Research Article - Biomedical Research (2022) Volume 33, Issue 7
Formulation and development of topical dosage form of piroxicam using mixed solvency concept and its evaluations.
Anirudha Padiyar*, Maheshwari RK
Department of Pharmacy, Shri Govindram Seksaria Institute of Technology and Sciences, Indore 452003, Madhya Pradesh, India
- Corresponding Author:
- Anirudha Padiyar
Department of Pharmacy
Shri Govindram Seksaria Institute of Technology and Sciences
Indore 452003
Madhya Pradesh
India
Accepted date: September 13, 2022
Abstract
Solubilizing drugs which are poorly water-soluble is a challenging factor and really vital issue in screening studies of new drug moieties as well as formulation development research. It leads to difficulties in several developmental, manufacturing and administrative processes. Furthermore, due to their poor pharmacokinetics the clinical trials of these drugs have witnessed a great failure. The aim of our research work is to promote mixed solvency concept by formulating the topical solution (lotion) and topical gel of poorly water-soluble drug piroxicam by decreasing the individual solubilizer concentration in small proportion for expected enhancement of drug solubility in water. For poorly water soluble drug piroxicam, solubilizers such as sodium benzoate, sodium citrate, sodium caprylate, arginine, valine, benzoic acid, poloxamer 407 and niacinamide has been used in combinations as mixed solvent systems. The procured sample of piroxicam was characterized by melting point, UV spectroscopy, IR and DSC studies. The formulations were evaluated for various properties of solution and gels such as pH, freeze thaw study and thin layer chromatography, physical appearance, etc. Mixed solvency concept was successfully utilized for formulation development of marketable product of piroxicam.
Keywords
Solubility, Piroxicam, Solution, Gel.
Introduction
Delivery of drugs through topical route of administration is a most convenient and easy approach for local and systemic effect of different drugs. A topical solution is a monophasic system of two or more substances. Absorption of drugs from solution dosage is rapid and very high. The solution which is applied directly to skin is called topical solution. Major key pre-formulation parameters that need to be taken under the consideration while formulating the solutions are solubility and stability. Topical solution acts locally and targets at the site of allergy and inflammation resulting in reduced side effects and toxicity to other organs. Low solubility in aqueous medium is the main problem for the formulation development of the various drugs which are used locally. Water is the major solvent used for liquid pharmaceutical formulations. Various solubility improvement techniques are used for solubility enhancement of poorly water soluble drugs. Mixed solvency concept is novel technique which used for the purpose of solubility enhancement. As per the mixed solvency concept proposed by Maheshwari each and every substance present on the earth has got solubilizing property i.e. all the liquids, gases and solids possess solubilizing power. As per his statement each substance is a solubilizer. Any substance is just solvent for some and non- solvent for another. The aqueous solution containing various water soluble substances may act as good solvent for poorly water soluble drugs. Such concentrated solutions may show synergistic or additive solubilizing actions on solubility of practically insoluble drugs. Each and every weaker solvent using various solid solubilizers [1-6].
Materials and Methods
Drug characterization
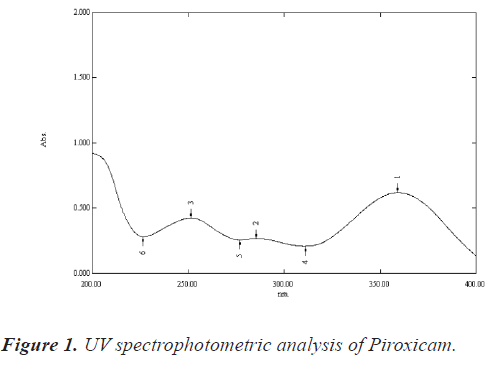
UV spectrophotometric analysis of piroxicam in DM water: About 50 mg of Piroxicam was accurately weighed and transferred to a volumetric flask (50 ml). Drug was dissolved using 40 ml of ethyl alcohol, and then the volume was made up to 50 ml with DM water to prepare a 1000 μg/ml solution. The solution thus produced was sufficiently diluted with DM water to obtain 15 μg/ml solution. The absorbance of solution was noted at 354 nm on a UV-visible spectrophotometer (Shimadzu® 1700) between wavelength 200 nm-400 nm and the UV spectrum was recorded and shown in figure 1 [7].
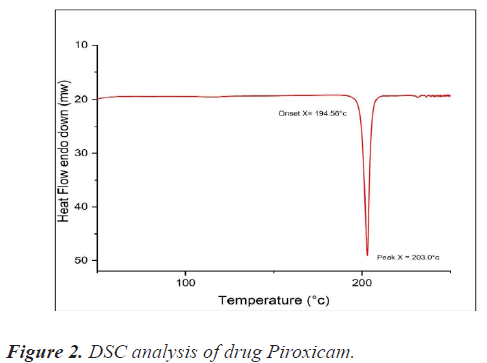
DSC study of piroxicam drug sample: A Pyris 6 DSC (Jade DSC) differential scanning calorimeter with thermal analyzer was used for the DSC investigation. Samples were accurately weighed (about 3 mg) and kept in a sealed aluminium pan before being heated at a scanning rate of 10°C per minute from 25°C to 350°C under nitrogen flow (20 ml/min). As a reference, an empty aluminium pan was used. The data was analysed and compared to the literature. Figure 2 shows the DSC spectrum of piroxicam drug sample.
