Research Article - Biomedical Research (2017) Volume 28, Issue 6
Factors affecting survival in non-small cell lung cancer invading the chest wall
Abidin Sehitogullari1, Yusuf Aydemir2* and Fuat Sayir3
1Department of Thoracic Surgery, Sakarya University, Sakarya, Turkey
2Department of Pulmonology, Sakarya University, Sakarya, Turkey
3Department of Thoracic Surgery, Yuzuncu Yil University, Van, Turkey
- *Corresponding Author:
- Yusuf Aydemir
Department of Pulmonology
Training and Research Hospital
Sakarya University, Turkey
Accepted date: November 09, 2016
Abstract
Aim: The current study aimed to evaluate the factors affecting survival in non-small cell lung cancer invading the chest wall.
Method: A total of 45 cases operated on for Non-Small Cell Lung Cancer invading the chest wall (NSCLC) were followed-up for five years. The effects of factors such as depth of tumor invasion of the chest wall (parietal pleura, extra pleural fatty tissue, intercostal muscles, and rib involvement), perinodal involvement, “N” involvement, surgical margin of the resection, and adjuvant chemotherapy on prognosis and survival were evaluated.
Results and Discussion: The number of males and females among the cases was 38 (84%) and seven (16%), respectively, with a mean age of 55 ± 8 years (42-74). Chest wall resection and extra-pleural resection was performed in 36 (80%) and nine (20%) cases, respectively. In the multivariate analysis, factors positively affecting survival were depth of invasion, tumor dimension less than 5 cm, N0 lymph node status, complete resection, and complete adjuvant chemotherapy. Full-thickness resection of the chest wall was an important prognostic factor for long-term survival in all patients with NSCLC invading the chest wall.
Conclusion: The stage of the tumor and histopathological factors such as lymphatic involvement, extrapleural invasion, and rib invasion have been shown to gain importance in improvement of survival, in addition to advancements in surgical techniques. Although there is no consensus on the surgical approach in presence of chest wall invasion, we suggest that “en bloc” resection should be preferred to extra-pleural resection.
Keywords
Lung cancer, Chest wall invasion, Extra-pleural invasion, Rib invasion, Survival
Introduction
Invasion of the parietal pleura or chest wall is detected in 5-8% of patients operated on for lung cancer [1]. Chest wall invasion was regarded as a criterion of inoperability before 1947, while Coleman reported in that year that performing chest wall resection in addition to lung resection is possible and may result in a better survival [2]. Surgery is an important part of the multimodal treatment most of the time. However, the type of resection is debatable in presence of chest wall invasion. Even limited invasion of the parietal pleura constitutes an indication for en bloc thoracic wall resection for many surgeons, while according to others; pleural striping is adequate against the possibility of increased local recurrence. The main factor determining the prognosis is distant metastasis rather than local recurrence [3]. The current study evaluated the prognostic factors following lung and chest wall resections in cases with Non-Small Cell Lung Cancer (NSCLC) with chest wall invasion.
Method
A total of 45 patients were operated on for NSCLC with chest wall or parietal pleura invasion between January 2005 and July 2010 at the chest surgery divisions of our clinics. No surgical treatment was performed in cases with severe pulmonary failure, unstable angina pectoris, or congestive heart failure. Patients with abnormal pulmonary function tests (FEV1<60%, DLCO<60%) were evaluated with lung perfusion scan and cardiopulmonary tests and the patients who were found suitable were operated on.
All patients were evaluated using thoracic Computed Tomography (CT), fiber optic bronchoscopy, respiratory function tests, and Positron Emission Tomography (PET-CT). Mediastinoscopy was performed in the presence of a suspicious lymphadenopathy according to PET-CT or a mediastinal lymphadenopathy with a short diameter of more than 1 cm according to the thoracic CT. Induction chemotherapy was started in two patients.
