Research Article - Current Pediatric Research (2017) Volume 21, Issue 3
Factors associated with outcomes of severe pneumonia in children aged 2 months to 59 months at jimma university specialized hospital, southwest Ethiopia.
Firaol Bekele1, Melese Sinaga1, Javed Ahsan Quadri2, Abhay Kumar3, A Shariff2, Tabarak Malik4
1Nutrition Unit, Department of Population and Family Health, College of Health Science, Jimma University, Ethiopia.
2ENS Lab, Department of Anatomy, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
3Department of Transplant Immunology, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
4Department of Biomedical Sciences, College of Health Science, Jimma University, Ethiopia.
- *Corresponding Author:
- Tabarak Malik
Department of Biomedical Sciences
College of Health Science, Jimma University
Ethiopia.
Tel: +0251960263441
E-mail: malikitrc@gmail.com
Accepted date: May 29, 2017
Abstract
Background: Pneumonia is inflammation of the lungs parenchyma due to various etiologies and is a major cause of childhood morbidity and mortality worldwide. Every year approximately 158 million cases of pneumonia reported worldwide and out of which, 154 million pneumonia cases occurring in developing countries. The clinical presentation of childhood pneumonia varies depending upon the causative factors and host susceptibility and immune status of the subjects. Treatment options of pneumonia depend on severity of disease, causative factors and resistivity of pathogens (MDR) for drugs. Aim: To investigate the factors associated with treatment outcome of severe non multidrug resistant pneumonia in children less than 5 years of age in the Jimma region of Ethiopia. Methodology: A cross sectional study, included children aged 2-59 months with severe pneumonia. Data was collected using standard questionnaires recorded by face to face interview with parents/caretakers and all the clinical history of the patients were recorded from treatment data sheets. Results: Out of 107 children with severe pneumonia, 22 (20.6%) were lived in urban and 85 (79.4%) in rural areas. The male to female ratio were 1.18:1. Only 52.34% of the subjects were vaccinated as per WHO guidelines. 61.7% of the children were lived in houses with attached kitchen in living room. 6 (5.6%) were sero-positive for HIV. Malnutrition was found significantly high in subjects with pneumonia (p<0.05). A remarkable number (48.6%) of the children had family member with symptoms of URTI and 12 (11.2%) of the children had previous history of pneumonia. Only 8 (7.5%) of the children had history of cigarette smokers in the house. Conclusion: The risk factors for poor outcome in childhood pneumonia include rural residence and poor infrastructure, parental literacy rate, vaccination, nutritional status, household environment, co-residents with upper respiratory tract infections (URTI). Parental education, diet counseling, avoidance to contact with infected person will reduce the prevalence of pneumonia and will be helpful in better hospitalization outcomes.
Keywords
Severe pneumonia, Treatment outcomes, Hospitalization period, Malnutrition, Jimma.
Introduction
Pneumonia is inflammation of the lung parenchyma and is a substantial cause of childhood morb idity and mortality in in developing countries [1-4]. About 20% of all deaths in children under five years of age has been reported to be happen due to acute lower respiratory infection (ALRI) which includes: pneumonia, bronchiolitis, and bronchitis. About 90% of ALRI associated deaths takes place due to severe pneumonia [2]. Although most of the cases of pneumonia are caused by microorganisms, non-infectious causes include aspiration of food or gastric acid, foreign bodies, hydrocarbons, and lipoid substances, hypersensitive reactions and drug- or radiation-induced pneumonitis. The cause of pneumonia in an individual patient is often difficult to determine because direct culture of lung tissue is invasive and rarely performed in primary and secondary health care setups. Sputum and upper respiratory tract swab culture often fails to determine the nature of lower respiratory tract infection. Streptococcus pneumoniae (pneumococcus) infection is the most common in 3 weeks to 4 years old children. While, Mycoplasma pneumoniae and Chlamydophila pneumoniae infections are the most common in children above 5 years of age. Some time pneumonia may reoccur, called as recurrent pneumonia and defined as 2 or more episodes in a single year or 3 or more episodes ever, with radiographic clearing between occurrences.