Melting point determination of piroxicam drug sample: The melting point of piroxicam was determined using open capillary method. The powered drug sample was packed in capillary and the melting point was measured by Analog melting point testing apparatus. The melting point was found to be 200°C-202°C, similar as reported in literature.
Preformulation studies
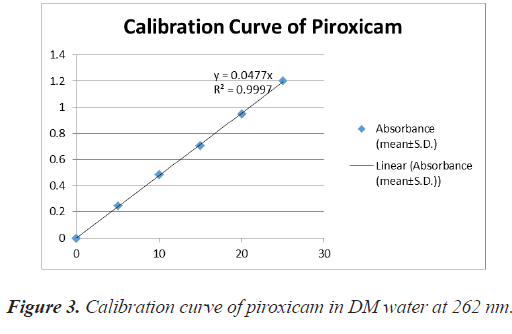
Preparation of calibration curve of piroxicam using DM water: The calibration curve was prepared in R.O. water in presence of sodium caprylate. About 20% w/v sodium caprylate solution was prepared in DM water. For calibration curve, 50 mg drug was taken in 50 ml volumetric flask and dissolved in 10 ml of 20% sodium caprylate solution and then the volume was made up to 50 ml with DM water. This stock solution of 1000 μg/ ml was appropriately diluted with DM water to prepare solutions of different concentrations (5 mcg/ml-25 mcg/ ml). The absorbances of these solutions were noted at 354 nm against respective reagent blanks. It was repeated three times [8-11] (Table 1 and figure 3).
| S.no | Concentration (µg/ml) | Absorbance (mean ± SD) |
|---|---|---|
| 1. | 0 | 0 ± 0 |
| 2. | 5 | 0.246 ± 0.0023 |
| 3. | 10 | 0.485 ± 0.0040 |
| 4. | 15 | 0.707 ± 0.0121 |
| 5. | 20 | 0.947 ± 0.0036 |
| 6. | 25 | 1.200 ± 0.0056 |
Table 1. Absorbance data for calibration curve of piroxicam in DM water (in presence of sodium caprylate) at 354 nm (n=3).
Equillibirium solubility studies in different solvent systems: Equillibirium solubility studies in different solvent systems were achieved by adding excess amount of drug in 10 ml of respective mediums, it was then screw capped and kept on mechanical shaker for 24 hrs. It was centrifuged in centrifuge tubes at 2000 rpm for about 5 min and filtered using Whatman grade 41 filters then diluted with suitable respective medium. The absorbance was measured at 354 nm on a double beam UV/Visible spectrophotometer (Shimadzu-1700) against reagent blank and noted in table 2 [12].
| S.no | Solvent system | Equilibrium solubility (mg/ml) |
Description |
|---|---|---|---|
| 1. | DM Water | 0.0512 | Practically Insoluble |
| 2. | Hydrochloric acid buffer pH 1.2 | 0.112 | Practically insoluble |
| 3. | Hydrochloric acid buffer pH 2.0 | 0.115 | Practically insoluble |
| 4. | Acid phthalate buffer pH 3.0 | 0.145 | Practically insoluble |
| 5. | Acid phthalate buffer pH 4.0 | 0.243 | Practically insoluble |
| 6. | Neutralized phthalate buffer pH 5.0 0.340 Practically insoluble | 0.340 | Practically insoluble |
| 7. | Phosphate buffer pH 6.0 | 0.463 | Practically insoluble |
| 8. | Phosphate buffer pH 7.0 | 0.641 | Very slightly Soluble |
| 9. | Phosphate buffer pH 8.0 | 0.789 | Very slightly Soluble |
| 10. | Alkaline borate buffer pH 9.0 1.632 Slightly soluble | 1.632 | Slightly soluble |
| 11. | Alkaline borate buffer pH 10.0 | 2.593 | Slightly soluble |
Table 2. Equillibrium solubility of piroxicam in different solvent systems.
Approximate solubility of piroxicam in different blends: Different concentrations of solubilizers in small quantity were used to prepare 10 ml blend of various composition and concentration and labelled as Blend 1 to Blend 20 [13].