All patients underwent posterolateral thoracotomy. Extra-pleural resection was performed in nine cases (20%) according to the intraoperative finding of limited invasion up to parietal pleura, confirmed by frozen section examination. Chest wall resection was performed in 36 cases (80%) due to the finding of presence of extended invasion through the parietal pleura and up to the chest wall. Chest wall resection was performed so that a tumor free tissue of 4 cm was included in the resection and the resection included one healthy rib each at the upper and lower margins according to the localization of the invasion. In two cases, healthy tissue measuring approximately 2 cm was included in the rib that was close to the vertebra. It was accepted as complete resection when no macroscopic or microscopic tumor was present at the resection margins and incomplete resection when macroscopic or microscopic tumor was present at the margins.
Patients who died up to 30 days after the operation were included in the operative mortality group. Cases were followed-up at the outpatient clinic every three months in the first year after the operation, every six months during the second year, and annually starting from the third year postoperatively. Patients were evaluated with physical examination, routine biochemical tests, PA chest x-ray, and thoracic CT at follow-up visits. Related organs were examined in cases with the suspicion of a metastasis. Follow-up of the cases was terminated in June 2015.
For statistical analysis, postoperative survival was calculated using the SPSS 7.5 program and Kaplan-Meier and log-rank tests. Cox regression analysis was used to calculate the effects of the variables on survival and p<0.05 was accepted as significant.
Findings
Among the cases, 38 were males (84%) and seven were females (16%) with a mean age of 55 ± 8 years (range: 42-74). Localization of the tumor was the right lung in 28 (62%) cases and the left lung in 17 (38%) cases. Chest wall resection (en bloc) (80%) and extra-pleural resection was performed in 36 and nine cases (20%), respectively. Local recurrence was found in four of all cases, two of them had undergone extra-pleural resection. The cases in which recurrence in the chest wall was detected underwent reoperation (incomplete resection=2). One of those cases was N2 positive. The remaining two patients were those with resection, including approximately 2 cm of healthy tissue in the rib close to the vertebrae. Local recurrence was found in those two cases. They were directed to chemoradiotherapy since a simultaneous brain metastasis was found in one of them and the other was pathological stage IIIA with N2.
Pneumonectomy and lobectomy were performed in 13 (29%) and 32 (71%) patients, respectively. According to the cell type, 26 cases had epidermoid carcinoma (59%), 13 cases had adenocarcinoma, three had large cell carcinoma (6.5%), and three had undifferentiated carcinoma (6.5%). Pathological stages of the operated cases were Stage IIB in 27 cases, Stage IIIA in 14 and N2 Stage IIIA in four cases. Survival by stage was as follows: five-year survival was 38%, 27%, and 0% in Stage IIB, IIIA, and IIIA N2, respectively (Table 1). Except for the four cases with N2, survival was statistically similar in the groups with stages IIB and IIIA (p=0.72).
| n (%) | IIB (T3N0M0) | IIIA (T3N1M0) | IIIA (T3N2M0) | |
|---|---|---|---|---|
| Squamous cell carcinoma | 26 (58) | 17 | 7 | 2 |
| Adenocarcinoma | 13(29) | 8 | 4 | 1 |
| Large cell carcinoma | 3 (6.5) | 1 | 2 | 0 |
| Undifferentiated carcinoma | 3 (6.5) | 1 | 1 | 1 |
| Total | 45 | 27 | 14 | 4 |
Table 1: Average survival and 5-year survival rates according to pneumonectomy and lobectomy.
Operative procedures performed on the patients and postoperative data are shown in Table 2. When the survival was evaluated according to the chest wall invasion, no significant differences were found between the rib involvement, intercostal involvement, extra parietal pleural fatty tissue involvement and parietal pleura involvement (p=0.148). When rib involvement and parietal pleura involvement was compared, rib involvement seemed to cause better survival compared to pleural involvement, though not statistically significant (p=0.150). Similarly, when extra-pleural fatty tissue involvement and parietal pleural involvement were compared, survival was better in extra-pleural fatty tissue involvement, though not significant (p=0.148). Lymphatic invasion of the tumor negatively affected survival and was statistically significant (p=0.034).
