Review Article - Journal of Biochemistry and Biotechnology (2021) Volume 4, Issue 5
Food product shelf stability overview of sourdough-risen flatbread
Melaku Tafese Awulachew*
Department of Food Engineering, Addis Ababa University, Addis Ababa, Ethiopia
- Corresponding Author:
- Melaku Tafese Awulachew
Department of Food Engineering
Addis Ababa University
Addis Ababa, Ethiopia
E-mail: melakutafese12@gmail.cm
Accepted date: August 30, 2021
Citation: Awulachew MT. Food product shelf stability overview of sourdough-risen flatbread. J Biochem Biotech. 2021;4(5):14-18.
Abstract
This paper aims to provide an overview of food preservation related to the shelf-life and stability of food products including sourdough-risen flatbread (injera). Understanding the properties and composition of injera products enables one for a better option for maintaining food quality at desirable level of properties or nature for their maximum benefits. Food quality loss can be described in terms of as environmental factors which include temperature, relative humidity, light, mechanical stress and total pressure such as compositional factors, concentration of reactive species, microorganism levels, catalysts, reaction inhibitors, pH and water activity, as well. There are a range of points in the food chain where manufacturers can influence the mix of intrinsic and extrinsic factors which affect shelf-life. Advances in processing and packaging materials and techniques have increased the options available for maintaining quality and for improving the shelf-life of foods.
Keywords
Injera, Shelf life, Food quality, Stability.
Introduction
Injera is an Ethiopia traditional tin flat bread made from water, flour, and starter (ersho), which is a fluid saved from previously fermented dough. Teff is the most popular grain for making injera, although other grains such as sorghum, maize, barley, wheat and finger millet are sometimes used. Teff [Eragrostis tef (Zucc) Trotter] has the largest share of area (23.42%, 2.6 million hectares) under cereal cultivation and third (after maize and wheat) in terms of grain production (18.57%, 29.9 million quintals) in Ethiopia (Central Statistical Agency of Ethiopia, 2008). A mixture of ingredients then let to ferment and baked on clay plate. Then it served with a variety of stews (wot) or sometimes salads (Cooking of Science) [1]. The shelf-life of a food is the period for which it remains safe and suitable for consumption. Those, the foods such as injera has not deteriorated by any means of enzymatically, biological, physical or other means in quality or spoiled in any way that the consumer would find unacceptable. The more recent IFST guidelines provide a more workable definition of shelf-life: shelf-life is the time during which the food product will: remain safe; be certain to retain desired sensory, microbiological characteristics, chemical, physical and physical; comply with any label declaration of nutritional data, when stored under the recommended conditions [2].
Literature Review
Factors affecting shelf stability of food
Food quality loss can be described in terms of compositional factors: microorganism levels, concentration of reactive species, catalysts, reaction inhibitors, water activity and pH, and also, environmental factors: temperature, relative humidity, light, mechanical stress and total pressure [3]. Such factors can influence shelf-life, and can be categorized into intrinsic and extrinsic factors (IFST, 1993). The Intrinsic factors are influenced by such variables as raw material type and quality, and product formulation and structure. They include the following: Water Activity (WA) (available water); pH value and total acidity; type of acid; Redox potential (Eh); Available oxygen; Nutrients; Natural microflora and surviving microbiological counts; Natural biochemistry of the product formulation (enzymes, chemical reactants) and use of preservatives in product formulation (e.g., salt) whereas the extrinsic factors are those factors the final product encounters as it moves through the food chain. They include the following: Time-temperature profile during processing; pressure in the headspace; Temperature control during storage and distribution; Relative Humidity (RH) during processing, storage and distribution; Exposure to light (UV and IR) during processing, storage and distribution; Environmental microbial counts during processing, storage and distribution; Composition of atmosphere within packaging; Subsequent heat treatment (e.g. reheating or cooking before consumption) and Consumer handling.
All these factors can operate in an interactive and often unpredictable way, and the possibility of interactions must be investigated. A particularly useful type of interaction occurs when factors such as reduced temperature, mild heat treatment, antioxidant action and controlled atmosphere packaging operate in concert to restrict microbial growth, the so-called ‘hurdle effect’. This way of combining factors which, individually, are unable to prevent microbial growth but, in combination, provide a series of hurdles which do so, allows manufacturers to use milder processing techniques which retain more of a product’s sensory and nutritional properties. The interaction of such intrinsic and extrinsic factors as these either inhibits or stimulates a number of processes which limit shelf-life.
