Research Article - Journal of Environmental Waste Management and Recycling (2023) Volume 6, Issue 2
Extraction and characterization of protein from cottonseed meal.
Dehenenet Flatie1*, Tamrat Tesfaye1,2,3, K Murugesh Babu1, Magdi Gibril3 and Fangong Kong31Department of Textile Chemistry Research and Innovation Center, Ethiopian Institute of Textile and Fashion Technology, Bahir Dar University, Bahir Dar, Ethiopia
2Biorefinery Research Center, Ethiopian Institute of Textile and Fashion Technology, Bahir Dar University, Bahir Dar, Ethiopia
3State Key Laboratory of Bio based Material and Green Papermaking, Qilu University of Technology, Jinan, Shandong, China
- *Corresponding Author:
- Dehenenet Flatie
Department of Textile Chemistry Research and Innovation Center
Ethiopian Institute of Textile and Fashion Technology
Bahir Dar University, Bahir Dar, Ethiopia
E-mail: desecure575@gmail.com
Received: 01-Mar-2023, Manuscript No. AAEWMR-23-90699; Editor assigned: 03-Mar-2023, PreQC No. AAEWMR-23-90699(PQ); Reviewed: 17-Mar-2023, QC No. AAEWMR-23-90699; Revised: 21-Mar-2023, Manuscript No. AAEWMR-23-90699(R); Published: 28-Mar-2023, DOI:10.35841/aaewmr-6.2.140
Citation: Flatie D, Tesfaye T, Murugesh Babu K, Gibril M, Kong F. Extraction and characterization of protein from cottonseed meal. Environ Waste Management Recycling. 2023;6(2):140
Keywords
Cottonseed Meal, Cottonseed Protein, Cottonseed Protein Flour, Extraction, Protein Characteristics, Precipitation.
Introduction
The growing challenges of greenhouse gas emissions, climate change, the increasing gap between economic growth and environmental sustainability, and dwindling fossil resources have enforced attention towards the development of sustainable bio-based platforms [1]. However, the ranking of the agricultural sector as the second-highest contributor to greenhouse gas emissions has focused interest on the optimal valorization of agricultural waste residues as an alternative to their burning or dumping in landfills [2].
Cotton (from the Arabic quote) is an agricultural crop which uses to produce spinnable fibers (lint) on the seed coats [3]. According to the world count report in 2020, around 27 million tons of cotton are reproduced globally in a year. Cottonseed contains, on average 45 percent hulls and linters and 55 percent types of meat [4]. Nearly 45-55% of this meal is a protein [5].
Cottonseed meal (CSM) is a by-product of the oil industry. Globulins (salt soluble, vicilin, and legumin families) are the major dominant storage proteins in cottonseed and account for 60%–70% of seed proteins. Albumins (water soluble) and gliadins (alkali-soluble) are in low concentrations. It is a rich source of protein characterized by a high concentration of amino acids. However, a relatively low concentration of lysine restricts the quality of protein in CSM. Its use as feed material in poultry nutrition is limited mainly due to the presence of free gossypol, as well as high variability in nutrient concentration [6]. 80% of defatted cottonseed is used as fertilizer or substrates for edible mushrooms while only 5-10% is used as feed materials, which results in a substantial wastage of protein.
Proteins are long-chain poly amino acids arranged in a linear chain and joined together by peptide bonds (─CO─NH─) between the carboxyl and amino groups of the next amino acid residues. The presence of amino and carboxylic acid imparts protein hydrophilicity, which is recognized as a surface-active component. Recently, a protein, used as a non-petroleum, biodegradable and renewable resource was focused on by many researchers. Carboxyl, amido, amide, hydroxy, and sulfhydryl groups exist in the main chain of the protein molecule. Chemical modification such as grafting, copolymerization, and crosslinking could result in biomaterial production like hydrogel of proteins [7].
Based on the above considerations, an attempt has been made in this research mainly to extract protein from the cottonseed flour. The effect of different extraction parameters was investigated and the resulting protein extraction would be taken for a further investigation like modification, such as graft copolymerization with other monomers, and to prepare different functional cottonseed protein derivatives or composite materials.
Materials and Methods
Materials
Cottonseed meal: Dry cottonseed meal was provided by Teshome Eshetu Edible Oil Factory, Adama, Ethiopia.