| Surgical procedures | n | Mean survival* (95% CI) | 5-year survival (± SD%) | P-value | |
|---|---|---|---|---|---|
| Type | Pneumonectomy | 13 | 21.8 (14.2-61.6) | 68.3 ± 16.7 | 0.043 |
| Lobectomy | 32 | 14.5 (15.3-31.3) | 54.3 ± 14.8 | ||
| Resection | Chestwall resection | 36 | 16.7 (17.6-32.4) | 54.3 ± 14.8 | 0.148 |
| Extra-pleural resection | 9 | 23.5 (11.3-55.2) | 38.9 ± 12.8 | ||
| Depth of invasion | Parietal pleural resection | 9 | 23.5 (10.4-53.5) | 48.4 ± 6.1 | 0.088 |
| Rib Resection (1-2 rib) | 7 | 12.3 (6.3-39.1) | 29.1 ± 24.1 | ||
| Rib Resection (3-5 rib) | 29 | 18.2 (15.6-35.3) | 42.8 ± 11.8 | ||
| Completeness | Complete | 43 | 28.8 (20.1-35.9) | 49.9 ± 11.2 | 0.001 |
| Incomplete | 2 | 13.2 (12.4 -14.0) | 0 | ||
| Invasion | Parietal pleura | 9 | 24.8 (15.1-58.4) | 49.1 ± 13.7 | 0.148 |
| Soft tissue and bone | 36 | 19.6 (16.8-31.8) | 41.2 ± 13.8 | ||
| *Month | |||||
Table 2: Average survival and 5-year survival rates according to surgical procedure.
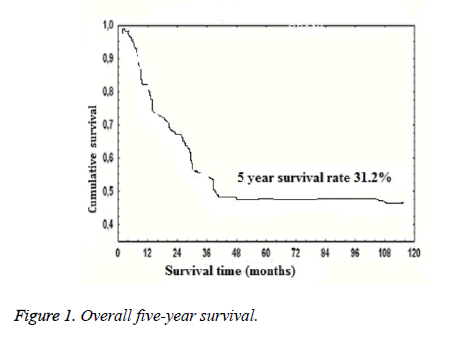
Five-year mortality was higher in patients who underwent pneumonectomy compared to the patients who underwent lobectomy (68% and 54%, respectively, p=0.043). Overall five-year survival was 31.2% (Figure 1). According to the N status, five-year survival was 38.6%, 23.4%, and 0% in N0, N1, and N2, respectively (p=0.034). Prognostic factors affecting survival in the univariate analysis were the extent of resection (lobectomy or pneumonectomy, p=0.043), tumor dimension (>5 cm or ≤ 5 cm, p=0.007), lymph node status (N0 or N1 and N2, p=0.034), complete resection (p<0.001), and complete adjuvant chemotherapy (p<0.004). Independent prognostic factors affecting survival in multivariate analysis were depth of invasion (deep or superficial, p=0.042), tumor dimension (>5 cm or ≤ 5 cm; p=0.015), lymph node status (p=0.001), complete resection (p=0.002), and complete adjuvant chemotherapy (p=0.002) (Table 3).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| P-value | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | |
| Pneumonectomy vs. lobectomy | 0.043 | 1.52 | 1.06-2.41 | 0.39 | 1.32 | 0.95-1.96 |
| Extra-pleural resection vs. chest wall resection | 0.148 | 0.69 | 0.41-1.18 | 0.266 | 1.48 | 0.76-1.58 |
| Tumor size (≤ 5 cm/>5 cm) | 0.007 | 0.43 | 0.36-0.64 | 0.015 | 0.35 | 0.28-0.76 |
| Lymphovascular invasion (no/yes) | 0.142 | 0.64 | 0.33-1.21 | 0.112 | 0.56 | 0.25-1.12 |
| N2 lymph node involvement (no/yes) | 0.034 | 0.61 | 0.29-0.83 | 0.001 | 0.27 | 0.21-0.58 |
| Complete resection | 0.001 | 0.32 | 0.20-0.54 | 0.002 | 0.28 | 0.16-0.52 |
| Depth of invasion (superficial/deep) | 0.144 | 0.69 | 0.48-1.14 | 0.042 | 0.6 | 0.36-0.98 |
| Completion of chemotherapy (no/yes) | 0.004 | 3.21 | 1.84-5.32 | 0.002 | 4.68 | 2.54-8.92 |
Table 3: Univariate and multivariate analysis for long term survivals.