One ml of blend-A solution was taken in volumetric flask. Five mg of drug piroxicam was added and shaken vigorously for about 15-20 minutes on vortex. When clear solution was formed then again the process was continued until turbidity appears. The approximate solubilities were noted in table 3.
| S.no | Blend–code | Composition of blends | Approximate solubility |
|---|---|---|---|
| 1. | Blend 1 | PVP K-30 (5%) Lignocaine HCl (5%) Niacinamide (5%) Sodium benzoate (5%) |
10 mg/ml |
| 2. | Blend 2 | PVP K-30 (5%) Niacinamide (5%) Sodium benzoate (5%) |
10 mg/ml |
| 3. | Blend 3 | PVP K-30 (5%) Sodium citrate (5%) Niacinamide (5%) Sodium benzoate (5%) |
15 mg/ml |
| 4. | Blend 4 | PVP K-30 (5%) Niacinamide (5%) Sodium benzoate (10%) |
20 mg/ml |
| 5. | Blend 5 | PVP K-30 (5%) Sodium citate (5%) Sodium acetate (5%) |
10 mg/ml |
| 6. | Blend 6 | PVP K-30 (5%) Caffeine (5%) Niacinamide (5%) Sodium benzoate (5%) |
5 mg/ml |
| 7. | Blend 7 | Caffeine (5%) Niacinamide (5%) |
15 mg/ml |
| 8. | Blend 8 | Sodium citrate (2.5%) Sodium benzoate (5%) L-Arginine (10%) Benzoic acid (5%) Niacinamide (2.5%) HP-β cyclodextrin (5%) |
30 mg/ml |
| 9. | Blend 9 | Sodium citrate (5%) Sodium benzoate (5%) L-Arginine (10%) Benzoic acid (5%) Niacinamide (2.5%) |
30 mg/ml |
| 10. | Blend 10 | Sodium citrate (2.5%) Sodium benzoate (5%) L-Arginine (10%) DL-Valine (5%) Sodium caprylate (5%) Benzoic acid (5%) Niacinamide (2.5%) |
40 mg/ml |
| 11. | Blend 11 | Sodium citrate (5%) Sodium caprylate (5%) HP-β cyclodextrin (5%) L-Arginine (10%) Benzoic acid (5%) |
30 mg/ml |
| 12. | Blend 12 | L-Arginine (10%) Poloxamer 407 (5%) Sodium caprylate (5%) Sodium benzoate (5%) Sodium citrate (5%) Benzoic acid (5%) |
20 mg/ml |
| 13. | Blend 13 | Sodium caprylate (5%) L-Arginine (10%) DL-Valine (5%) Poloxmer 407 (5%) Benzoic acid (5%) |
20 mg/ml |
| 14. | Blend 14 | Sodium caprylate (5%) Sodium benzoate (5%) Sodium citrate (5%) L-Arginine (10%) Poloxamer 407 (5%) Lycine Hcl (10%) |
40 mg/ml |
| 15. | Blend 15 | Glycine (10%) Poloxamer 407 (5%) Sodium caprylate (5%) Sodium benzoate (5%) Sodium citrate (5%) |
20 mg/ml |
| 16. | Blend 16 | L-Arginine (10%) Glycine (10%) |
10 mg/ml |
| 17. | Blend 17 | Sodium caprylate (5%) Sodium benzoate (5%) Sodium citrate (5%) Niacinamide (2.5%) |
15 mg/ml |
| 18. | Blend 18 | Sodium acetate (5%) Sodium benzoate (5%) Sodium citrate (5%) Niacinamide (2.5%) |
5 mg/ml |
| 19. | Blend 19 | Sodium acetate (5%) Sodium citrate (5%) |
5 mg/ml |
| 20. | Blend 20 | Sodium acetate (5%) Sodium benzoate (5%) Sodium citrate (5%) Niacinamide (5%) |
5 mg/ml |
Table 3. The approximate solubility of piroxicam in different blends.
Selection of blends for formulation of topical solutions and gel: Two blends were considered as they provide great solubility to piroxicam for formulation of topical solution. As a result, these mixtures were chosen for further research. These studies were conducted based on visual appearance, which included precipitation, crystal development, and clarity. Prepared blends with acceptable medication concentrations were visually observed in these experiments. For clarity and precipitation, these solutions were observed. Blend-9 and Blend-11 were used for preparation of aqueous topical solution of drug as they showed maximum solubility [14-17].
To prepare aqueous topical gels, same blends were chosen. Caropol 934 has been found as suitable gelling agent for formulations. The visual appearance of the samples was considered in the selection experiments, which included clarity, spreadability, and grittiness. Two blends, Blend-9 and Blend-11, with required quantities of solubilizers were chosen for the production of aqueous topical gel formulations, and they were used in the preparation of aqueous topical gel formulations containing 1% piroxicam.
Preparation of topical solutions using selected blends: About 1% Topical solution of piroxicam using mixed solvency concept was prepared, for this initially all the solubilizers, were taken and weighed accurately according to quantities decided in a 100 ml clean and calibrated volumetric flask and sufficient amount of water was added to dissolve them. When drug dissolves totally, volume was made up with remaining water. This gave 100 ml of blend having 25% / 30% concentrations of solubilizers [18].