Chemicals: Sodium hydroxide (NaOH, Ethanol solution, Hydrochloric acid (HCl), and other auxiliary chemicals were used for protein extraction.
Equipment: Different size beakers, digital balance, thermometer, litmus paper, oven dryer, centrifuge, non-woven heat-sealable pouch, petri dish, porous filter plate, filter paper, Infrared Analysis (FT- IR), Freeze dryer.
Methods
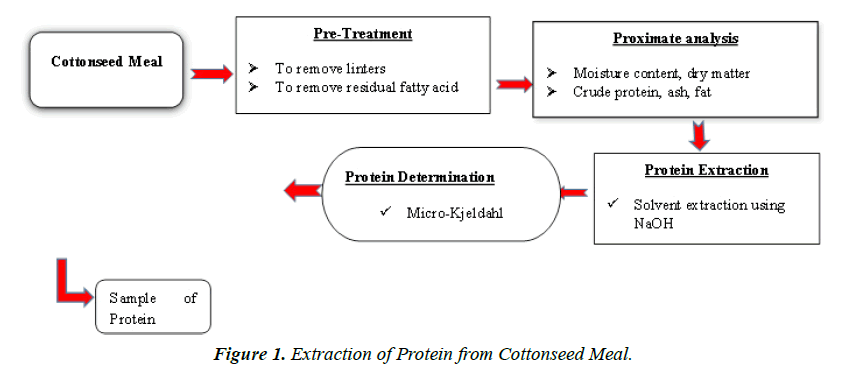
The general flow for the extraction of protein from cottonseed flour is presented in Figure 1.
Pretreatment & proximate analysis of defatted cottonseed meal
Defatting
The cotton seed meal was defatted using the method described by [8]. Meal (1 kg) was added to diethyl ether and stirred at room temperature for 60 min, and then left standing, until natural sedimentation of the meal and organic solvent Mararation occurred. Recovery of organic solvents was then completed, and the precipitated cottonseed meal was added to diethyl ether, and this procedure was repeated three times. The fat percentage was then calculated using equation 1.
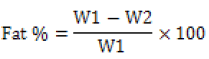 (1)
(1)
Where W1 & W2 are weights (g) of the sample before and after defatting respectively.
Moisture Content
Moisture content was determined after drying 5 grams of cottonseed meal in an oven at 1050C for 3 hours according to AOAC (2000) using equation 2.
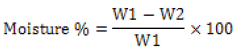 (2)
(2)
Where W1 & W2 are weights (g) of the sample before and after drying respectively.
Crude Protein
Crude protein was quantified by the standard micro-Kjeldahl Nitrogen. 0.5g of each sample was measured and placed in the digestion flasks. Then 1g of Kjeldahl catalyst and 6 ml of sulfuric acid were added and the sample was allowed to digest. The sample was digested at 3500C for 180 min. After digestion, it was distilled with water, 40% sodium hydroxide, 4% of boric acid, and, reagents followed by titration. According to AOAC (2000) crude protein was estimated by multiplying the nitrogen content with a factor of 6.25 using equation 3.
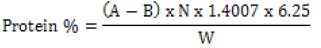 (3)
(3)
Where, A = Volume (ml) of 0.2 N HCl used in sample titration
B = Volume (ml) of 0.2 N HCl used in blank titration
N = Normality of HCl
W = Weight (g) of sample
14.007 = Atomic weight of nitrogen
6.25 = Protein-nitrogen conversion factor for fish and its by-products
Ash Content
Ash content was determined as the weight of the residue after 2 grams of the sample with pre weighted crucible which was ashed at 575°C in a muffle furnace overnight AOAC (2000).
 (4)
(4)
Protein Extraction
Protein extraction was carried out using alkaline solution which was of sodium hydroxide (NaOH). Central composite designs (CCD) with three factors (concentration, temperature, and time) and a constant solvent-to-flour ratio were used to determine the number of runs and combinations. Protein purity and flour dissolution percentage have been taken as a response.