Tumor histology, differentiation, neoadjuvant therapy, type of neoadjuvant therapy, and diameter of resection did not have an effect on survival. Survival was statistically significantly better in cases that received adjuvant RT and/or chemotherapy compared to the cases who did not receive those therapies.
Operative mortality occurred in a case that had undergone pneumonectomy (2.2%), and no operative mortality occurred in cases with lobectomy. Postoperative complications developed in 12 out of 45 cases. Complications were pleural space (n=4), atelectasis (n=3), empyema (n=1), prolonged air leak (n=2), pneumonia (n=1), and wound infection (n=1).
Discussion
Chest wall invasion is associated with a high morbidity and mortality in en bloc resections and chest wall reconstructions; therefore, preoperative detection of the condition is important. Kawaguchi et al. reported that mean survival was 46 months in 532 patients with chest wall invasion among 11,663 patients with lung cancer [4]. The most important CT findings of pleural involvement are bone destruction or presence of a mass in the chest wall [5]. However, since it does not demonstrate invasion directly, it is difficult to predict pleural involvement by CT [6]. Three-dimensional thoracic CT was used in the preoperative evaluation of the chest wall invasion in the present study. Thoracic wall invasion was detected correctly in all but two patients by the imaging method. In the remaining two patients, invasion was considered to be positive since the tumor was found to adhere the chest wall tightly.
Chest wall invasion in NSCLC, once considered as a contraindication for surgery, is currently considered to carry a good survival with an acceptable operative mortality [7]. Nevertheless, various factors affect long term survival in such cases. These factors are completeness of the resection, lymph node status, histopathological stage, and the extent of resection [8].
The study of Downey et al. reported that survival was dependent on the depth of chest wall invasion, in addition to a high rate of complete resection and lymph node negativity [7]. They found the five-year survival in T3N0M0 cases with parietal pleural invasion alone as 62% and with bone and muscle invasion as 35%. Nevertheless, N0 and N1 disease has been reported to be similar in terms of survival in various studies and depth of invasion has been reported not to effect survival provided that complete resection is performed [9,10]. Performing an incomplete resection or leaving a residual tumor, even at the microscopic level, has been reported to cause no curative benefit and survival has been known to be equal in cases that undergo incomplete resection and no surgical procedure at all [7].
In the 45 cases in this present study, local recurrence was found in four patients, although the surgical margins were negative. Two cases underwent extra-pleural resection and reoperations were performed on these two cases with detection of recurrence in the chest wall (incomplete resection=2). N2 was positive in one of those cases and they were referred for chemotherapy. In the remaining two patients, approximately 2 cm of healthy tissue was resected in the rib close to the vertebrae. Local recurrence was found in the two of the cases. They underwent chemoradiotherapy since one of them had simultaneous brain metastasis and the other was pathological Stage III A with N2.
According to the depth of invasion of the chest wall, survival was similar in rib involvement, intercostal involvement, extra parietal pleural fatty tissue, and parietal pleural involvement (p=0.148). However, survival was better in bone and soft tissue invasion.
When performing a chest wall resection, a healthy tissue in the lateral side should be resected as much as possible, in addition to one upper and one lower rib together with the invaded rib [10]. A tight adherence to the chest wall suggests intrathoracic fascia is passed and chest was invaded and requires a chest wall resection. Santos et al. in their patients with lung cancer and endothoracic facia invasion reported that “bird cage resection” was less aggressive and resulted in similar morbidity and mortality when compared to en bloc resection and that it did not negatively affect the patients in terms of curative results [11]. Treatment in tumors adhered to the parietal pleura is debatable. Malignant invasion of the parietal pleura is clinically difficult to differentiate from fibrosis due to inflammation. Some authors demonstrated that the long-term patient survival following extra-pleural excision was similar to the survival following chest wall excision in tumors involving only the parietal pleura [7-12]. However some other authors [13-15] observed a high incidence of positive histological margin following extra-pleural excision and reported that survival following en bloc resection was significantly better compared to extra-pleural excision of these lesions.
In the present study, survival was better in rib involvement compared to other levels of invasion. No tumor remnants could be seen in the intercostal muscles with frozen section in any of the nine cases received extra-pleural dissections with the suspicion of parietal pleural involvement. In the present study, rib involvement was found to result in better survival rates compared to parietal pleural involvement; however, it was not statistically significant (p=0.150).
Another important prognostic factor is the TNM stage. The five-year survival was found to be 66% in patients with stage IA disease. T3N0 disease (for example Stage IIB) has a fiveyear survival of 35-50% [7]. On the other hand, survival drops to 23% in Stage IIIA [15]. In this present study, postoperative stages of the cases that were operated on were Stage IIB in 27 cases, Stage IIIA in 14 cases and N2 Stage IIIA in four cases. Five-year survival was longest in Stage IIB. No five-year survival was observed in cases with N2 Stage IIIA.
Lymph node status is a determining factor in predicting the prognosis [16-18], Burkhart et al. [19] found the five-year survival in 95 patients with chest wall invasion was 44.3%, 26.3%, and 0% in cases with N0, N1, and N2, respectively. Also, Roviaro et al. [20] found the five-year survival to be 78.5% in N0 cases and 7.2% in N1 cases in a series of 43 patients with chest wall invasion. Lee et al. found a five-year overall survival rate of 26.3%. According to the lymph node status, the five-year survival was 37.4%, 21.1%, and 4.6% in stages N0, N1, and N2, respectively [21]. In this present study the overall survival was 31.2%. According to the N status, five-year survival was 38.6%, 23.4%, and 0% in N0, N1, and N2, respectively. Also, five-year mortality was higher in patients who underwent pneumonectomy compared to the patients who underwent lobectomy. We consider the reason for better survival in cases with a pneumonectomy originated from the fact that all N2 cases were in the group who underwent lobectomy and extra-pleural resection.
Lymphatic invasion and perineural invasion were important prognostic factors in the univariate analysis in a study of 82 cases [22]. Ruffini reported the presence of perineural invasion to be marker of aggressiveness of the tumor [23]. Also, in the present study, lymphatic invasion of the tumor negatively and significantly affected survival (p=0.034). However, perineural invasion of the tumor did not have a statistically significant impact on survival (p=0.63).
There is no consensus on the administration of adjuvant radio-chemotherapy in cases with chest wall resection. Postoperative adjuvant Radiotherapy (RT) has been suggested in “N” involvement or in cases of R1, while chemotherapy (ChT) has been suggested in “N2” cases [24]. In many studies, RT was reported to have no effect of survival [7,8], while Patterson [25] and Facciolo [24] reported that survival was better in cases with the administration of postoperative adjuvant RT, though not significant. The five-year survival rate in patients who completed the adjuvant chemotherapy was higher in cases who did not receive it [20]. Similarly, in the present study, a significant difference was found in survival in patients who received and did not receive adjuvant RT and/or ChT (p<0.004).
Operative morbidity developed due to pneumonia in one case in which a pneumonectomy was performed (n=1; 2.2%). Voltolini et al. reported a 30 day mortality rate of 4% [3]. In that study, causes of mortality were detected as bronchopleural fistula and myocardial infarct.
Conclusion
Nodal state, depth of invasion, and adjuvant chemotherapy are prognostic factors for long-term survival in patients with NSCLC with chest wall invasion. Although there is no consensus on the type of resection to be performed in non-small cell lung cancer, “en bloc” resection should be preferred over extra-pleural resection.
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
References
- Matsuoka H, Nishio W, Okada M, Sakamoto T, Yoshimura M. Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 2004; 26: 1200-1204.
- Coleman FP. Primary carcinoma of the lung, with invasion of the ribs: pneumonectomy and simultaneous block resection of the chest wall. Ann Surg 1947; 126: 156-168.
- Voltolini L, Rapicetta C, Luzzi L, Ghiribelli L. Lung cancer with chest wall involvement: Predictive factors of long-term survival after Surg ical resection. Lung Cancer 2006; 52: 359-364.
- Kawaguchi K, Miyaoka E, Asamura H, Nomori H, Okumura M, Fujii Y. Modern Surg ical results of lung cancer involving neighbouring structures: a retrospective analysis of 531 pT3 cases in a Japanese lung cancer registry study. J Thorac Cardiovasc Surg 2012; 144: 431-437.
- UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: The new TNM staging system and potential imaging pitfalls. Radiograph 2010; 30: 1163-1181.
- Glazer HS, Duncan-Meyer J, Aronberg DJ, Moran JF, Levitt RG. Pleural and chest wall invasion in bronchogenic carcinoma: CT evaluation. Radiol 1985; 157: 191-194.
- Downey RJ, Martini N, Rusch VW, Bains MS, Korst RJ. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg 1999; 68: 188-193.
- Chapelier A, Fadel E, Macchiarini P, Lenot B, Le Roy Ladurie F. Factors affecting long-term survival after en-bloc resection of lung cancer invading the chest wall. Eur J Cardiothorac Surg 2000; 18: 513-518.
- Martin LW, Walsh GL. Vertebral body resection. Thorac Surg Clin 2004; 14: 241-254.
- Magdeleinat P, Alifano M, Benbrahem C, Spaggiari L, Porrello C. Surg ical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg 2001; 71: 1094-1099.
- Andrade Santos HT, Lopes AJ, Higa C, Nunes RA, Saito EH. Lung cancer with chest wall invasion: retrospective analysis comparing en-bloc resection and resection in birdcage. J Cardiothorac Surg 2014; 9: 57-65.
- McCaughan BC, Martini N, Bains MS, McCormack PM. Chest wall invasion in carcinoma of the lung. Therapeutic and prognostic implications. J ThoracCardiovasc Surg 1985; 89: 836-841.
- Pairolero PC. Extended resections for lung cancer. How far is too far? Eur J Cardiothorac Surg 1999; 16: 48-50.
- Albertucci M, DeMeester TR, Rothberg M, Hagen JA, Santoscoy R. Surg ery and the management of peripheral lung tumors adherent to the parietal pleura. J Thorac Cardiovasc Surg 1992; 103: 8-12.
- Ucvet A, Gursoy S, Erbaycu A, Kul C, Tozum H, Anar S. Factors affecting the prognosis of non-small cell lung cancer with chest wall/parietal pleural invasion (T3) following resection. Int J Hematol Oncol 2010; 20: 89-95.
- Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in Surg ically managed non-small cell lung cancer. J Thorac Oncol 2009; 4: 792-801.
- Scaglia NC, Chatkin JM, Pinto JA, Tsukazan MT, Wagner MB. Role of gender in the survival of Surg ical patients with non-small cell lung cancer. Ann Thorac Med 2013; 8: 142-147.
- Baltayiannis N, Chandrinos M, Anagnostopoulos D, Zarogoulidis P, Tsakiridis K. Lung cancer Surg ery: an up to date. J Thorac Dis 2013; 5: 425-439.
- Burkhart HM, Allen MS, Nichols FC 3rd, Deschamps C, Miller DL. Results of en bloc resection for bronchogenic carcinoma with chest wall invasion. J ThoracCardiovasc Surg 2002; 123: 670-675.
- Roviaro G, Varoli F, Grignani F, Vergani C, Pagano C. Non-small cell lung cancer with chest wall invasion: evolution of Surg ical treatment and prognosis in the last 3 decades. Chest 2003; 123: 1341-1347.
- Lee CY, Byun CS, Lee JG, Kim DJ, Cho BC. The prognostic factors of resected non-small cell lung cancer with chest wall invasion. World J Surg Oncol 2012; 10: 9.
- Sayar A, Turna A, Kiliçgün A, Solak O, Urer N, Gurses A. Prognostic significance of Surg ical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg 2004; 25: 434-438.
- Ruffini E, Rena O, OliaroA, Filosso PL. Lung tumors with mixed histologic pattern. Clinico-pathologic characteristics and prognostic significance. Eur J Cardiothorac Surg 2002; 22: 701-707.
- Facciolo F, Cardillo G, Lopergolo M, Pallone G, Sera F. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 2001; 121: 649-656.
- Patterson GA, Ilves R, Ginsberg RJ, Cooper JD, Todd TR. The value of adjuvant radiotherapy in pulmonary and chest wall resection for bronchogenic carcinoma. Ann Thorac Surg 1982; 34: 692-697.