Intrinsic factors: Intrinsic parameters are properties that exist as part of the food product itself. These parameters are: pH, Moisture content, Oxidation-reduction potential (Eh), Nutrient content, antimicrobial constituents, and biological structures. Under a set of conditions, these parameters promote microbiological growth [4].
pH: The growth and metabolism of microorganisms are influenced by pH [5]. pH is an important factor affecting growth of microorganisms in food because it affects: Microbial energy metabolism involving the buildup of hydrogen ion concentration gradients across membrane and Microbial enzyme activity and stability of cellular macromolecules [6]. Bacteria tend to be more fastidious (complex nutritional or cultural requirements for growth) in their relationships to pH than molds and yeasts, with the pathogenic bacteria being the most fastidious [7]. Most foods are at least slightly acidic, since materials with an alkaline pH generally have a rather unpleasant taste. Some foods are characterized by inherent acidity; others owe their acidity or pH to the actions of certain microorganisms. The latter type is referred to as biological acidity and is displayed by products such as fermented milks, sauerkraut, and pickles [3]. The acidity of a product can have important implications for its microbial ecology and the rate and character of its spoilage (Adams and Moss) [8].
Moisture content: The water requirement of microorganisms is described in terms of the water activity (aw) in the environment. This parameter is determined by the ratio of the water vapor pressure of food substrate to the vapor pressure of pure water at the same temperature: aw=p/po, where p is the vapor pressure of the solution and po is the vapor pressure of the solvent (usually water). Those the water activity of pure water is 1.00 [9]. This concept is related to Equilibrium Relative Humidity (ERH) in that ERH=100 × aw. The aw of most fresh foods is above 0.99. Every microorganism has a limiting aw value below which it will not grow, form spores, or produce toxic metabolites. Water may influence chemical reactivity in different ways; may also change the mobility of the reactants by affecting the viscosity of the food systems and form hydrogen bonds or complexes with the reacting species. Thus, a very important practical aspect of water activity is monitoring and/or controlling unwanted enzymic and chemical reactions that reduce the shelf life of foods [10].
Redox potential, Eh: The oxidation-reduction potential (also referred to as the redox potential and abbreviated Eh or ORP) is a physicochemical parameter that determines the oxidizing or reducing properties of the medium, and it depends on the composition of the food, pH, temperature, and, to a large extent, the concentration of Dissolved O2 (DO). An oxidation-reduction (redox) reaction occurs as the result of a transfer of electrons between molecules or atoms. In living cells an ordered sequence of both electron and hydrogen transfer reactions is an essential feature of the energy generation and electron transport chain by oxidative phosphorylation. The tendency of a medium to donate or accept electrons, to oxidize or reduce, is termed as redox potential (O/R potential) which is expressed by the symbol Eh [8].
Nutrient content: In order to function and grow normally, the microorganisms of concern in the food factory require: water, source of energy, source of nitrogen, vitamins and related growth factors, and minerals. As sources of energy, foodborne microorganisms may utilize sugars, alcohols, and amino acids. Some microorganisms are able to utilize complex carbohydrates such as starches and cellulose as sources of energy by first degrading these compounds to simple sugars. Fats are used by microorganisms as sources of energy, even though these compounds are attacked by are comparatively small number of microbes in foods [4].
Antimicrobial constituents: As a line of defense to attack by microorganisms, the product tissues may contain antimicrobial components, local concentration of which usually increases as a result of physical damage. Many natural constituents of plant tissues such as pigments, alkaloids and resins have antimicrobial properties. Antimicrobial components differ in their spectrum of activity and potency, they are present at varying concentrations in the natural product, and are frequently at levels too low to have any effect [8].
Biological structures: The properties and the natural covering of certain food sources provide superior protection against the entry and subsequent damage by spoilage organisms. Examples of such protective structure are testa of seeds, the outer covering of fruits, the shell of nuts, the hide of animals, and the shells of eggs [9]. They are usually a composed of macromolecules relatively resistant to degradation and provides an inhospitable environment for micro-organisms by having a low aw, a shortage of readily available nutrients [8].