Now, 1 gm of piroxicam drug was weighed and transferred to a 100 ml volumetric flask. After this about 60 ml of blend was added to dissolve the drug, when a clear solution was obtained, volume was made up using remaining blend solution. Hence, the topical solution using Blend 9 and Blend 11 was prepared and stored in an air tight container.
The quantities required for formulation composition are shown in tables 4 and 5.
| S.no | Ingredients | Quantity for 100 ml | Uses |
|---|---|---|---|
| 1. | Piroxicam | 1 gm | Active ingredient |
| 2. | L-Arginine | 10 gm | Solubilizer |
| 3. | Sodium citrate | 2.5 gm | Solubilizer |
| 4. | Sodium benzoate | 5 gm | Solubilizer |
| 5. | Benzoic acid | 5 gm | Solubilizer |
| 6. | Niacinamide | 2.5 gm | Stabilizer |
| 7. | Propylene glycol | 10 ml | Humeactant |
| 8. | Water (milli-Q water) | q.s. 100 ml | Vehicle |
Table 4. Formulation composition of topical solution PLF1.
| S.no | Ingredients | Quantity for 100 ml | Uses |
|---|---|---|---|
| 1. | Piroxicam | 1 gm | Active ingredient |
| 2. | L-Arginine | 10 gm | Solubilizer |
| 3. | Sodium caprylate | 5 gm | Solubilizer |
| 4. | Sodium citrate | 5 gm | Solubilizer |
| 5. | Cyclodextrin | 5 gm | Solubilizer |
| 6. | Benzoic acid | 5 gm | Solubilizer |
| 7. | Propylene glycol | 10 ml | Humeactant |
| 8. | Water (milli-Q water) | q.s. 100 ml | Vehicle |
Table 5. Formulation composition of topical solution PLF2.
Preperation of topical gel
For preparation of gel using mixed solvency concept, firstly blend solution was prepared using decided quantity of solubilizers. All the solubilizes were taken in a 100 ml volumetric flask and 60 ml of milli-Q water was added to dissolve through shaking for about 10-15 minutes the solubilizers, now 1 g of piroxicam was added to it with continues shaking until drug gets dissolve. Twenty ml of water was added and about 3.5 g of carbopol 934 was added gradually with a continuous stirring in a water bath at 40°C-50°C with avoidation of bubble formation. When transparent gel was formed, the blend solution was added gradually with continuous stirring. The gel was cooled completely using ice bath and the consistency was checked. Now the gel was stored in an air tight container [19-27].
The quantities required for formulation composition are shown in tables 6 and 7.
| S.no | Ingredients | Quantity for 100 ml | Uses |
|---|---|---|---|
| 1. | Piroxicam | 1 gm | Active ingredient |
| 2. | L-Arginine | 10 gm | Solubilizer |
| 3. | Sodium citrate | 2.5 gm | Solubilizer |
| 4. | Sodium benzoate | 5 gm | Solubilizer |
| 5. | Benzoic acid | 5 gm | Solubilizer |
| 6. | Niacinamide | 2.5 gm | Stabilizer |
| 7. | Propylene glycol | 10 ml | Humeactant |
| 8. | Carbopol 934 | 3.5 gm | Gelling agent |
| 9. | Water (milli-Q water) | q.s. 100 ml | Vehicle |
Table 6. Formulation composition of topical gel PLF3.
| S.no | Ingredients | Quantity for 100 ml | Uses |
|---|---|---|---|
| 1. | Piroxicam | 1 gm | Active ingredient |
| 2. | L-Arginine | 10 gm | Solubilizer |
| 3. | Sodium caprylate | 5 gm | Solubilizer |
| 4. | Sodium citrate | 5 gm | Solubilizer |
| 5. | Cyclodextrin | 5 gm | Solubilizer |
| 6. | Benzoic acid | 5 gm | Solubilizer |
| 7. | Propylene glycol | 10 ml | Humeactant |
| 8. | Carbopol 934 | 3.5 gm | Gelling agent |
| 9. | Water (milli-Q water) | q.s. 100 ml | Vehicle |
Table 7. Formulation composition of topical gel PLF4.
Evaluation of topical solutions and gels
pH determination of formulations: About 5 ml of topical solution was taken in a 25 ml dry and clean beaker and the pH determined using cyber scan 510 pH meter fitted with electrode and data was recorded.
About 5 g of topical gel was taken in 25 ml dry beaker and diluted up to 25 ml using milli-Q water, then was recorded using cyber scan 510 pH meter fitted with an electrode.
Table 8 shows pH of selected topical solutions and gels.
| S.no | Selected topical solution | pH |
|---|---|---|
| 1. | F1 | 6.90 |
| 2. | F2 | 6.82 |
| 3. | F3 | 5.40 |
| 4. | F4 | 5.23 |
Table 8. pH of selected topical solutions and gels.
