy B, Verma V, Dey S, et al. Three levels face centered central composite design of colon targeted micro-particulates system of celecoxib: screening of formulations variables and in vivo studies. Curr Drug Deliv. 2014;11(5):621-35.
- Mastiholimath VS, Dandagi PM, Jain SS, et al. Time and pH dependent colon specific, pulsatile delivery of theophylline for nocturnal asthma. Int J Pharm. 2007;328(1):49-56.
- Abraham S, Srinath MS. Development of Modified Pulsincap Drug Delivery System of Metronidazole for Drug Targeting. Indian J Pharm Sci. 2007;69(1).
- Abraham S, Srinath MS. Development of Modified Pulsincap Drug Delivery System of Metronidazole for Drug Targeting. Indian J Pharm. Sci. 2007;69(1).
- Sahoo SK, Mallick AA, Barik BB, et al. Formulation and in vitro Evaluation of Eudragit® Microspheres of Stavudine. Trop J Pharm. Res. 2005;4(1):369-75.
- Sangalli ME, Maroni A, Gazzaniga A, et al. Different HPMC viscosity grades as coating agents for an oral time and/or site-controlled delivery system: a study on process parameters and in vitro performances. Eur J Pharm Med Res. 2004;22(5):469-76.
- Bussemer T, Peppas NA, Bodmeier R. Evaluation of the swelling, hydration and rupturing properties of the swelling layer of a rupturable pulsatile drug delivery system. Eur J Pharm Biopharm. 2003;56(2):261-70.
- Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6(1):33-66.
- Bussemer T, Bodmeier R. Formulation parameters affecting the performance of coated gelatin capsules with pulsatile release profiles. Int. J. Pharm. 2003;267(1-2):59-68.
- Ross AC, Macrae RJ, Walther M, et al. Chronopharmaceutical drug delivery from a pulsatile capsule device based on programmable erosion. Indian J Pharm Pharmacol. 2000;52(8):903-9.
- Krishnaiah YS, Karthikeyan RS, Sankar VG, et al. Three-layer guar gum matrix tablet formulations for oral controlled delivery of highly soluble trimetazidine dihydrochloride. J Control Release. 2002;81(1-2):45-56.
- Stevens HN, Wilson CG, Welling PG, et al. Evaluation of Pulsincap™ to provide regional delivery of dofetilide to the human GI tract. Int J Pharm. 2002;236(1-2):27-34.
- Fukui E, Miyamura N, Uemura K, et al. Preparation of enteric coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int J Pharm. 2000;204(1-2):7-15.
- Ross AC, Macrae RJ, Walther M, et al. Chronopharmaceutical drug delivery from a pulsatile capsule device based on programmable erosion. J Pharm Pharmacol. 2000;52(8):903-9.
- Marvola M, Nykanen P, Rautio S, et al. Enteric polymers as binders and coating materials in multiple-unit site-specific drug delivery systems. Eur J Pharm Biopharm. 1999;7(3):259-67.
- Matsui T, Miyamoto K, Yamanaka K, et al. Cell-based two-dimensional morphological assessment system to predict cancer drug-induced cardiotoxicity using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Appl Pharmacol. 2019;383:114761.
- Çelebier M, KAYNAK M, Altınoz S, et al. UV spectrophotometric method for determination of the dissolution profile of rivaroxaban. Dissolution Technol. 2014;21(4).
- Halsas M, Ervasti P, Veski P, et al. Biopharmaceutical evaluation of time-controlled press-coated tablets containing polymers to adjust drug release. Eur J Drug Metab Pharmacokinet. 1998;23:190-6.
- Sandeep M, Kishore VS, Sudheer B, et al. Design and development of chronopharmaceutical drug delivery of lansoprazole. Asian J. Pharm. Res. 2013:63-70.
- Bojja R, Satyanandam R. Design and Development of Pulsincap for Chronopharmaceutical Drug Delivery of Losartan Potassium. Asian J Pharm Res. 2014:78-86.
- Patel DM, Jani RH, Patel CN. Design and evaluation of colon targeted modified pulsincap delivery of 5-fluorouracil according to circadian rhythm. Int J Pharm. Investig. 2011;1(3):172.
- Reddy BV, Navaneetha K, Reddy KV, et al. Formulation and evaluation of fast dissolving tablets of losartan potassium. Indo Am. j. pharm. sci. 2014;4(5):2573-84.
- Patel DM, Jani RH, Patel CN. Design and evaluation of colon targeted modified pulsincap delivery of 5-fluorouracil according to circadian rhythm. Int J Pharm. Investig. 2011;1(3):172.
- Kumar AA, Rajyalakshmi K. Formulation and evaluation of Metoprolol Succinate pulsatile drug delivery system for chrono biological disorder: anti hypertension. Int J Pharm Sci Res. 2012;3(10):4004.
- Higuchi TJ. Mechanism of sustained‐action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(12):1145-9.
- Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60(4):110-1.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref