Research Article - Biomedical Research (2017) Volume 28, Issue 6
Fabrication and in vitro degradation behaviour of TCP/CS composite bioceramics
Bin Xu1*#, Hanyuan Zhang1# and Weijun Fang21Department of Sports Medicine and Arthroscopic Surgery, the First Affiliated Hospital of Anhui Medical University, Hefei, PR China
2College of Basic Medicine, Anhui Medical University, Hefei, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Bin Xu
Department of Sports Medicine and Arthroscopic Surgery
The First Affiliated Hospital of Anhui Medical University, PR China
Accepted date: November 4, 2016
Abstract
Ca3 (PO4)2 (TCP) bio-ceramic materials are known as the ideal bone repair materials because they possess good biocompatibility and biodegradability. In this study, β-TCP bio-ceramic materials were prepared by wet process through controlled process conditions. CaSiO3 and β-TCP bioceramics were ground and mixed to prepare the TCP/CaSiO3 (TCP/CS) bioceramic embryonic bodies. TCP/CS porous composites were obtained by sintering at 800°C. TCP/CS bioceramics possessed the best strength after sintering for 2 h; the sample strength was about 122 MPa, which is approximately consistent with the strength of human bone. The degradation behaviour of porous TCP/CS bioceramics were investigated by static soaking and dynamic dissolution experiments in physiological saline to control the degradation rate of TCP/CS. The degradability of TCP/CS samples reached 10% within 12 days in vitro, suggesting an excellent in vitro bioactivity and biodegradability of TCP/CS composite bioceramics. The TCP/CS composite bioceramics might become potential biomaterials as good mechanical strength, bioactive and biodegradable for bone tissue repair applications.
Keywords
Tricalcium phosphate, Calcium silicate, Biocompatibility, Biodegradability, Bioactivity.
Introduction
In these two decades, bone tissue engineering scaffold materials have been successfully applied in reconstruction as the carrier materials for bone tissue (including alveolar bone) due to controllable biodegradability, appropriate threedimensional structure with connected porosity, good mechanical strength and acceptable biocompatibility [1,2]. Porous calcium phosphate TCP (Ca3 (PO4)2) bioceramic contains good biodegradability, biocompatibility and biological safety. It has been widely investigated by scientists and engineers all over the world as a potential bone tissue engineering scaffold material [3-12].
A major problem hindering the clinical application of porous TCP ceramics clinical is the difficulty to coordinate the degradation rate and the growth rate of newly formed bone [3,4,7]. TCP has two types of crystal forms, alpha phase and beta phase, which have different water solubility and in vivo degradation rate. The degradation rate of α-TCP is too high and cannot be applied clinically [6,7]. Moreover, the bone formation of β-TCP material is slow, and the integration of bone is not perfect [8,9]. Recent studies have shown that calcium silicate powder or ceramic has good biological activity and induced deposition of bone like hydroxyapatite layer in vitro [10-12]. Silicon has been considered as a medium for promoting new bone formation. The formation of hydroxyl apatite is very helpful for the promotion of bone conduction and bone regeneration in biomaterials, and it can promote the formation of chemical bonding between hard/soft tissues and materials, indicating that calcium silicate is a potential and promising bioactive material having great clinical applications. Previous studies also showed that CaSiO3 (CS) possesses a good ability to induce the deposition of hydroxyapatite, acceptable biocompatibility and bone binding/repair properties [13-15]. Recently, calcium silicate ceramics have been studied as bioceramics for new bone regeneration [16].
In this study, porous α/β dual-phase composite TCP bioceramics was prepared using α-TCP and β-TCP powder materials respectively obtained by initial wet process, then TCP/CS bioceramics was prepared by a certain proportion of TCP bioceramics and CS powder. The in vitro degradation behaviours of porous TCP/CS bioceramics were investigated in physiological saline to control degradation rate of TCP bioceramics. This study provided a fundamental research for the potential clinical application of TCP/CS as one of the most important tissue engineering scaffold materials.
Materials and Methods
Preparation of TCP/CS nanocomposite bioceramics
Based on Ca (OH)2-H3PO4 system, calcium hydroxide suspension was added drop wise into phosphoric acid solution with stirring at room temperature, the reaction proceeded for 5 hours followed by filtering and drying at 85°C to prepare TCP powder.
Porous TCP ceramics was prepared by stearic acid particle filling. The high purity spherical particles of stearic acid were divided into different mesh with sample sieve, the stearic acid particles with appropriate particle size and TCP powder were mixed, and Polyvinyl Alcohol (PVA) solution was added as binder. After molding, stearic acid was molten at 80°C then dried to obtain the porous body.