The clinical presentation of childhood pneumonia varies depending upon the responsible pathogen and host severity. The clinical symptoms are non-specific; no single symptom or sign is pathognomonic for pneumonia in children. Viral and bacterial pneumonias are often preceded by days of symptoms of an upper respiratory tract infection, typically rhinitis and cough. Tachypnea is the most consistent clinical manifestation of pneumonia. Increased work of breathing accompanied by intercostal, subcostal, and suprasternal retractions, nasal flaring, and use of accessory muscles is common. Severe respiratory infection may be accompanied by cyanosis and respiratory fatigue, especially in infants. Auscultation of the chest may reveal crackles and wheezing, but it is often difficult to localize the source of these adventitious sounds in very young children with hyper resonant chests [2].
Bacterial pneumonia in adults and older children typically begins suddenly with a shaking chill followed by high grades of fever, cough, and chest pain. Other symptoms that may be seen include drowsiness with intermittent periods of restlessness; rapid respirations; anxiety; and, occasionally, delirium. Circumoral cyanosis may be observed. Physical findings depend on the stage of pneumonia. Early in the course of illness, diminished breath sounds, scattered crackles, and rhonchi are commonly heard over the affected lung field. With the development of increasing consolidation or complications of pneumonia such as effusion, empyema, and pyopneumothorax, dullness on percussion is noted and breath sounds may be diminished. A lag in respiratory excursion often occurs on the affected side [1].
Treatment of Severe Pneumonia
In case of severe Pneumonia child needs immediate hospitalization. In case of respiratory distress (severe lower chest wall indrawing or a respiratory rate of ≥ 70/ min), oxygen support may be given to maintain saturation. During first line of treatment benzyl penicillin (50 000 units/kg IM or IV, 6 hourly) will be administered for at least 3 days. High grades of fever will be managed with paracetamol. If wheezing sound is present, rapidacting bronchodilator gives relief. Hydration should be maintained with fluids. After symptomatic relief, patients may be switched to oral amoxicillin (25 mg/kg twice a day). The total course of antibiotic treatment takes 5 to 7 days. In case non-response to treatment up to 48 hours, or patients starts deteriorating treatment modalities should be chosen accordingly. If there are no apparent complications observed, patients may be switch to chloramphenicol (25 mg/kg every 8 h) until the child has improved [3]. Despite of hospitalization and available treatment options for pneumonia, it is a leading cause of infant death in developing countries.
In developing countries, respiratory tract infections are not only more prevalent but severe also, accounting for more than 2 million deaths annually; pneumonia is the number one killer of children [4-6]. In Ethiopia, pneumonia is a leading single disease killing under-five children. It is estimated that 3,370,000 children encounter pneumonia annually which contributes to 20 percent of all causes of deaths (over 40,000 under-five children) [7]. Many factors have been suggested as influencing factor for severity of childhood pneumonia but to the best of our knowledge, very few reports are available on socio-demographic/ socio-economic factors of the Jimma region of Ethiopia, affecting the outcome of the treatment of severe pneumonia in children under five years of age.
At JUSH, Jimma town also, pneumonia is one of the leading causes of hospital admission and cause child mortality. From September 2000-August 2000 E.C the leading cause of admission was severe pneumonia and out of 639 hospitalization, 228 (44.4%) were pneumonic children with recorded death of 3 children [8]. In 2004 a total of 340 children were admitted with severe pneumonia and 11 deaths were recorded [9].
Methodology
The Jimma town and its periphery were chosen for the study. Jimma town is located 354 km away from Addis Ababa in south western Ethiopia and has population of 207573 (2012 Gc). The residents of the city comprise of different ethnic groups Oromo being most common followed by Amara, SNNPR and others. The data collection was done in 2016 (after getting ethical Clarence from JUSH, Jimma University ethical committee) from pediatric OPD, JUSH, an super-specialty centre serving the Jimma town and its surroundings. Guardians (Mother and fathers) of children were explained about the purpose of the study and written consent was obtained prior to the data collection. Study subjects sampled from all under 5 sick children who are visiting JUSH, Pediatrics OPD. All the confirmed cases of severe pneumonia (as per WHO criteria), aged from 2 to 59 month were included in the study. The neonates were not included in the study because the child at this age is at highest risk of multiple infections and other complications, as confounding factors. The patients with history of congenital defects, genetic defects, anemia, tuberculosis, malignancies, recurrent pneumonia and other chronic diseases were excluded from the study.
The nutritional statuses of the subjects were evaluated by the measuring Mid-Upper Arm Circumference (MUAC). MUAC is the circumference of the left upper arm, measured at the mid-point between the tip of the shoulder and the tip of the elbow.
The recruited patients examined and parents/caretakers were interviewed to know the living conditions of the patients, socioeconomic status, associated risk factors and their clinical and treatment history were recorded to evaluate the treatment outcome with respect to variables as follows:
Age, sex, place of residence, family income, maternal occupation, paternal occupation, maternal educational status, paternal education status, housing/living conditions, nutritional status, immunization status, sero-positivity for HIV, parental smoking, URTI status in older family member, breast feeding status, duration of illness, duration of hospital stay.
Validation and Accuracy of Data Collection
Before the actual data collection, the format was pretested for its consistency so that the personal variations on interpretation of the questions will be minimized. A structured questionnaire was pretested on 5% of the study population that were not included in the main survey, to ensure clarity of questions and required amendment was made. The data collection was supervised to check for completeness, accuracy, inclusion/exclusion criteria to ensure quality of the data. The questionnaire and interviews were taken in the respondent’s language (Tables 1 and 2).
| Pneumonia | Severe Pneumonia | Non-Pneumonia |
|---|---|---|
| On examination, the child has cough or difficult breathing and fast breathing: ?age 2–11 months: ≥ 50/min ?age 1–5 years: ≥ 40/min ?Child with none of the signs of severe pneumonia ?Other signs of pneumonia (on auscultation) may be present, including crackles, reduced breath sounds, or an area of bronchial breathing (3) |
Pneumonia with at least one of the following: ?Central cyanosis ?Inability to breastfeed or drink, or vomiting everything ?Convulsions, lethargy or unconsciousness ?Grunting (in young infants) ?Lower chest wall in drawing ?Head nodding (3) |
No signs of pneumonia or severe pneumonia |
Table 1. Classification of pneumonia, severe pneumonia and no pneumonia based on clinical parameters
| Sl. | Parameters | Definition |
|---|---|---|
| 1. | Illiterate | A person who is unable to read or write |
| 2. | URTI in the house | Any family member or person living in the same house with the child in question who has symptoms of upper airway disease: cough, sneezing, rhinorrhea and/or difficulty swallowing |
| 3. | Severe acute malnutrition | A child with one of the following ?Weight for height (W/H) <70% ?Middle Upper Arm Circumference (MUAC) <11 cm (for 6 months-5 years.) ?Bilateral pitting edema ?Visible severe wasting (3) |
| 4. | Moderate malnutrition | ?W/H between 70 and 80 percentile ?MUAC between 11 and 12.5 cm |
| 5. | Mild malnutrition | ?W/H between 80 and 85 percentile ?MUAC between 12.5 and 13.5 cm |
| 6. | Well nourished | ?W/H above 85 percentile ?MUAC above 13.5 cm |
| 7. | Immunization status | ?Fully vaccinated: were vaccinated as per WHO guidelines ?Partially Immunized: were not vaccinated as per WHO guidelines with absence of few episodes of vaccinations ?Not Immunized: were not received any vaccination |
| 8. | Previous history of pneumonia | ?Severe Pneumonia within the preceding 6 months as documented on the child’s chart |
Table 2. Represents variables assessed and their definitions
Statistical Analysis
The obtained data was analyzed using SPSS software version 20 and chi square test. Appropriate frequency distributions were presented as means and percentages. A difference at p<0.05 was considered statistically significant.