Extrinsic factors
The extrinsic parameters of foods are independent of substrate concertation. The extrinsic parameters are those properties of the environment (Storage and processing) that exist outside of the food product; affect both the foods and their microorganisms [9]. Those of greatest importance to the welfare of food borne organisms are temperature of storage, relative humidity of environment, concentration and presence of gases, and activities of other microorganisms [4].
Temperature of storage: Microorganisms, individually and as a group, grow over a very wide range of temperatures. Therefore, it is well to consider the temperature of growth ranges for organisms in order to select the proper temperature for the storage of different types of foods [9]. Microbial growth can occur at a temperature range from about -8°C up to 100°C, and at atmospheric pressure [8]. Microbial growth is accomplished through enzymatic reactions. As temperature influences enzyme reactions (with every 10°C rise in temperature, the catalytic rate of an enzyme doubles and reduced to half by decreasing the temperature by 10°C), it has a crucial role in microbial growth in food [7]. On the basis of their temperature of growth important microorganisms in foods are divided into three groups of with an optimum temperature and a temperature range of growth: thermophiles (grow at relatively high temperature), with optimum at 55°C and range 45 to 70°C; mesophiles (grow at ambient temperature), with optimum at 35°C and range 10 to 45°C; and psychrophiIes (grow at cold temperature), with optimum at 15°C and range -5°C to 20°C (Ray and Bhunia) [7]. When the foods are exposed to temperatures beyond the maximum and minimum temperatures of growth, microbial cells die rapidly at higher temperatures and relatively slowly at lower temperatures. Those, understanding influence of temperature on viability is important in reducing food spoilage and microbial growth, in food bioprocessing and enhancing safety against pathogens.
Relative humidity of environment: The Relative Humidity (RH) of the storage environment is essential both from the standpoint of aw within foods and the growth of microorganisms at the surfaces [4]. RH is a measure of the water activity of the gas phase. When food commodities having a low water, activity are stored in an atmosphere of high relative humidity water will transfer from the gas phase to the food and the foods pick up moisture until equilibrium has been established. Similarly, foods with a high-water activity lose moisture when placed in an environment of low RH [8].
Presence and concentration of gases: Oxygen comprises 21% of among the earth’s atmosphere and is one of the most important gases in contact with food under normal circumstances. Its presence and its influence on redox potential are important determinants of the microbial associations that develop and their rate of growth (Adams and Moss) [8]. Carbon Dioxide (CO2) is the single most important atmospheric gas that is used to control microorganisms in foods. A reduction in the proportion of oxygen achieved by an increase in the proportion of carbon dioxide within specified limits maintains the original product quality extend the product shelf life. This is achieved by: inhibiting mould and bacterial growth, reducing oxidative changes and controlling biochemical and enzymatic activity to slow down senescence and ripening (Fellows) [11]. The CO2 inhibits microbial activity in one of the two ways: That the first way is CO2 dissolves in water in the food to form mild carbonic acid and of lowers the pH of the product; and the second way is CO2 has negative effects on enzymic and biochemical activities in cells of both micro-organisms and foods [12].
Presence and activities of other microorganisms: The inhibitory effect of some members of the food microbiota on other microorganisms is well established. Some foodborne organisms produce substances that are either lethal or inhibitory to others; include bacteriocins, antibiotics, organic acids and hydrogen peroxide (Table 1) [9].
| Intrinsic | Extrinsic |
|---|---|
| Microbiological of raw materials | Prerequisites of Hygiene and Good manufacturing practices |
| Raw material history | Hazard analysis control point |
| Food assessment and structure | Food processing |
| PH | Storage temperature |
| Types of acid present | Gas atmosphere |
| Water activity (aw) | Relative humidity |
| Redox potential (Eh) | Packaging |
| Biological structure | Retail practices |
| Oxygen availability | Customer practice |
| Nutritional content and availability | |
| Antimicrobial constituent | |
| Natural or artificial microflora of the food |
Table 1. Intrinsic and Extrinsic properties determining the quality loss of the food.