The porous body was placed in a sintering furnace, heated up to 300°C, and heat was preserved for 1 h in order to remove residual moisture, PVA, stearic acid and other residues. The porous body was then heated up to 800°C for 1 h and to 1000°C and 1200°C for 3 h, cooled down to room temperature to obtain β-TCP and α-TCP bioceramics, respectively.
The prepared α-TCP and β-TCP bioceramics were ground and mixed with CS powder according to different mass ratios. The powder mixtures were calcined at 800°C for 2 h to yield TCP/CS nanocomposite powder. Polyethylene Glycol (PEG) 350-400 μm was used as pore forming agent, TCP/CS powder and PEG particles were homogeneously mixed according to a mass ratio of 4:6, PVA solution (5% wt) was added as the binder to obtain biscuits. The biscuits were sintered at 800°C to prepare porous α-TCP/β-TCP dual phase TCP/CS composite bioceramics.
Composition and structure analysis
Ca and P contents in synthesized TCP powder were determined by potassium permanganate method and quinoline phosphomolybdate gravimetric method, respectively. In the experiments of in vitro degradation of physiological saline, Ca2+ concentration was measured with PW-2404 (Philips, Holland) atomic absorption spectrophotometer. The crystal phase composition of samples was analysed by MAX 2550V type (Rigaku Co., Japan) X-ray diffraction analysis. The morphology changes of the samples before and after degradation were observed with JSM-6700F (JEOL, Japan) Scanning Electron Microscope (SEM). The mechanical properties of TCP/CS bioceramics were measured with AG-IS type Shimadzu Universal Testing Machines.
In vitro static dissolution experiment
The various porous TCP/CS bioceramics (shape: column; length: 15 mm; diameter: 5 mm) were immersed into saline filled in sealed jar, the normal saline was prepared according to a solid/liquid ratio (g/ml) of 1/100. The system was loaded in a water bath thermostat to maintain a temperature of 37°C. The soaking liquid was taken after different time periods for determination of Ca2+ concentration.
In vitro dynamic degradation experiment
The various porous TCP/CS bioceramics (shape: column; length: 15 mm; diameter: 5 mm) were immersed into saline filled in sealed jar, the normal saline was prepared according to a solid/liquid ratio (g/ml) of 1/100. The saline was controlled to flow through the porous TCP/CS bioceramics with a fixed circulation flowing volume by a peristaltic pump, the temperature of circulation system was at 37°C. The circulation liquid was taken for determination of Ca2+ concentration. The degradation rates of TCP/CS bioceramics were calculated according to Equation 1.
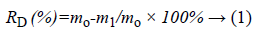
Where RD is rate of degradation, m0 is the mass of sample before soaking and m1 is the mass of sample after soaking.
Results and Discussion
The linear shrinkage, relative density and bending strength of TCP/CS bioceramics were listed in Table 1. It can been seen that linear shrinkage and relative density improved with an increase of sintering time from 0.5 h to 2 h. The relative density reached 95.36% after 2 h, but remained constant when the time was longer than 2 h. The sintering experiments of TCP/CS bioceramics indicated that the sample possessed the best strength after sintering for 2 h; the sample strength was about 122 MPa, which is approximately consistent with the strength of human bone. TCP/CS bioceramics prepared in this paper is obviously better than that reported in literatures, whose relative density was only around 92% after sintering for 2 h at 800°C, the mechanical strength was less than 50 MPa.
| Sintering time (h) | 0.5 | 1 | 2 | 5 |
| Linear shrinkage (%) | 21.34 | 23.67 | 25.84 | 25.92 |
| Relative density (%) | 90.54 | 93.28 | 95.36 | 95.41 |
| Bending strength (MPa) | 94.51 | 103.48 | 122.21 | 122.56 |
Table 1. Effect of the sintering time on linear shrinkage, relative density and bending strength of the samples.
This study showed that the basic requirement of high-strength bioceramic is high density, because only high density can guarantee the strength of the bioceramics. Compared with general ceramics, the TCP/CS nano composite bioceramics have some advantages such as large surface area, good sintering activity, fine particles and so on. Therefore, high strength TCP/CS composite bioceramic materials can be prepared at lower sintering temperature. However, the relative density of prepared TCP/CS composite powder even under 800°C and sintering for 5 h can only reached about 95%. The reason was attributed to the poor sintering property of CS component, which is difficult to achieve complete densification. Further increasing the sintering temperature and time might improve the sintering density of samples, but that will make β-TCP phase transfer to α-TCP phase, thus properties of TCP/CS bioceramic degradation will change.