Drug content
About 1.0 ml of topical solution and 1.0 g for topical gel was placed in a 100 ml volumetric flask to determine the drug content. About 10 ml of 20 % sodium caprylate solution was added, and the flask was sonicated for 15 minutes and the volume was make up to 100 ml with DM water. The resulting solution was filtered via Whatman filter paper no. 41. The filtrate was properly diluted and spectrophotometrically examined [28-34].
The results are summarized in table 9 below.
| S.no | Topical solution formulation | Percent drug content (% w/v) |
|---|---|---|
| 1. | F1 | 1.02 % |
| 2. | F2 | 1.08 % |
| 3. | F3 | 1.02% |
| 4. | F4 | 1.03% |
Table 9. Drug content of topical solutions and gels.
Freeeze-Thaw testing
Freeze-thaw stress testing used to study the chances of precipitation in formulation. Both the selected topical solutions were filled in a vial and capped properly. The vials were stored at temperature of 2°C-8°C in a refrigerator for 24 hours and then in oven at temperature 40°C for another 24 hour. After oven, the vials were then placed in a room temperature. This is the first cycle of testing. Likewise 7-7 such cycles were performed alternately at 2°C-8°C then 40°C oven and then room temperature to observe the precipitation and turbidity.
As result, no precipitation was found in both the vials containing aqueous topical solution.
TLC (Thin Layer Chromatography) analysis of topical solutions
The analysis study was performed to find any possibility of interactions between the solubilizers and the drug. A plate of silica gel GF 254 was activated at the temperature 110°C for 1 hour. A spot of drug solution was made using 2% solution of drug in ethanol and on another side topical solution was spotted. Now, the plate was allowed to dry [35,36].
The aqueous solvent system was prepared using 20% solution (having 10% sodium benzoate, 10% sodium caprylate) in a beaker. After the drying of spot the plate was placed in a solvent medium and allowed to run for about 5 cm. The plate was dried and the spot was observed under UV light chamber. Rf values were recorded in table 10.
| Solvent system | Absorbent | Rf Value | Rf value | ||
|---|---|---|---|---|---|
| Pure drug | Drug in blend 9 | Pure drug | Drug in blend 11 | ||
| 20% Blend solution (10% sodium benzoate, 10% sodium caprylate) | Silica Gel GF 254 | 0.55 | 0.56 | 0.75 | 0.75 |
Table 10. TLC analysis of pure drug and selected topical solutions.
Physical evaluation of topical gels
By examining the gel against a white background, the clarity and color of the topical gels were determined visually. Small sized polythene bag were used to fill the prepared gels. The capability of the gel to extrude from the gel when pushing through the polythene bag determines the gel's extrudability. The prepared gels were applied to human skin, and the spreadability of the gels was investigated.
Physical evaluation studies were recorded in following table 11.
| Gel formulations | Color | Clarity | Spreadibility | Extrudibility |
|---|---|---|---|---|
| F3 | White | Clear | Good | Good |
| F4 | White | Clear | Good | Good |
Table 11. Physical evaluation of topical gels.
In-vitro drug release study of topical solution of piroxicam
The in-vitro drug release studies were performed using dialysis membrane to measure amount of drug released in a respective medium. The dialysis membrane was firstly activated and humectant glycerol can be removed by washing the membrane with running water for 3-4 hours. The sulphate salts were removed by soaking the tubing in a 0.3 percent (w/v) sodium sulphide solution for 1 minute at 80°C. It was washed for 2 minutes with hot water (60°C), then acidified with a 0.2 percent (v/v) sulfuric acid solution, then rinsed with hot water to remove the acid [37-40].
The experiment was performed under certain conditions like, the release medium having phosphate buffer pH 7.4 of volume 200 ml and rotated at 50 rpm at temperature 37°C.
Method used for in-vitro drug release study
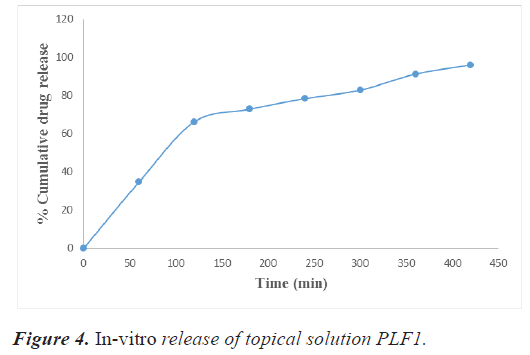
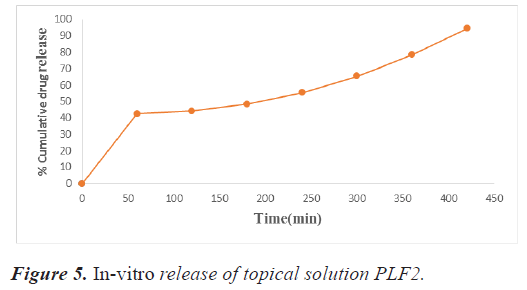
The percent drug release of the developed topical solution was calculated by placing 1 ml of the developed topical solution in a dialysis membrane placed in a 250 ml beaker containing 200 ml of phosphate buffer of pH 7.4. Ten ml of sample was taken at regular intervals (1 hr, 2 hr, up to 7 hr) and replenished with an equal volume of phosphate buffer of 7.4 pH for maintaining sink condition. The samples were examined by a double beam UV-visible spectrophotometer (Shimadzu 1700) at 262 nm. The gel/ solution's area of contact with the medium was 15 cm2. Tables 12 and 13 showing in-vitro release data of PLF1, PLF2 and graphically represented in figures 4 and 5.
| S.no | Time (min) | % Cumulative drug release (%) |
|---|---|---|
| 1. | 0 | 0 |
| 2. | 60 | 34.73 |
| 3. | 120 | 66.32 |
| 4. | 180 | 72.93 |
| 5. | 240 | 78.48 |
| 6. | 300 | 82.91 |
| 7. | 360 | 91.39 |
| 8. | 420 | 96.11 |
Table 12. Absorbance data for in vitro drug release study of topical solution PLF1.
| S.no | Time (min) | % Cumulative drug release (%) |
|---|---|---|
| 1. | 0 | 0 |
| 2. | 60 | 42.43 |
| 3. | 120 | 44.09 |
| 4. | 180 | 48.31 |
| 5. | 240 | 55.39 |
| 6. | 300 | 65.38 |
| 7. | 360 | 78.36 |
| 8. | 420 | 94.38 |
Table 13. Absorbance data for in vitro drug release study of topical solution PLF2.
In-vitro drug release study of topical gels
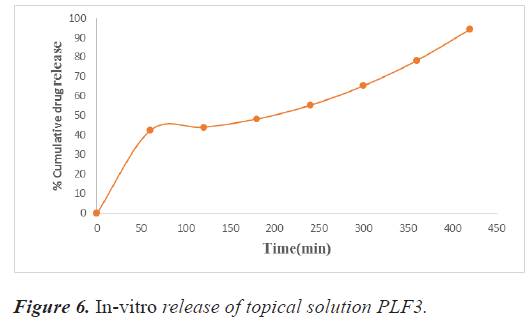
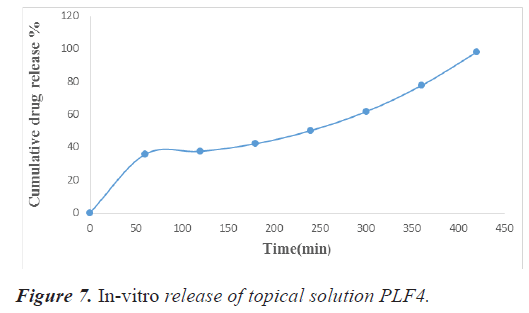
The release studies were carried out by inserting 1 g of the formulated gel in the dialysis bag in a 250 ml beaker containing 200 ml of phosphate buffer 7.4 pH. 10 milliliters of sample were taken at 1, 2, 3, 4, 5, 6, and 7, hours. In order to keep the sink condition, this was replaced with an equal volume of buffer. Tables 14 and 15 shows the drug release statistics and represented graphically in figures 6 and 7.
| S.no | Time (min) | %Cumulative drug release (%) |
|---|---|---|
| 1. | 0 | 0 |
| 2. | 60 | 42.40 |
| 3. | 120 | 44.09 |
| 4. | 180 | 48.31 |
| 5. | 240 | 55.39 |
| 6. | 300 | 65.38 |
| 7. | 360 | 78.36 |
| 8. | 420 | 94.38 |
Table 14. Absorbance data for in vitro drug release study of topical gel PLF3.
| S.no | Time (min) | %Cumulative drug release (%) |
|---|---|---|
| 1. | 0 | 0 |
| 2. | 60 | 35.65 |
| 3. | 120 | 37.44 |
| 4. | 180 | 42.10 |
| 5. | 240 | 50.09 |
| 6. | 300 | 61.63 |
| 7. | 360 | 77.46 |
| 8. | 420 | 97.84 |
Table 15. Absorbance data for in vitro drug release study of topical gel PLF4.
Results and Discussion
The motivation behind this investigation was to see if mixed solvency concept may help improve transdermal penetration and formulation of aqueous topical gels and solutions for a weakly water-soluble medication.
Piroxicam is a non-selective cyclooxygenase inhibitor and Non-Steroidal Anti-Inflammatory Drug (NSAID) used to treat musculoskeletal problems, dysmenorrhea, and postoperative pain. A sample of the bulk drug was obtained and characterized using UV, DSC, and melting point studies. The outcomes of the experiments were consistent with those described in official compendia; hence the sample obtained was employed for further research.
In the current study, the piroxicam drug sample was subjected to various characterization parameters. The characterization result of the drug sample obtained confirmed that it was piroxicam and it was used for further research.
The calibration curve of the drug, Piroxicam in DM water was developed during the preformulation studies. The absorbances at 354 nm of solution or pure drug and drug with solubilisers are nearly same therefore no interference was shown by the excipients in the UV visible estimation of 354 nm.