Results
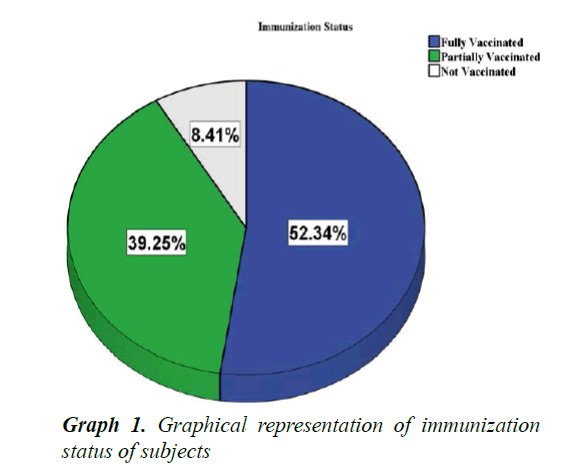
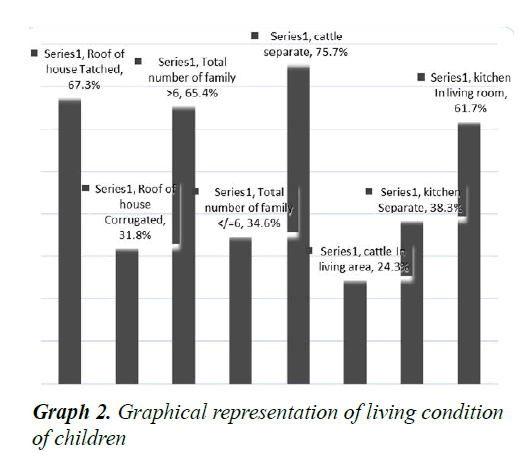
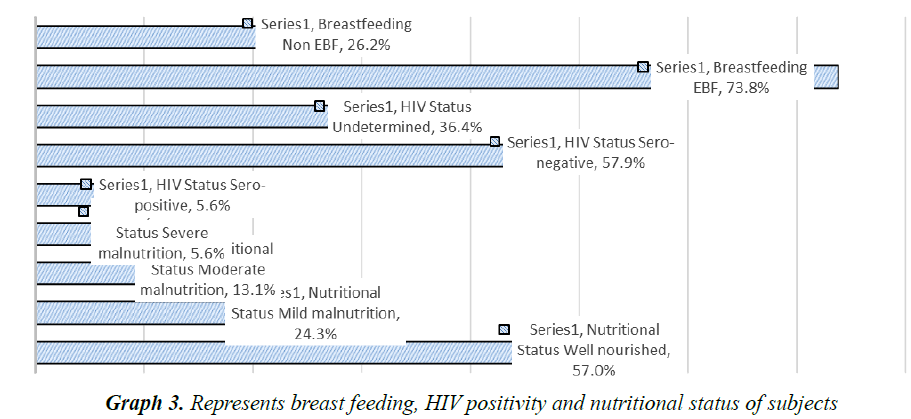
A total of 107 children with severe pneumonia were included in study and their parents/caretakers were interviewed. Out of 107, 22 (20.6%) were lived in urban and 85 (79.4%) were in rural areas (p<0.05). The male patients were accounted for 58 (54.2%) with male to female ratio of 1.18:1. It was found that the majority of the mothers were illiterate, housewives and fathers had learned up to 6th grade (Table 3). Among 107 children, 56 (52.34%) were fully vaccinated as per WHO guidelines, 42 (39.25%) were partially vaccinated and 9 (8.41%) were not vaccinated at all (Graph 1). Among the children 67.3% lived in thatched roof houses, while 31.8% lived in corrugated iron roofed houses and only 1 child was homeless. Majority of children lived in a family of greater than 6 people living together. Children were lived in houses with kitchen in the living room accounted 61.7% (Graph 2). Among the children, 79 (73.8%) were received exclusive breast feeding. It was also observed that 6 subjects (5.6%) were sero-positive for HIV. Malnutrition was prevalent in the subjects and about 43.0% (46) were suffering from malnutrition and 5.6% (6/107) with severe malnutrition (Graph 3). It was interesting to see that about 48.6% (52) of the pneumonic children had at least one family member with symptoms of URTI and 11.2% (12) had history of previous history of pneumonia. Only 7.5% (8/107) of the children had history of cigarette smoker coresidents.
| Variable | Category | Number of subjects | Percentage | p-values |
|---|---|---|---|---|
| Sex | Male | 58 | 54.2 | P=0.32 |
| Female | 49 | 45.8 | ||
| Place of residence | Rural | 85 | 79.4 | p<0.05 |
| Urban | 22 | 20.6 | ||
| Maternal literacy | Illiterate | 49 | 45.8 | p<0.05 |
| 1st-6th | 36 | 33.6 | ||
| 7th-12th | 15 | 14.0 | ||
| Higher education | 7 | 6.5 | ||
| Paternal literacy | Illiterate | 22 | 20.6 | p<0.05 |
| 1st-6th | 46 | 43.0 | ||
| 7th-12th | 27 | 25.2 | ||
| Higher education | 12 | 11.2 | ||
| Maternal Occupation | Housewife | 82 | 76.6 | p<0.05 |
| Farmer | 9 | 8.4 | ||
| Merchant | 8 | 7.5 | ||
| Government employee | 4 | 3.7 | ||
| Other | 4 | 3.7 | ||
| Paternal Occupation | Farmer | 74 | 69.2 | p<0.05 |
| Merchant | 17 | 15.9 | ||
| Government employee | 9 | 8.4 | ||
| Other | 7 | 6.5 |
Table 3. Represents socioeconomic characteristics of subjects
Among the 107 children, 87.9% (94) were discharged in improved conditions, 4.7% (5) died during hospitalization and 3.7% (4) were leave hospital against medical advice. Discharge information was unknown for 3.7% (4) cases. Majority of children stayed less than 5 days before hospital visit. Duration of hospital stay was less than 4 days for the majority (Table 4).