Defatted cottonseed meal protein was extracted with a solventto- flour ratio of 20:1 using 0.1-0.5 M NaOH in a water bath using AUTOWASH 2762 after the desirable temperature, pH, and time were adjusted. After extraction, the solution was filtered immediately. Then the extract was acidified with 1N hydrochloric acid and then kept steady at 5℃ for 24 hours until the isoelectric value was attained (precipitation). Generally, the isoelectric point (pI) of plant oil proteins ranges from pH 4 to 5. Due to different techniques of oil extraction, the highest precipitation for cottonseed protein from various sources usually occurs at a pH between 3.5 to 5.5. Later, the precipitated protein was washed with 50% 2-propanol and then freeze-dried.

Protein Characterization
Solubility
The solubility of extracted protein was determined according to the method described by [9]. In brief, 0.5 g of protein was dispersed in 50 ml of deionized water which was maintained at different pH values (pH 3.0, 5.0, 7.0, 9.0, and 11.0). The mixture was then stirred for 1h and centrifuged at 1700×g for 30 min. The protein content of the supernatants was determined using the Kjeldahl method. Protein solubility was expressed as the percentage ratio of supernatant protein content to the total protein content.
Water Absorbency
Water absorbency was determined using the method described by [10]. The protein sample (0.5 g) was dispersed in distilled water in a 10 ml pre-weighed centrifuge tube. The dispersion was shaken for 1 min, allowed to stand for 30 min, and centrifuged at 1700×g for 30 min at room temperature. The supernatant was decanted, excess water in the upper phase was drained for 10 min, and the tube containing the protein residue was weighed again to determine the amount of water retained per gram of sample.
FTIR Analysis
The functional groups of sample protein were characterized using an FTIR spectrophotometer (JASCO FT/IR - 6600). KBr pellets were prepared using 1-2 mg of protein powder which was observed under IR. Transmittance values were recorded at wave numbers between 4,000 and 400 cm-1.
Results and Discussion
Composition of Cottonseed Meal
The proximate composition of cottonseed meal was determined in terms of moisture content, fat content, residual oil content, crude protein content, and total ash %. Analytical data on the cottonseed flours before protein extraction are shown in Table 1.
| Flour source | Moisture (%) | Total fat (%) | Residual oil (%) | Crude protein (%) | Ash (%) |
|---|---|---|---|---|---|
| Standard as per FSSR, 2011 | < 8% by wt. | < 3% dry wt. | - | > 45% dry wt. | < 7% dry wt. |
| Cottonseed flour in current study |
4.68% | 2.83 | 6.23 | 35.47 | 5.90 |
Table 1: Standard and obtained values of CSM
The cottonseed meal cake for this study was obtained from one round mechanical press technique. It may be observed from Table 1 that, it gives lower oil yields than the scientific solvent oil extraction i.e., the remaining oil in mechanical press meal is high. The meal can leave the press with up to 20% of the original oil still present in it [11]. Hence, one can observe the presence of residual oil in the cake. Similarly, a lower crude protein content of CSM than the standard may be due to factors like varieties and location differences, varied agronomic practices (fertilizer), and differences in the degree of certification, the proportion of husk, and processing methods during oil extraction [6] and the lower fat content might be due to the defatting process.
Protein Extraction
To optimize the better combination of factors for a maximum protein yield and flour dissolution percentage i.e. protein in the form of solution before precipitation and maximum protein purity percentage, a central composition design was used. The maximum protein dissolution percentage was calculated using the residual undissolved impurities and the maximum protein purity percentage was quantified by the standard micro- Kjeldahl Nitrogen using the conversion factor of 6.25. The results are presented in Table 2.
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | |
|---|---|---|---|---|---|
| Run | A: Concentration (M) |
B: Time (Min) |
C: Temperature (°C) |
Dissolution (%) |
Protein purity (%) |
| 1 | 0.3 | 37.5 | 45 | 55.44 | 57.77 |
| 2 | 0.3 | 37.5 | 45 | 57.59 | 56.90 |
| 3 | 0.1 | 60 | 60 | 64.77 | 67.15 |
| 4 | 0.3 | 37.5 | 19.77 | 54.49 | 53.60 |
| 5 | 0.5 | 15 | 30 | 60.45 | 64.86 |
| 6 | 0.3 | 37.5 | 45 | 52.81 | 54.23 |
| 7 | 0.63 | 37.5 | 45 | 62.19 | 59.89 |
| 8 | 0.3 | 37.5 | 45 | 56.02 | 54.86 |
| 9 | 0.1 | 15 | 30 | 41.48 | 50.86 |
| 10 | 0.5 | 60 | 30 | 63.72 | 59.23 |
| 11 | 0.5 | 60 | 60 | 67.55 | 53.98 |
| 12 | 0.5 | 15 | 60 | 58.65 | 56.27 |
| 13 | 0.1 | 60 | 30 | 48.49 | 60.93 |
| 14 | 0.3 | 75.340 | 45 | 60.05 | 59.90 |
| 15 | 0.1 | 15 | 60 | 53.80 | 64.90 |
| 16 | 0.3 | 37.5 | 70.23 | 74.17 | 56.49 |
| 17 | 0.3 | 37.5 | 45 | 51.32 | 54.27 |
| 18 | 0.3 | 37.5 | 45 | 57.12 | 54.18 |
Table 2: Protein Purity and flour Dissolution Percentage
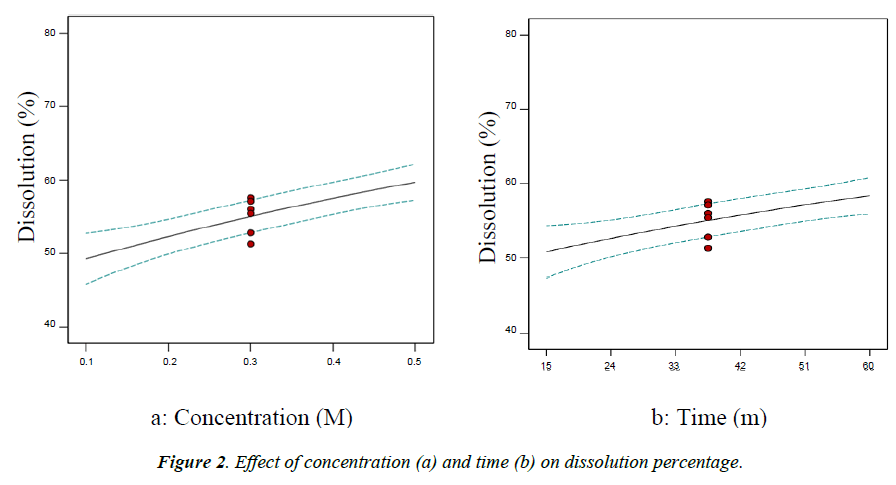
Effect of concentration on dissolution percentage
The effect of concentration on the dissolution percentage is clearly shown in Figure 2a. The lines in the Figure from top to bottom describe the relation between predicted, calculated and actual values of dissolution percentage respectively. Concentration has a significant effect on the dissolution percentage and as it increases the dissolution percentage also increases up to the optimum value. The degree of protein solubility in an aqueous medium is the result of electrostatic and hydrophobic interactions between protein molecules. At pH above or below isoelectric pH (pI), the proteins become negatively or positively charged depending on the pH, resulting in electrostatic repulsions between molecules and hydration of charged residues, contributing to the solubility of proteins [12]. However, cottonseed protein is prone to degradation and denaturation and difficult to be kept in strongly alkaline conditions [7].
Effect of time on flour dissolution percentage
Figure 2b shows the significant effect of time on the dissolution percentage. As time increases the dissolution percentage also increases. After 15 minutes, the extraction rate amounted to 50%. While after 40 minutes the extraction rate was seen slowly decreasing.
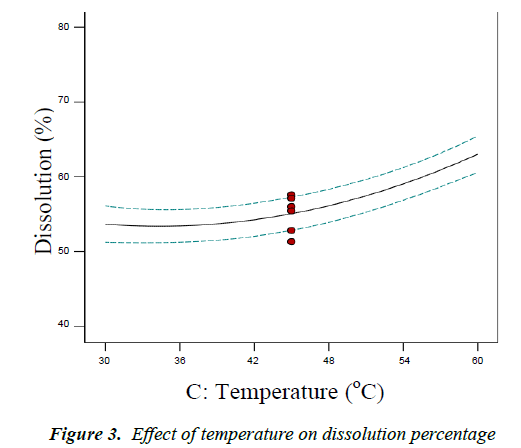
Effect of temperature on dissolution percentage
The temperature has a significant effect on the dissolution percentage of cottonseed flour. Figure 3 shows that, with the increasing temperature, the flour dissolution percentage increased. Dissolution is directly proportional to the increase in temperature. When the temperature is increased, molecules move fast and the mass transfer rate of the interface between solid and liquid is developed. Therefore, increasing temperature promotes mass transfer and solubility, reduces the viscosity of the solution, and thus increases the extraction rate. However, high temperature causes the reduction of protein activity and thermal denaturing of protein. Additionally, with the increase in temperature, the color of the protein extraction becomes dark.