Various aqueous solutions of mixed blend solubilisers were used to study the solubility of piroxciam. The drug's calibration curve was made in an aqueous solution of sodium caprylate (20% w/v, the linearity of the calibration curve revealed that the Beer Lambert's law was obeyed in the concentration range of 5-25 μg/ml at a maximum wavelength of 354.0 nm.
The clarity and precipitation study of blends were used to choose solubilizers for the formulation creation of an aqueous topical solution and gel. For the formulation development of aqueous topical solutions, two blends with a minimum concentration of solubilizers were chosen. Gels using Carbopol 934 were formed in less concentration with good clarity and organoleptic properties than other gels. These gels were further evaluated. The final batches of topical solution and gels were made, as well as evaluation trials. Gels were made from batches of the above-mentioned topical solution blends (Blend 11 and Blend 16).
Conclusion
The aforementioned study discovered that several solubilizers from the hydrotropes and cosolvents can improve transdermal penetration of a poorly water-soluble medication (propylene glycol). Carbopol as a gelling agent and sodium caprylate, sodium citrate, L-arginine, cyclodextrin, niacinamide and propylene glycol as solubilizers (all GRAS) can be used to make aqueous topical gels and solutions with good clarity, spreadability, extrudability, and stability.
References
- Maheshwari RK, Shilpkar R. Formulation development and evaluation of injection of poorly soluble drug using mixed solvency concept. Int J Pharma Biosci 2012; 3: 179-189.
- Solanki SS, Soni LK, Maheshwari RK. Study on mixed solvency concept in formulation development of aqueous injection of poorly water soluble drug. J Pharm 2013; 1-8.
[Crossref] [Google Scholar] [PubMed]
- Pawar PB, Rawat S, Mahajan YY, Galgatte UC, Maheshwari RK. Formulation development and evaluation of aqueous injection of poorly soluble drug made by novel application of mixed solvency concept. Int J Drug Deliv 2013; 5: 152-166.
- Wohlrab J. "Topical preparations and their use in dermatology." J Dtsch Dermatol Ges 2016; 14: 1061-1070.
[Crossref] [Google Scholar] [PubMed]
- Akhter SA, Barry BW. “Absorption through human skin of ibuprofen and flurbiprofen; effects of dose variation, deposited drug films, occlusion and penetration enhancer N-methyl pyrrolidone.” J Pharm Pharmacol 1985; 37: 27-37.
[Crossref] [Google Scholar] [PubMed]
- Solanki SS, Soni LK, Maheshwari RK. Solubilization of poorly water soluble drug using mixed solvency approach for aqueous injection. J Pharm Res Int 2014; 4: 549568.
[Crossref] [Google Scholar] [PubMed]
- Indian Pharmacopoeia. The Indian Pharmacopoeia Commission 2007.
- Gour V, Garg A, Shukla A, Yadav AK. Development and evaluation of metronidazole injection by mixed solvency approach. Asian J Biomat Res 2014; 2: 38-45.
- Gayakwad PS, Gavit AJ, Rajput PV, Bari MM, Barhate SD, Maheshwari RK. Formulation development and evaluation of an aqueous injection of gatifloxacin by novel mixed solvency technique. World J Pharm Res 2014; 3: 1642-1664.
- Solanki SS. Development of parenteral formulation of poorly water soluble drugs: the role of novel mixed-solvency concept. Asian J Pharm 2017; 11: 1-10.
- Maheshwari N, Maheshwari RK. Formulation development of dry injection for reconstitution of a poorly water soluble drug, candesartan cilexetil, using mixed solvency concept and their evaluations. Int J Sci Res 2018; 8: 1313-1320.
- Padiyar A, Maheshwari RK. Novel dry injection for reconstitution of aspirin using solid solubilisers. J Drug Deli Ther 2017; 7: 44-45.
- Khan MA. Enhancement of solubility of poorly water soluble drugs diclofenac sodium by mixed solvency approach. Res J Pharm Dosage Forms Tech 2013; 5: 39-41.
- Kendre PN, Pande VV, Chavan KM. Novel formulation strategy to enhance solubility of Quercetin. Pharmacophore 2014; 5: 358370.
- Kumar CJ, Kumar MD. Solubility enhancement of theophylline drug using mixed solvency approach. Int J Chem Sep Tech 2015; 1: 1-4.
- Gadade D, Lohade, TS, Lahoti SR, Rawat SS, Maheshwari RK. Solubility enhancement of ofloxacin by mixed solvency approach. Indian Drugs 2015; 55: 34-40.
- Jain S, Khan A, Jain SD, Maheshwari RK. Solubility enhancement of paracetamol by mixed solvency method. Res and Rev: J Comp Biol 2019; 8:19-22.