| Variables | Category | Number of subjects | Percentage | p-value |
|---|---|---|---|---|
| Patient discharge condition | Improved | 94 | 87.9 | p<0.05 |
| Self-discharge | 4 | 3.7 | ||
| Died | 5 | 4.7 | ||
| Unknown | 4 | 3.7 | ||
| Total duration of illness | <5 days | 98 | 91.6 | p<0.05 |
| 5-10 days | 7 | 6.5 | ||
| >10 days | 2 | 1.9 | ||
| Duration of hospital stay | = 3 days | 82 | 76.6 | p<0.05 |
| 4-10 days | 14 | 14.0 | ||
| >10 days | 10 | 9.3 |
Table 4. Represents treatment outcomes of subjects admitted with pneumonia
There were significant (p<0.05) associations between nutritional status of the child and status of discharge observed. Two of the five deaths occurred in two severely malnourished children and another death occurred in a moderately malnourished child. Status of discharge also tends to be affected by previous history of pneumonia, 3 of the 5 dead children had previous history of pneumonia and 3 of the 12 children with recurrent pneumonia died (Table 5). A Smoker in the house had significant association with status of discharge (Table 5).
| Parameters assessed | Status of discharge | Total | p-value | ||||
|---|---|---|---|---|---|---|---|
| Improved | Self-Discharge | Died | Unknown | χ2=18.82 P=0.27 | |||
| Nutritional Status | Well nourished | 55 | 1 | 2 | 3 | 61 | |
| Mild Malnutrition | 24 | 1 | 0 | 1 | 26 | ||
| Moderate Malnutrition | 11 | 2 | 1 | 0 | 14 | ||
| Severe Malnutrition | 4 | 0 | 2 | 0 | 6 | ||
| Smoker In the house | Yes | 6 | 0 | 2 | 0 | 8 | χ2=8.4 P=0.037 |
| No | 88 | 4 | 3 | 4 | 99 | ||
| Previous history of pneumonia | Yes | 8 | 1 | 3 | 0 | 12 | χ2=13.9 P=0.003 |
| No | 86 | 3 | 2 | 4 | 95 | ||
| Duration of hospital stay | <4 days | 72 | 4 | 2 | 4 | 82 | χ2=18.36 P=0.005 |
| 4-10 days | 15 | 0 | 0 | 0 | 15 | ||
| >10 days | 7 | 0 | 3 | 0 | 10 | ||
| Total | 94 | 4 | 5 | 4 | 107 | ||
Table 5. Status of discharge and its comparison with nutritional status, smoker in the house, previous history of pneumonia and duration of hospital stay
There were significant association observed between nutritional status and duration of hospital stay p<0.05. It was remarkable that 6 of the 10 children who were hospitalized over 10 days were severely malnourished and 2 were malnourished. 13 children were hospitalized for more than 3 days and were associated with moderate malnutrition. 3 of the 10 hospital stays more than 10 days were associated with sero-positivity for HIV. A Smoker in the house had significant association both between status of discharge and duration of hospital stay (Table 6). Duration of hospital stay was prolonged more than 10 days for 4 children with previous history of pneumonia (Table 6).