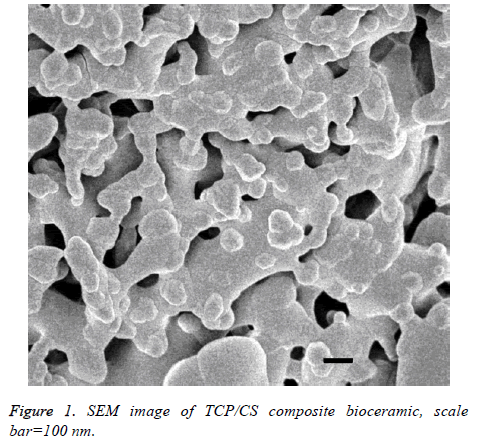
Figure 1 show that the prepared porous TCP/CS bioceramics have rich micro porous structure, the size of pore and its distribution can be controlled through the porogen particle size and dosage of PEG. These pores are mainly used to combine with osteoinductive Bone Morphological Protein (BMP), and it also helpful for new bone growth.
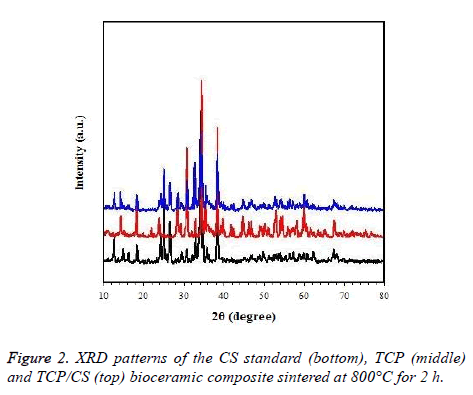
According to the XRD standard of TCP and CS, the purity of the TCP/CS bioceramic composite is high, and is in accordance with the requirements (Figure 2).
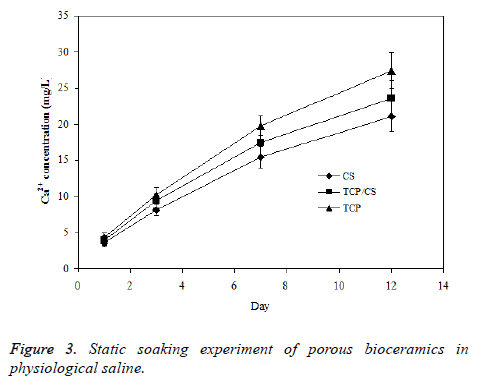
The solubility property of porous TCP/CS bioceramics in physiological saline solution was estimated based on the solubility of prepared TCP and CS bioceramics. The three ceramics samples were soaked in physiological saline respectively; the change of Ca2+ concentration in solution was acquired and shown in Figure 3. The degradation rate of TCP/CS bioceramics in saline is higher than TCP but lower than CS, suggesting that the method for regulation of degradation rate of porous bioceramics is feasible.
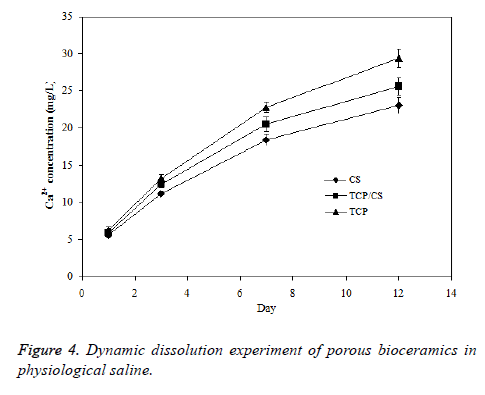
The skeleton of human or animal body is located within the tissue fluid, the body tissue fluid is about 13-15% wt of human body weight. The tissue fluid keeps constantly circulating to maintain a dynamic balance. As a result, the bioceramics implanted inside body will be in a dynamic equilibrium of the body fluid. In this study, the dynamic environment of porous TCP/CS bioceramics was simulated by dissolving the samples in a dynamic environment of organism, and the liquid membrane surface was continuously refreshed to ensure the significant power of dissolution. The data presented in Figure 4 shows that the results obtained from dynamic soaking experiment are similar with the results from static soaking experiment, but the dissolution rates in a dynamic environment are much larger than the rates in static conditions.