- Kushwaha A, Gupta MK, Goswami A. Formulation and evaluation of optimized batch in situ gel of ondansetron hydrochloride. Pharmacia: An International Journal of Pharmaceutical Sciences 2016; 3: 11-16.
- Kumar D. Domperidone nasal gel using fenugreek seed as mucoadhesive agent: water solubility problem sort-out. Trends in Pharmaceuticals and Nanotechnology 2019; 2: 1-11.
- Agrawal A, Maheshwari RK. Formulation development and evaluation of in situ nasal gel of poorly water soluble drug using mixed solvency concept. Asian J Pharm 2014; 5: 131-140.
- Maheshwari RK, Rajagopalan R. Formulation and evaluation of paracetamol syrup made by mixed-solvency concept. Der Pharmacia Lettre 2012; 4: 170-174.
- Maheshwari RK, Rajagopalan R. Formulation and evaluation of tinidazole syrup made by mixed solvency concept technique. Der Pharmacia Lettre 2011; 3: 266-271.
- Soni LK, Solanki SS, Maheshwari RK. Evaluation of analgesic, antiinflammatory and ulcerogenic liability of oral solution (syrup) formulation developed by novel mixed solvency concept. Adv Pharmacol Toxicol 2015; 16: 21-30.
- Gahlot N, Maheshwari RK. Formulation and development of vaginal films of poorly water soluble drug, metronidazole, using mixed solvency concept and their evaluations. J Drug Deli Ther 2018; 8: 41-48.
- Carpenter G, Maheshwari RK. Formulation and development of fast dissolving oral film of a poorly soluble drug, frusemide with improved drug loading using mixed solvency concept and its evaluation. J Drug Deliv Ther 2018; 8: 132-141.
- Mulani P, Maheshwari RK. Formulation development of aqueous topical solutions and gels of poorly water soluble drug nimesulide, using novel application of mixed solvency concept and their evaluations. Int J Sci Res 2018; 8: 1521-1529.
- Singh S, Maheshwari RK, Gahlot N. Formulation development of topical solutions of poorly water-soluble drug indomethacin employing novel application of mixed solvency concept and their evaluation. Int J Green Pharm 2018; 12: 73-79.
- Maheshwari RK, Chaklan N, Singh S. Novel pharmaceutical application of mixed solvency concept for development of solid dispersions of Piroxicam. Eur J Biomed Pharm Sci 2015; 1: 578-591.
- Mansuk AG, Deshmukh D, Maheshwari, RK, Pachpute T. Formulation optimization and characterization of solid dispersion of piroxicam prepared by novel application of mixed solvency concept. Pharm Res 2020; 2: 41-46.
- Bagel J, Maheshwari RK. Novel application of mixed solvency concept in the development of fast dissolving solid dispersion of poorly water-soluble drug, torsemide and its evaluations. World J Pharm Res 2020; 9: 1820-1839.
- Chouhan M, Gupta D, Choukse R, Maheshwari RK. Formulation and evaluation fast dissolving tablets of lovastatin using solid dispersion method. J Drug Del Ther 2019; 9: 2931.
- Agrawal R, Maheshwari RK. Novel application of mixed solvency concept in the development of oral liquisolid system of a poorly soluble drug, cefixime and its evaluations. J Drug Del Ther 2018; 8: 5-8.
- Barua D, Indurkhya A, Maheshwari RK, Kumar P. Formulation of rifabutin liquisolid system using mixed solvency concept and their evaluation. Int J Pharm Pharma Sci 2019; 4: 2455-2698.
- Jain S, Maheshwari RK. Formulation development of oral liquisolid system of poorly water soluble drug, piroxicam, using mixed solvency concept and their evaluations. Int J Sci Res 2018; 8: 1703-1711.
- Rathod M, Agarwal S. Development and evaluation of furosemide microspheres made by mixed solvency concept. Int J Pharma Erud 2013; 2: 22-31.
- Maheshwari RK, Chandna C. Mixed solvency concept in reducing surfactant concentration of self-emulsifying drug delivery systems of candesartan cilexetil using D-optimal mixture design. Asian J Pharma 2013; 7: 83-91.
- Maheshwari RK, Jain S, Padria A, Mulani P, Baghel JS, Maheshwari N. Eco-friendly extraction using solids: A novel application of mixed solvency concept. J Drug Del Ther 2019; 9: 244-249.
- Padiyar A, Maheshwari RK, Jain S. Formulation and development of high concentration diclofenac sodium injection using mixed solvency concept and its evaluation. Int J Adv Pharma 2016; 6: 78-84.
- Jat P, Maheshwari RK. Formulation development of dry powder injection for reconstitution of poorly water-soluble drug, indomethacin, using mixed solvency concept and their evaluations. Int J Pharma Res Appli 2021; 6: 314-326.
- Jain A, Maheshwari RK. Novel application of mixed solvency concept in formulation and development of fast dissolving oral films of poorly water soluble drug, amlodipine besylate and their evaluations. Int J Pharma Res Appl 2021; 6: 35-55.