| Clinical Features | Duration of Hospital Stay | Total | p-value | |||
|---|---|---|---|---|---|---|
| = 3 days | 4-10 days | >10 days | ||||
| Nutritional Status | Well nourished | 49 | 10 | 2 | 61 | χ2=63.8 P=0 |
| Mild Malnutrition | 22 | 2 | 2 | 26 | ||
| Moderate Malnutrition | 11 | 3 | 0 | 14 | ||
| Severe Malnutrition | 0 | 0 | 6 | 6 | ||
| HIV status | Sero-positive | 3 | 0 | 3 | 6 | χ2=13.6 P=0.009 |
| Sero-negative | 50 | 9 | 3 | 62 | ||
| Undetermined | 29 | 6 | 4 | 107 | ||
| Smoker in the child’s house | Present | 5 | 0 | 3 | 8 | χ2=8.7 P=0.12 |
| Absent | 77 | 15 | 7 | 99 | ||
| Previous history of pneumonia | Yes | 7 | 1 | 4 | 12 | χ2=9.2 P=0.01 |
| No | 75 | 14 | 6 | 95 | ||
| Status of discharge | Improved | 72 | 15 | 7 | 94 | χ2=18.36 P=0.005 |
| Self- discharge | 4 | 0 | 0 | 4 | ||
| Dead | 2 | 0 | 3 | 5 | ||
| Unknown | 4 | 0 | 0 | 4 | ||
| Total | 82 | 15 | 10 | 107 | ||
Table 6. Duration of hospital stay by and its association with nutritional status, HIV infection, smoker in the child’s house and status of discharge
Discussion
Based on the objective of determining factors associated with outcome of severe pneumonia in children 2 to 59 months old at JUSH, a total of 107 parents of children were included in the study. Among the children males accounted for 54.2% of the children and male to female ratio are 1.18:1. The finding is in agreement with the study conducted in Tanzanian and other part of the world [7,9]. Children suffering from severe pneumonia in rural area accounted for 79.4% compared to children in urban area (20.6%) which is similar to EDHS 2011 data showed that lower proportion of children from urban areas than rural tends to develop ARI including pneumonia [10-15].
Regarding parental educational status, 45.8% of the mothers were illiterate. Among the fathers 30.6% were illiterate, 33.6% had learned up to 6th grade and the rest had received either secondary or higher level of education. While reports from Este town showed 71.7% mothers and 42.5% fathers were illiterate which was higher than findings of the present study. But the findings of this study are in agreement to the report of EDHS 2011 said a lower proportion of ARI occurred in mothers with secondary or higher educational levels [7,16,17,19].
In the present study it was observed that 52.34% of children were fully vaccinated, 39.25% were partially vaccinated and 8.41% were not vaccinated at all. According to the previous reports from the Este town, 76.9% were fully vaccinated and 13.3% were partially vaccinated. The possible reason for the difference in observed values might be due to definitions of fully and partially vaccinated that are used in these studies [7].
The Living conditions including number of people residing together in the house, types of roof seemed to have and smokers in the family, have an impact on prevalence of pneumonia. The likely explanation for the findings is adverse impact of exposure to above mentioned living and environmental conditions. In a Kenyan study, 7.8% of the children were sero-positive and we found that 5.6% of the children were sero-positive for HIV. In contrast this finding in Tanzanian, 29.5% of the children were sero-positive for HIV which may be due to high prevalence of HIV infection in the study area. Due to weakness of immune system in HIV positive patients the chances of recurrent pneumonia increases. In the present study it was also observed that the prevalence of pneumonia and treatment outcomes are heavily influences by nutritional status of the patients, which is evident from the findings that, duration of hospital stay of 5.6% children had associated severe malnutrition and 43% were malnourished. Two of the five deaths occurred in two severely malnourished children and another death occurred in a moderately malnourished child and 6 of the 10 children who stayed greater than 10 days were severely malnourished and other 2 had mild malnutrition. Nutritional status of individual influence immunity, once the nutritional intake is compromised immunity gets compromised and subjects become prone to infections. Even the recovery/hospitalization period may prolong due to delayed clearance of infection in subjects with weak immune strength. In a Tanzanian study, 27.3% of the children had malnutrition [16]. The possible reason for the higher prevalence of malnourished children in the present study may be related to poor nutritional status in Jimma. Breast feeding improves immunity hence, it improves outcomes.
It was observed that 48.6% of adult residing with subjects was having URTI. It may be due to cross contamination from adult to child due to long chronic contact time. About 11.2% subjects had history of previous episode of severe pneumonia and to the best of our knowledge the same is not reported yet. Smokers in the house in 7.5% of the pneumonic children may be explained by passive smoking hazard to exposed children. In a report of EDHS 2011, children’s of smokers were greater than nonsmokers to have symptoms of ARI [7,17].