Shelf-life stability measurement
Shelf life of a food product is the time between the harvesting to processing or production and packaging of the product and the point at which it becomes unacceptable under defined environmental conditions. Distribution and Storage are necessary links in the food chain. Safety and quality considerations dictate the conditions and maximum duration of these links in the chain although most food deteriorations take place gradually. A total quality approach embraces all aspects of a food from its conception, through development and production to its consumption, and for a manufactured food product this will include: product design (including hazard analysis and risk assessment to ensure safety); specification and testing of ingredients and packaging materials; transport, storage and retail display; manufacturing processes; and storage at home and consumption. Because the food must be safe with an acceptable quality when consumed, the time for which this is maintained the shelf life is hence an essential aspect of product design the control of which is a requirement of good manufacturing Practice (IFST) and requirement of the international standard for quality systems, ISO 9001 standards (ISO). Shelf-life determination of a new product often requires storage for significant periods, and includes samples from early development stages as well as initial production runs. Through the evaluation of stored samples, potential storage problems can be identified and, either eliminated or controlled before the food goes into production. When in production, an ongoing quality assurance system is equally important, and involves assessment of freshly made products, typically before the production has been released into distribution.
Direct Method for shelf-life determination of foods: The common and direct way of determining the shelf-life is to carry out storage trials of the product under controlled conditions that simulate to encounter during distribution, storage, retail display and consumer use. Those the direct method of shelf-life determination of foods involves, Identification of causes for spoilage of food; Selection of suitable tests for determining spoilage of food; Planning of shelf-life study; Running the shelf-life study; Determination of the shelf life and monitoring the shelf life.
Indirect methods for determination of shelf life of food: The food industry has a great need to obtain, in a relatively short time. Consequently, procedures have been developed to predict or estimate shelf-lives quickly. Indirect methods attempt to predict the shelf life of a product without running a full-length storage trial; hence, they can be useful for products with long shelf lives. The two most common indirect methods are accelerated shelf-life studies and predictive modeling for shelf life. There are number of approaches to accelerated shelf-life testing but all are concerned with how to get reliable deterioration data in a short period, what model to use and how eventually to predict the actual shelf-life of the product. In principle, accelerated shelf-life testing is applicable to any deterioration process that has a valid kinetic model, that process may be biochemical, chemical, microbiological or physical. In practice, most accelerated tests have been done on deterioration processes that are chemical in nature. The basic idea is that the rate of a shelf-life limiting chemical reaction is increased at an elevated storage temperature. The end of shelf-life is thereby reached much quicker and the data obtained can be extrapolated to provide an estimate of the shelf-life at normal or ambient storage conditions, usually by using the Arrhenius relationship.
Accelerated shelf-life testing: Food manufacturers are under increasing pressures to introduce attractive new products into retail outlets with minimum delay, and legislation in many countries demands some form of sell by or use by labeling. While this is feasible for short shelf-life products, the introduction of new long shelf-life products requires knowledge of the storage characteristics over the intended shelf-life period, and can introduce unacceptable delays. The basic premise of an accelerated test is that by changing a storage condition, the chemical or physical process that leads to deterioration is accelerated, and that a predictive shelf-life relationship related to ambient conditions can be defined. The key to this premise is the assumption that the deteriorative process limiting shelflife remains the same under the two conditions. If this is not the case, and another deteriorative process dominates at the abuse condition, then a valid relationship is not attainable. It is also often (erroneously) assumed that accelerated deterioration can be achieved by raising the storage temperature, using an Arrhenius model [13].
Accelerated shelf-life testing: Food manufacturers are under increasing pressures to introduce attractive new products into retail outlets with minimum delay, and legislation in many countries demands some form of sell by or use by labeling. While this is feasible for short shelf-life products, the introduction of new long shelf-life products requires knowledge of the storage characteristics over the intended shelf-life period, and can introduce unacceptable delays. The basic premise of an accelerated test is that by changing a storage condition, the chemical or physical process that leads to deterioration is accelerated, and that a predictive shelf-life relationship related to ambient conditions can be defined. The key to this premise is the assumption that the deteriorative process limiting shelflife remains the same under the two conditions. If this is not the case, and another deteriorative process dominates at the abuse condition, then a valid relationship is not attainable. It is also often (erroneously) assumed that accelerated deterioration can be achieved by raising the storage temperature, using an Arrhenius model [13].