Conclusions
Porous TCP/CS bioceramics was prepared from CS and TCP ceramics by sintering at 800°C. TCP/CS bioceramics exhibited the best strength after sintering for 2 h, the sample strength was about 122 MPa, which is quite similar with the strength of human bone. The degradation behaviour of porous TCP/CS bioceramics were investigated by static soaking and dynamic dissolution experiments in physiological saline to control the degradation rate of TCP/CS. The degradability of TCP/CS samples reached 10% within 12 days in vitro, suggesting an excellent in vitro bioactivity and biodegradability of TCP/CS composite bioceramics. This study showed that CS and TCP ceramics could be used to prepare the biodegradable and bioactive TCP/CS composite bioceramic materials having good mechanical strength, which is expected to be used for hard tissues like bone repairing materials.
References
- Wang L, Barbieri D, Zhou H, Bruijn JDD, Bao C, Yuan H. Effect of particle size on osteoinductive potential of microstructured biphasic calcium phosphate ceramic. J Biomed Mater Res Part A 2015; 103: 1919-1929.
- Meng D, Dong L, Wen Y, Xie Q. Effects of adding resorbable chitosan microspheres to calcium phosphate cements for bone regeneration. Mater Sci Eng C 2015; 47: 266-272.
- Gamblin AL, Brennan MA, Renaud A, Yagita H, Lezot F, Heymann D. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: The local implication of osteoclasts and macrophages. Biomater 2014; 35: 9660-9667.
- Khan AF, Saleem M, Afzal A, Ali A, Khan A, Khan AR. Bioactive behaviour of silicon substituted calcium phosphate based bioceramics for bone regeneration. Mater Sci Eng C Mater Biol Appl 2014; 35: 245-252.
- Camargo NHA, Gemelli E, Moraes AND, Costa BDD, Oleskovicz N, Dallabrida AL. In vivo preliminary study on bone neoformation behavior of three types of calcium phosphate bioceramics. J Biosci Med 2014; 2: 36-42.
- Zhang J, Barbieri D, Hoopen HT, Bruijn JDD, Blitterswijk CAV, Yuan H. Microporous calcium phosphate ceramics driving osteogenesis through surface architecture. J Biomed Mater Res Part A 2014; 103: 1188-1199.
- Karbe E, Kramer H, Heide H. Experimental bone replacement with resorbable calcium phosphate ceramic. J Antimicrob Chemother 2014; 69: 2835-2840.
- Denry I, Kuhn LT. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater Offic Publ Acad 2015; 2012: 43-53.
- Manchon A, Alkhraisat M, Rueda-Rodriguez C, Torres J, Prados-Frutos JC, Ewald A. Silicon calcium phosphate ceramic as novel biomaterial to simulate the bone regenerative properties of autologous bone. J Biomed Mater Res Part A 2015; 103: 479-488.
- Mazon P, Garcia-Bernal D, Meseguer-Olmo L, Cragnolini F, Aza PND. Human mesenchymal stem cell viability, proliferation and differentiation potential in response to ceramic chemistry and surface roughness. Ceram Int 2015; 41: 6631-6644.
- Wang C, Lin K, Chang J, Sun J. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomater 2013; 34: 64-77.
- Jin Z, Qian H, Tan J. Dental follicle cells combined with beta-tricalcium phosphate ceramic: a novel available therapeutic strategy to restore periodontal defects. Med Hypoth 2010; 75: 669-670.
- Wang C, Xue Y, Lin K, Lu J, Chang J, Sun J. The enhancement of bone regeneration by a combination of osteoconductivity and osteostimulation using ß-CaSiO 3 /ß-Ca 3 (PO 4 ) 2, composite bioceramics. Acta Biomaterialia 2012; 8: 350-360.
- Kien PT, Maruta M, Tsuru K, Matsuya S, Ishikawa K. Effect of phosphate solution on setting reaction of a-TCP spheres. J Austr Ceram Soc 2010; 46: 63-67.
- Ikumoto H, Matsuzaka K, Inoue T, Uchiyama T, Yoshinari M. The behaviour of osteoblast-like cells on different crystal systems of calcium phosphate ceramics in vitro. Biomed Res 2003; 24: 239-248.
- Wiltfang J, Merten HA, Schlegel KA, Schultze-Mosgau S, Kloss FR, Rupprecht S, Kessler P. Degradation characteristics of a and ß tri-calcium-phosphate (TCP) in minipigs. J Biomed Mater Res 2002; 63: 115-121.