It was found that the status of discharge was influenced by the above mentioned risk factors and 4.7% of subjects were died and this finding is remarkably lower than 29.3% Tanzanian 3.2 [9]. This may be due to other factors like high prevalence of HIV positivity (29.5%) in pneumonic children in Tanzania. A Smoker in the house had significant association both between status of discharge and duration of hospital stay Status of discharge also tend to be affected by previous history of pneumonia. 3 of the 5 dead children had previous history of pneumonia and 3 of the 12 children with recurrent pneumonia died. Duration of hospital stay was prolonged greater than 10 days for 4 children with previous history of pneumonia. Statuses of discharge were significantly associated with duration of hospital stay. 3 of the children who died stayed in the hospital for greater than 10 days.
Conclusion
The risk factors for poor outcome in childhood pneumonia include rural residence and poor infrastructure, parental literacy rate, vaccinated, national status, household environment, co-residents with URTI. Parental education, diet counseling, avoidance to contact with infected person will reduce the prevalence of pneumonia and will be helpful in better hospitalization outcomes.
Acknowledgement
We are deeply grateful to University CBE office for providing the material and financial support for the success and completion of this research proposal. We would like to forward thanks to Jimma University coordinating office of medicine and Jimma University specialized hospital Director for allowing and gathering of necessary records and information.
References
- Kliegman R, Stanton B, St. Geme J, et al. Nelson Textbook of pediatrics. 19th Ed. Philadelphia: Elsevier Saunders 2008; 392: 1142-1151.
- Acute respiratory infections in children Basic facts, family and community health cluster (FCH). WHO-African region 2011.
- Ashworth A, Bickler S, Deen J, et al. WHO pocket book for hospital cares for children: Guidelines for the management of common illness with limited resources. Amsterdam: KIT press 2013; 68-83.
- Varinder S, Satinder A. Pediatric respiratory reviews. 12th ed. Philadelphia: Elsevier Science 2011; 11:128.
- Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86: 408.
- Wardlaw T, Salama P, Johansson EW, et al. Pneumonia: The leading killer of children. Lancet 2006; 368: 1048.
- Fekadu GA, Terefe MW, Alemie GA. Prevalence of pneumonia among under-five children in Este Town and the surrounding rural kebeles, north-west Ethiopia: A community based cross sectional study. Sci J Public Health 2014; 2: 150-155.
- Yordanos A. A retrospective Analyses of pattern and outcomes among JUSH pediatric emergency ward admissions. Jimma: Jimma University 2008; 5: 29-34.
- Ahmed A. A retrospective analyses of pattern and outcomes severe pneumonia in children at Jimma University Specialized Hospital. Jimma: Jimma University 2011; 5: 26-33.
- Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update. UK: BPP 2011; 66: 112-114.
- Margolis P, Gadomski A. The rational clinical examination: Does this infant have pneumonia? JAMA 1998; 279: 308.
- Jokinen C, Heiskanen L, Juvonen H, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol 1993; 137: 977.
- Boyer KM. Non-bacterial pneumonia. In: Feigin RD, Cherry JD, Demmler-Harrison GJ, Kaplan SL (Eds). Textbook of Pediatric Infectious Diseases. Philadelphia: Saunders 2009; 289.
- Pelton SI, Hammerschlag MR. Overcoming current obstacles in the management of bacterial community-acquired pneumonia in ambulatory children. Clin Pediatr (Phila) 2005; 44: 1.
- Nahya S. Masoud. Factors related to severity and outcome of severe pneumonia in children aged 2 months and 59 months. Tanzania: Muhimbili University of health and allied sciences 2008; 6: 38-51.
- Onyango D, Kikuvi G, Amukoye E, et al. Risk factors of severe pneumonia among children aged 2-59 months in western Kenya: A case control study. Pan Afr Med J 2012; 13: 45.
- Abelti G, Ahmed J, Seyuom E, et al. Ethiopian demographic and health survey. Addis Ababa, Ethiopia: Central Statistical Agency 2011; 10: 142-143.
- Green GM, Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med 1967; 276: 421.
- Dagnew M, Dawit S, Daniel B. Analysis of admissions to the pediatric emergency ward of AH in Addis Ababa Ethiopia, Ethiopia Med JH Dev 2007; 21: 84-89.