Characterization of cottonseed protein
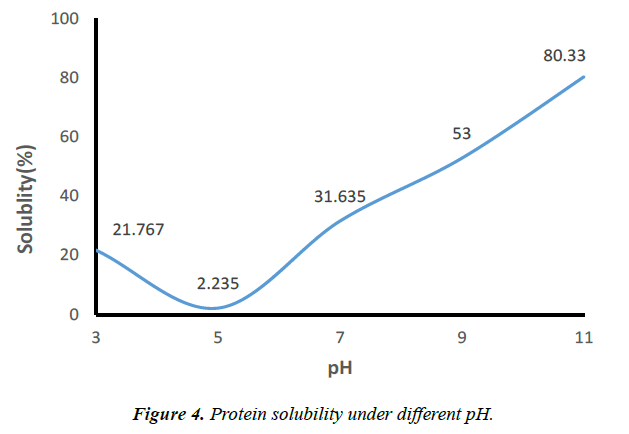
Protein solubility
During an increase or a decrease in the pH of the protein solution, the side chains can assume strong positive or negative charges, resulting in strong repulsive forces between proteins that pull proteins apart from each other. These strong positive or negative charges will favor the protein-water interactions thus causing the solubility of proteins. Figure 4 shows the protein solubility at different pH conditions. The maximum protein solubility occurs at alkaline conditions i.e., at pH 11, whereas, the least protein solubility occurs at an acidic pH of 5 [13]. This result was due to the reduced interaction between protein and water, and this phenomenon enhances protein-protein interactions, resulting in protein aggregation and precipitation. The degree of protein solubility in an aqueous medium is the result of electrostatic and hydrophobic interactions between protein molecules. At pH above or below isoelectric pH (pI), the proteins become negatively or positively charged depending on the pH, resulting in electrostatic repulsions between molecules and hydration of charged residues, contributing to the solubility of proteins [13, 14]. Strong electrostatic repulsions among protein molecules and the increased protein-solvent interaction at this pH could have contributed to this increased solubility [12].
Protein water absorbency
Protein water absorbency is the index of the ability of proteins to absorb and retain water. It directly influences the waterholding capacity of a synthesized material from proteins for various applications. The average water-holding capacity of the extracted cottonseed protein was 1.624g/g and this result indicates the presence of more soluble proteins and higher availability of polar amino acids in the cottonseed protein isolate. It may be concluded based on the results that, the cottonseed protein demonstrates a desirable ability to absorb and retain water [15].
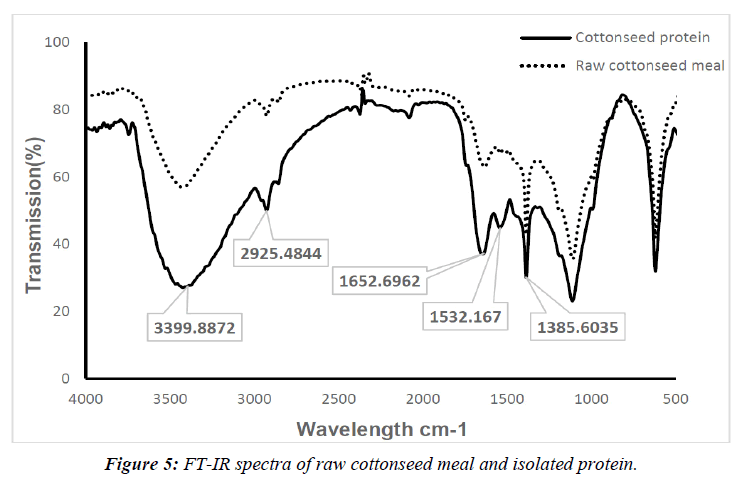
FTIR Analysis
FTIR spectra of raw cottonseed meal and extracted protein are presented in Figure 5. It may be observed that both the raw cottonseed meal and protein isolate samples display similar relevant peaks. The characteristic peak at 1653 cm-1 for protein isolate can be attributed to amide I. Amide I corresponds to the C=O stretching vibration with a minor contribution from the C-N stretching vibration. Similarly, the peak at 1532 cm-1 can be attributed to amide II which arises from N-H bending and C-N stretching vibration. The broad peak observed between 3300–3400 cm-1 results from the overlapping contributions of the stretching vibrations of both O-H and N-H groups. Furthermore, the area of the peak at 1385 cm-1 is related to the symmetric stretching vibrations of COO- groups due to the carboxyl group vibrations of aspartic and glutamic acids and belongs to the C–N for amide III [16]. Interestingly, the presence of the peak at 1532 cm-1 (amide II) in the protein isolate sample which was not present in the raw sample indicates that the extraction was highly targeted to the amide groups in proteins or protein-like compounds isolation. The transmission peak at 1652 cm-1 which is related to C=O stretching vibration with a minor contribution from C-N stretching vibration in the cottonseed protein isolate sample demonstrates that the extraction was significantly focused on isolating the protein-like compounds from the raw sample.