Predictive models: The food industry has long been interested in ways of predicting rates of deteriorative change resulting from differing combinations of intrinsic and extrinsic factors. With the increasing capabilities and availability of personal computers, predictive modelling, particularly of microbiological behaviour, has become a major area of research. Such models look for statistical and mathematical relationships between three sets of variables: intrinsic (product related) factors; extrinsic (environmental) factors; and implicit factors, the characteristics of the microorganism itself and how it behaves in the presence of combinations of intrinsic and extrinsic factors.
Measuring shelf-life
Sensory panels: Measurement of the changes in eating quality on storage requires the use of sensory techniques. There are substantial difficulties in ensuring high quality sensory data over long test periods, and instrumental methods can be an important back-up to sensory methods, provided that their limitations are recognized.
Instrumental methods: Sensory measures of quality changes on storage are an essential measure of perceived quality, but are expensive and time-consuming to operate. They also suffer from high variability when carried out over long time periods, requiring regular panel calibration. If valid instrumental methods are available, they can be of great value in augmenting sensory data.
Physical measurements: The most commonly used physical tests measure the changes in the texture of products. These changes may be the result of chemical reactions occurring in the product, such as those caused by interaction of ingredients or by environmental influences, such as moisture migration through the packaging. Methods of measurement for texture have to be chosen carefully so that the results correlate well with the textural changes as perceived by the use of sensory panels measuring attributes such as hardness, crispness and snap are commonly used during shelf-life testing [14].
Chemical measurements: Chemical analyses play a vital role in shelf-life testing as they can be used either to measure the end points of chemical reactions occurring in food during storage, or to confirm the results obtained by the sensory panels. Some examples of product deterioration caused by chemical reactions within the food.
Microbiological measurements: There are two important aspects to be considered in determining the microbiological stability of a product: microbial growth, which leads to the spoilage of a food product; and the growth of microbial pathogens that affect the safety of the product. The water activity, storage temperature, time and pH can be used to predict to a large extent the micro-organisms that are likely to grow in the product. The ‘time to spoilage’ can be determined by storing the product at the appropriate temperature and measuring the microbial load at staged intervals. The time to reach a pre-determined level of microbial count (total count and level of individual microbes) will be considered to be the end-point. Since it is advisable to leave a safety margin in setting the shelf-life, generally 70% of the time to spoilage is taken to be the storage life [15,16].
Reaction modeling principles
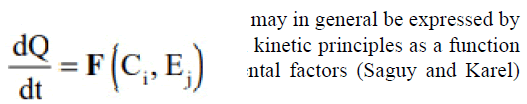
Were:
• Ci are composition factors, such as concentration of inorganic catalysts, reactive compounds, enzymes, reaction inhibitors, pH, water activity, as well as microbial populations and
• Ej are environmental factors, such as temperature, relative humidity, total pressure and partial pressure of different gases, light and mechanical stresses.
The established methodology consists of first identifying the chemical and biological reactions that influence the quality and the safety of the food and through a careful investigation of the food components and the process, the reactions judged to have the most critical impact on the deterioration rate, are determined (Labuza) [17].
Extending of shelf-life
There are a range of points in the food chain where manufacturers can influence the mix of intrinsic and extrinsic factors which affect shelf-life [18-20]. These include: Raw material selection and quality; Product formulation and assembly; the processing environment; Processing and preservation techniques; Packaging; Storage and distribution and Consumer handling. While all of these points are important, two of the most dynamic areas of research are in new processing methods and packaging techniques [21-23].
Influence of processing: The initial quality of a food product is determined by the quality of the raw materials and the processing methods used during the manufacture of the product [24]. A wide range of processing techniques is used in the food industry to achieve the required level of sensory and microbiological quality. In the case of a perishable product, the extent to which microbial growth can be controlled after processing and packaging determines the final shelf-life [25].
Packaging: There are many factors to be considered in choosing the optimal packaging form and material for any particular product, including the product characteristics, processing considerations, shelf-life required and overall cost. Advances in packaging materials and techniques have increased the options available for maintaining quality and for improving the shelflife of foods [26].
Discussion and Conclusion
Food preservation is generally any method of maintaining or an action taken to food at desirable level of properties or nature for their maximum benefits. Preservatives are additives that primarily contribute to food safety and the prevention of food spoilage. Advanced packaging materials also contribute to for improving and maintain quality the shelf-life of foods. In this regard, both processing and Packaging have recommended to enhancing the shelf-life of foods products including Injira (sourdough-risen flatbread).
References
- Cooking of Science: Ethiopian injera. Retrived from. 2008.
- IFST Shelf Life of Foods – Guidelines for its Determination and Prediction. Food Sci Technol. Institute of Food Science & Technology, London. 1993.
- Cadwallader KR, Weenen H. Freshness and shelf life of foods. J Am Chem. 2002.
- Jay JM, Loessner MJ, Golden DA. Modern food microbiology. Springer Science & Business Media. 2008.
- Bohra A, Parihar P. Food Microbiology. India: Agrobios. 2006.
- Lucas J. Integrating MAP with new germicidal techniques. In: Lucas J (eds) Novel food packaging techniques. Wood head Publishing Limited, Boca Raton, USA, 2003:pp.312-336.
- Ray B, Bhunia A. Fundamental food microbiology. CRC press, Boca Raton, USA. 2008.
- Adams MR, Moss MO. Food Microbiology. The Royal Society of Chemistry, Guildford, UK. 2000.
- Jay JM. Modern Food Microbiology. (6th edn), An Aspen Publication and Aspen publishers, Gaithersburg, Maryland. 2000.
- Robertson GL. Food Packaging Principles and Practice. (2nd edn), CRC Press, Boca Raton, Florida. 2006.
- Fellows P. Food Processing Technology. Wood head Publishing Limited, Cambridge, England. 2000.
- Dixon NM, Kell DB. The inhibition by CO2 of the growth and metabolism of micro‐organisms. J Appl Bacteriol. 1989;67(2):109-136.
- Labuza TP, Schmidl MK. Accelerated shelf-life testing of foods. Food technology (USA). 1985.
- Rodel, W. Water activity and its measurement in food. In: E. Kress Rogers. Instrumentation and Sensors for the Food Industry. Wood head Publishing, Cambridge. 1993.
- Patrick M. Leatherhead Food RA, personal communication. 2000.
- Saguy I, Karel M. Modeling of quality deterioration during food processing and storage, Food Technol. 1980;34(2): 78-85.
- Labuza TP, Schmidl MK. Accelerated shelf-life testing of foods. Food Technology.1985;134: 57-64.
- Anon HACCP: A Practical Guide. Campden Food and Drink Research Association Technical Memorandum. 1992:38.
- Cole MB, Davies KW, Munro G, et al. The effect of pH, NaCl concentration and temperature on the thermal inactivation of Listeria monocytogenes, in Predictive Microbiology and Computer Modeling for the Food Industry. In: Buchanan RL, Palumbo SA, Whiting RC (eds) in press, Macmillan, New York. 1994.
- Jones JE, Walker SJ. Advances in modeling microbial growth. J Ind Microbiol. 1993;12(3):200-205.
- Gould GW. Predictive mathematical modelling of microbial growth and survival in foods. Food Sci. Technol. 1989;3:89-92.
- McClure PJ, Blackburn CD, Cole MB, et al. Modelling the growth, survival and death of microorganisms in foods: the UK Food Micromodel approach. Int J Food Microbiol. 1994;23(3-4):265-275.
- Maxcy RB, Wallen SE. Heterogeneity of samples as a problem in shelf-life prediction. J food protec. 1983;46(6):542-544.
- ISO 9001:1987. Quality Systems – Model for Quality Assurance in Design/Development, Production, Installation and Servicing. International Organization for Standardization. 1987.
- Central Statistical Agency of Ethiopia. Agricultural Sample Survey: Report on Area and Production of Crops. Addis Ababa. 2008.
- McMeekin TA, Olley JN, Ross T, et al. Predictive Microbiology: Theory and Application, Taunton, Research Studies Press Ltd. 1993.