Conclusion
This work focused on the extraction of protein from cottonseed meal using NaOH as a solvent with a solvent-to-flour ratio of 20:1. Several factors were investigated to determine their effects on the extraction of proteins from cottonseed meal and to optimize the extraction protocol. The optimized parameters used for extraction were a concentration of 0.1M NaOH, a temperature of 67°C, pH 12, and a time of 90 minutes. Under these optimum conditions, it was possible to extract about 81% of protein from the raw cottonseed meal. The flour dissolution percentage indicates the amount of dissolved flour that can be precipitated for protein isolation. The temperature has a significant effect on flour dissolution since the energy of the molecule increases as the temperature increases which promotes mass transfer and solubility. Furthermore, these studies provided basic information on the characteristics of extractable proteins. The micro-Kjeldahl Nitrogen result indicated that the extractable proteins had a better protein purity as this may be useful for the production of proteinbased bio-products. The protein was also characterized using FTIR and the protein solubility was also determined. It may be concluded that, the extracted protein could be directly used for any chemical modification without any further treatments.
References
- Nizami AS, Rehan M, Waqas M, et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresource technology. 2017;241:1101-17.
- Didaskalou C, Buyuktiryaki S, Kecili R, et al. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: From materials to sustainable process design. Green Chem. 2017;19(13):3116-25.
- Lee JA, Fang DD. Cotton as a world crop: origin, history, and current status. Cotton. 2015;57:1-23.
- Adams R, Geissman TA, Edwards JD. Gossypol, a pigment of cottonseed. Chem Rev. 1960;60(6):555-74.
- Blankenship DC, Alford BB. Cottonseed--the new staff of life
- Swiatkiewicz S, Arczewska-Wlosek A, Jozefiak D. The use of cottonseed meal as a protein source for poultry: an updated review. Worlds Poult Sci J. 2016;72(3):473-84.
- Zhang B, Cui Y, Yin G, et al. Alkaline extraction method of cottonseed protein isolate. Mod Appl Sci. 2009;3(3):77-82.
- Timilsena YP, Adhikari R, Barrow CJ, et al. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food chem. 2016;212:648-56.
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino acids. 2009;37:1-7.
- Ajibola CF, Malomo SA, Fagbemi TN, et al. Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. Food Hydrocoll. 2016;56:189-200.
- Kristoferson LA, Bokalders V. Renewable energy technologies: their applications in developing countries. Elsevier; 2013.
- Yasothai R, Giriprasad R. Acid/Alkaline solublization method of processing protein. Int J Environ Sci Technol. 2015;4(1):96-100.
- Li C, Xue H, Chen Z, et al. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res Int. 2014;57:1-7.
- Ivanova P, Chalova V, Koleva L, et al. Amino acid composition and solubility of proteins isolated from sunflower meal produced in Bulgaria. Int Food Res J. 2013;20(6):2995-3000.
- Adebowale YA, Adeyemi IA, Oshodi AA. Functional and physicochemical properties of flours of six Mucuna species. Afr J Biotechnol. 2005;4(12).
- Cuadri AA, Bengoechea C, Romero A, et al. A natural-based polymeric hydrogel based on functionalized soy protein. Eur Polym J. 2016;85:164-74.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref