- Biomedical Research (2009) Volume 20, Issue 2
Expressions and localization of cyclin D1 and cyclin-dependent kinase inhibitor p27Kip1 in gliomas and meningiomas
Farizan Ahmad1*, Jafri Malin Abdullah1, Hasnan Jaafar2
2Department of Pathology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia.
- *Corresponding Author:
- Farizan Ahmad
Department of Neurosciences
School of Medical Sciences
Universiti Sains Malaysia 16150 Kubang Kerian
Kelantan, Malaysia
Phone: +609-7664240
Fax: +609-7648613
Accepted March 16 2009
Abstract
Various studies have shown contradicting results in p27 and cyclin D1 protein expression and the association with brain tumors. This study was thus performed to address new information of both markers’ expression in gliomas and meningiomas cases, among Malaysian brain tumors patients. For these purposes, 24 meningiomas and 23 gliomas were analyzed by immunohistochemistry and immunogold electron microscopy analyses to determine both protein expression and localization, respectively. From the results, we observed high expression level of p27 in all types of tumors including low and high grades of gliomas, and meningiomas. Our findings revealed that p27 was overexpressed in all cases including meningiomas (82.6%), low grades gliomas (80%) and high grades gliomas (84.6%). We subsequently found high level of cyclin D1 expression in meningiomas (70.8%), equal expression of cyclin D1 in low grades of gliomas, and the expression showed to decrease in higher malignancy of the cancer (76.9% as low expressors). Immunogold staining showed that p27 and cyclin D1 confined in nuclear and cytoplasm in both cancer types independently of survival of the patients (p27 miclocalization in meningiomas, p=0.6299; gliomas, p=0.2233 and cyclin D1 miclocalization in meningiomas, p=0.5627; gliomas, p=0.8230). Our results showed that elevation of p27 level did not inhibit proliferation of cancer cells. Via immunogold staining using electron microscopy technique, we have detected mislocalization of the p27 protein in cytoplasm of both meningiomas and gliomas cases which might cause the inactivation of the p27 protein.
Keywords
Cyclin D1, p27, protein expression, localization, glioma, meningiomas
Introduction
Cell cycle is tightly controlled by three families of key cell cycle regulatory proteins: cyclins, their catalytic subunits known as cyclin-dependent kinase (CDK), and cyclindependent kinase inhibitor (CDKI). Various CDKs, cyclins and CDKIs involve in regulating cell cycle progression which differ from stage to stage [1].
Cyclins are recognizing specific kinases (CDKs), bind to and form complexes which allow progression of cell cycle. At the G1-S transition, it is initially governed by cyclin D1-3 complexes, and continued by cyclin E, by activating CDK4-6 and CDK2, respectively [2,3]. The complexes activities are controlled by phosphorylation-dephosphorylation mechanism and of cyclin-dependent kinase inhibitors (CDKIs). Of all CKIs, p27/Kip1 is one of the most important regulators which bind to complexes with cdk4, cdk6 or cdk2 and brings cells to S phase of cell cycle regulation [4]. It will negatively regulate the early G1 phase as well as late G1 phase at necessary points [5].
Protein expression level of cyclin-dependent kinase inhibitor, p27 and cyclin D1 in cell cycle have been remarked as the important markers for determining tumorigenesis in various types of tumors [1,6-9]. It has been established that the information gathered is a valuable predictors to be correlated with the prognosis of the cancers [3,4,10,11]. Various studies have shown contradicting results in p27 and cyclin D1 protein expression and the association with brain tumors. This study was thus performed to address new information of both markers’ expression in gliomas and meningiomas cases, among Malaysian brain tumors patients.
Materials and methods
Tissue samples
We examined a series of tissue specimens that has been collected from surgical excision from 24 glioma and 23 meningioma cases referred to the Hospital Universiti Sains Malaysia, Kelantan between the years of 1997 to 2000. This study was approved by the Research Development and Human Ethics Committee, School of Medical Sciences, University Science Malaysia and informed consent was obtained from all patients. The histology of the specimens was determined by hematoxylin and eosin staining and the classification of the diseases were done according to the WHO classification [12]. All histopathological examination were observed by at least three histopathologist and discussed in our neuropathology conference and all p27 and cyclin D1 specimens were analyzed twice to rule out false or negative results. In our study meningioma study, we included 22 WHO grade I, 1 with WHO grade II and 1 with WHO grade III. In addition, in gliomas, there were 3 with WHO grade I, 7 with WHO grade II, 6 with WHO grade III and 7 with WHO grade IV. Parameters like age, sex, classification of the tumors and presenting complaints such as headache, hemiplegia, epilepsy as well as radiological images i.e. site, size, consistency of tumor, midline shift and vascularity were compared with their management, p27 and cyclin D1 protein expression, relapse rate and outcome. All patients were operated on and had a Karnofsky score of more than 70 prior to the inclusion of this study.
Immunohistochemistry of p27 and cyclin D1
The resected specimens were routinely fixed at 10% formalin and embedded in paraffin. Then, 4 μm of thick sections were cut from the paraffin block and placed on slides coated with Poly-L-Lysine. Immunohistochemical staining was performed using steptavidin-biotin-HRP complex method of Immunoperoxidase Secondary Detection System (Chemicon International, USA) according to the manufacturer’s protocols. Briefly, the sections were deparaffinized at 60°C for a minimum of 60 minutes, followed by incubation in xylene and hydration in a series of decreasing concentration of ethanol of 100%, 70% and 30%. After that, the sections were placed in a microwavable plastic ware and filled with antigen retrieval solution. Two different solutions were used, 0.01M citrate (pH 6.0) buffer for p27 and 0.1M EDTA for cyclin D1. They were boiled in microwave for 14 minutes for the antigen retrieval purpose. The sections were washed in TBS buffer (pH 7.6), and immersed in 3% hydrogen peroxide and 100% methanol for 10 min to inhibit endogenous peroxidase activity. Primary antibodies of P27KIP1 Epitope Specific Rabbit Antibody (Lab Vision, USA) and Cyclin D1 (Clone SP4) Rabbit Monoclonal Antibody (Lab Vision, USA) were used in this study. The slides were incubated with primary antibodies for 30 minutes at dilution of 1: 50 at room temperature with P27KIP1 Epitope Specific Rabbit Antibody and overnight at dilution of 1: 50 at 4°C with Cyclin D1 (Clone SP4) Rabbit Monoclonal Antibody. After incubation with secondary antibody (available in the staining kit), the sections were washed with TBS and covered with peroxidase-conjugated streptavidin. The peroxidase activity was developed with diaminobenzidine as a chromogen, and the sections were then counterstained with hematoxylin, dehydrate, coversliped, and mounted with DPX mounting media. Human breast carcinoma and human benign prostatic hyperplasia were used as the positive controls for cyclin D1 and p27 markers respectively.
P27 and cyclin D1 scoring of immunohistochemistry analysis
Sections stained for p27 and cyclin D1 were assessed on Microscope Leitz Labolux20 (Leitz, Germany) and the percentage of immunostained cells was determined. The percentages of p27 and cyclin D1 immunopositive tumor cells were counted in 5 consecutive microscopic fields (magnification 100x) per tumor sample in area that showed the highest density of these cells. In each field, 100 tumor cell nuclei were evaluated and the mean for every 5 fields were calculated. A distinct granular brown nuclear stain was scored as positive. A cutoff value of <5% immunopositive cells was considered negative, and >5% immunopositive cells was considered positive. The positive samples were scored according to the frequency of p27 and cyclin D1 immunopositive cells as 5%, 25%, 26% to 50%, 51% to 75%, and >75%, as previously Samples from patients with <50% cyclin D1 and p27-positive tumor cells were considered low expressors, whereas those with >50% cyclin D1 and p27-positive tumor cells were considered high expressors. [3]
For immuno-localization of p27 and cyclin D1 protein, percentages of immunopositive cells in 100 cells were counted in 5 different microscopic fields (magnification 100x) and the mean of were evaluated according to Viglietto et al. (2002). P27 and cyclin D1 were scored ‘nuclear’ when more than 50% of p27 and cyclin D1-expressing cells presented nuclear staining, and ‘cytoplasmic’ when there was nucleo-cytoplasmic or exclusively cytoplasmic staining in at least 35% of p27 and cyclin D1-expressing cells. [13]
Electron microscopy analysis
Fresh tissue specimens which were obtained from the surgical procedure at Hospital Universiti Sains Malaysia, Malaysia were cut into small pieces (1mm3) and placed in a vial containing fixative buffer of 4% paraformadehyde 3% glutaraldehyde for 2 hours. The tissue was then dehydrated and infiltrated in series concentration of ethanol and resin. Finally, they were embedded in 100% pure resin for the polymerization step.
Ready samples were cut into 60-90nm thickness and placed onto grids. They were then stained using primary antibody (same as immunohistochemistry) and gold-conjugated secondary antibody. Viewing of the samples was done using Transmission Electron Microscope, LEO 912AB, Omega (German) with Soft Imaging System.
Results
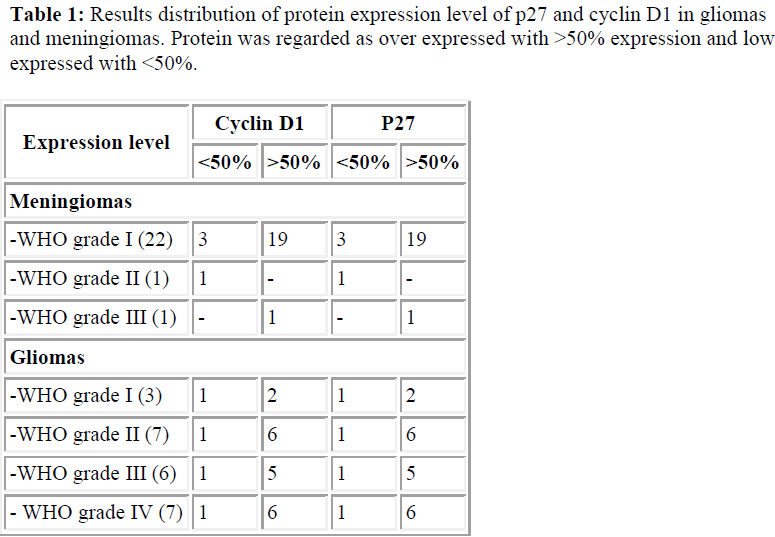
Our immunohistochemistry findings revealed that p27 was overexpressed in all cases including meningiomas, low grades gliomas and high grades gliomas. See Table 1 and Figure 1.
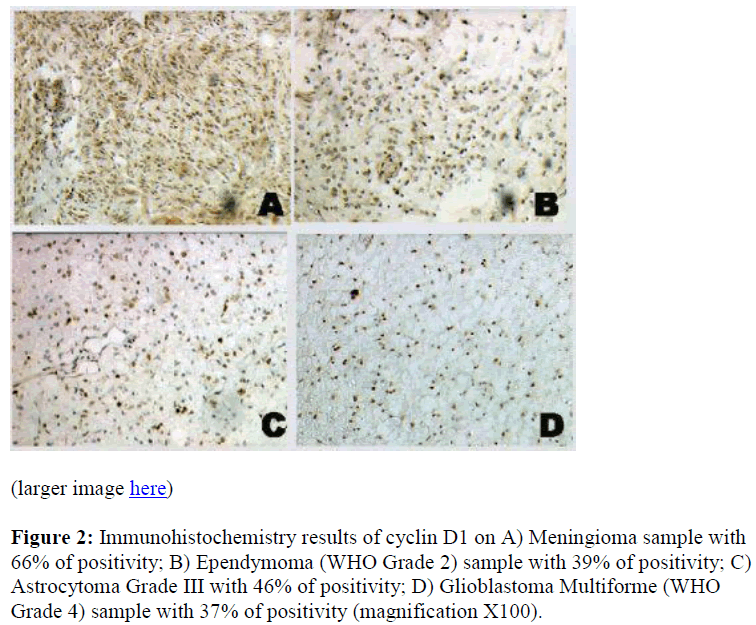
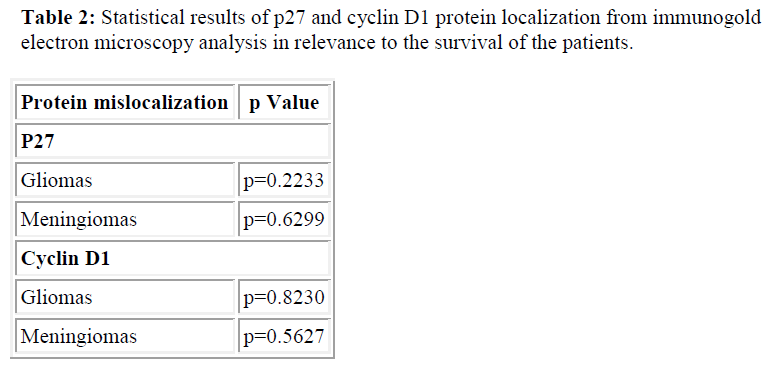
We also detected cytoplasmic localization of p27 and cyclin D1, 79% in meningiomas cases and 58% and 50% in gliomas cases, respectively (Table 1). This finding was further confirmed by the immunogold electron micros copy analysis where we subsequently detected p27 protein in the nucleus and cytoplasmic of meningiomas and gliomas cases (Figure 3). However, p27 and cyclin D1 proteins mislocalization was not found to be significantly correlated with the survival of the patients (Table 2).
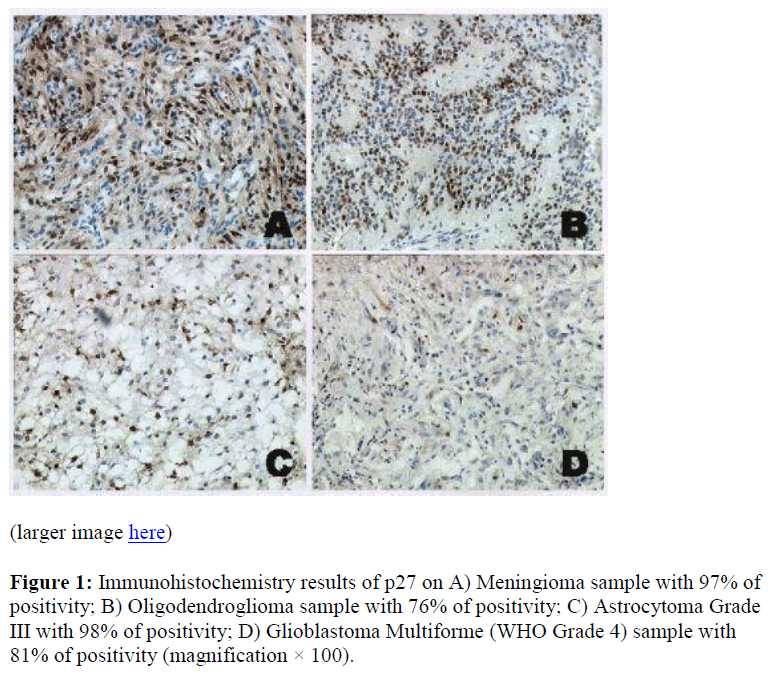
Figure 2: Immunohistochemistry results of cyclin D1 on A) Meningioma sample with 66% of positivity; B) Ependymoma (WHO Grade 2) sample with 39% of positivity; C) Astrocytoma Grade III with 46% of positivity; D) Glioblastoma Multiforme (WHO Grade 4) sample with 37% of positivity (magnification X100).
Figure 3: p27 immunogold staining of glioma tissue. Immunogold labeling is represented by discrete black spots (arrows) scattered in the nucleus and cytoplasm of the cell. The spots were seen at the dense and loose area of nucleus chromatin and along cytoplasmic area. N, Nucleus; C, Cytoplasm. (Magnification × 16 000).
Discussion
Low expression level of p27 protein have been associated with worse prognosis of many tumor types including breast, gastric, prostate, non-small cell lung, ovarian, colorectal, endocrine and head and neck tumors [3,14,15]. In general, p27 has inverse correlation of the protein expression and cell cycle progression [4,16]. Contrary to p27 protein expression, cyclin D1 is frequently reported to be overexpressed in higher grade tumors. Significant correlation of cyclin D1 overexpression in higher malignancy of tumors has been viewed in breast cancer, colorectal cancer, parathyroid adenoma, prostate cancer, melanoma and lymphoma [17].
To further discover prognostic association of p27 and cyclin D1 proteins expression in brain tumors, 24 meningiomas, 10 low grade gliomas and 13 high grade gliomas were analyzed immunohistochemically. The results thus suggested p27 protein elevation in both benign and malignant brain tumors, independently of tumor grades. Others [4,16] have reported contrary findings to ours, by reporting downregulation of p27 protein expression in brain tumors. Interestingly, our results were similar to those found by Zagzag et al. [3]. In a study which included 26 astrocytomas, they found approximately 50% of the high grades astrocytomas were low expressors of p27 and 50% were high expressors. Besides brain tumors, p27 protein elevation has also been found in small cell lung carcinoma [14], breast cancer [18], and in both normal and abnormal muscle fibres [15].
Our overall findings of cyclin D1 protein expression showed that cyclin D1 was highly expressed in benign and low grades gliomas of brain tumors nevertheless decreased in higher grades of tumors.
The patterns of p27 and cyclin D1 protein expression in our study might be influenced by the hypoxia condition in the tumor series. Hypoxia has been shown to contribute to p27 protein increment in various types of cancer [3,19]. In our study, we detected increasing percentage of p27 protein expression in low grades to high grades gliomas. Regardless to that, we observed decrement of cyclin D1 protein as the malignancy increased. Krtolica et al., [20] has reported similar p27 and cyclin D1 expression pattern in human ovarian carcinoma cells.In the study, they exposed ovarian cancer cells to hypoxia relatively increasing in time. As the time increased, hypoxic condition became more dominant with the decreasing availability of the oxygen. They monitored the expression pattern of p27 and cyclin D1 as well as several other proteins according to certain time points. They subsequently found p27 protein elevation in more hypoxic condition. Nevertheless, they revealed a lowering in cyclin D1 protein abundance whilst the hypoxia became more dominant However, further studies need to be carried out to address the issue.
Our results showed contradiction to the normal functions of p27 as one of the cyclin dependent kinase inhibitors. Instead of being strongly found in quiescent cells, we discovered high level of p27 protein in highly proliferating cancer cells. Our findings was consistent with the results reported by Fredersdorf et al. [18], Sui et al. [21], and Viglietto et al. [13]. In their studies, they also found elevation of p27 proteins in highly proliferating cells of breast and colorectal cancers, epithelial ovarian tumors, and breast cancer respectively. In our studies, the brain tumor cells were highly proliferating instead of having high p27 protein. Concerning to the original functions of p27 protein, to inhibit cells proliferation, we are assuming that certain mechanisms should involve which allow cancer cells to circumvent p27 growth inhibition.
To address the issue, we subsequently assessed the localization of p27 protein using immunostaining with antip27. Interestingly, we detected p27 protein to be exclusively confined in the cytoplasm in majority of the samples. Cytoplasmic localization of p27 and cyclin D1 occurred in 79% in meningiomas cases and 58% and 50% in gliomas cases, respectively. The mislocalization of the protein was further confirmed by the immunogold electron microscopy analysis where we subsequently found p27 protein to be confined in nucleus and cytoplasmic of meningiomas and gliomas cases (Figure 3). It clearly showed that even though high p27 protein level was found, the cancer cells maintained its’ high proliferate capacity due to the inactivation of the cyclin-dependent kinase inhibitor protein. Generally, p27 is regarded as a nuclear protein which is encoded by CDKN1B gene, which is located on chromosome 12q13 [18,22,23]. In many types of cancer cases, p27 oftenly reported to be mislocalized in the cytoplasm of the cells [24]. Various studies have shown that p27 mislocalization occurs in breast cancer [13], lung and colorectal cancers [25], and choroidal melanoma tumors [26]. p27 nuclear localization is essential for growth inhibiting functions of the protein. Thus, the p27 protein mislocalization in cytoplasm is believed to produce inactivated cyclin dependent kinase inhibitor protein, which is no more functioning to restrain the proliferation of abnormal cells [13,15,24]. Therefore, cancer cells with high level of p27 protein were able to survive and actively proliferate. Singh et al. [15] have reported loss of p27 functions in Barrett’s associated adenocarcinoma due to the protein mislocalization. They detected poor survival in patients with inactivated p27 proteins. In our cases however, p27 and cyclin D1 proteins mislocalization was not found to be significantly correlated with the survival of the patients (p27 miclocalization in meningiomas, p=0.6299; gliomas, p=0.2233 and cyclin D1 miclocalization in meningiomas, p=0.5627; gliomas, p=0.8230).
Conclusion
Immunohistochemistry study has given us new information of the level of protein expression of both p27 and cyclin D1 proteins in meningiomas and gliomas in Malaysia. P27 protein was reported to be overexpressed in all cases including meningiomas, low grades gliomas and high grades gliomas. Contrary to p27, cyclin D1 showed decrement of protein expression with increasing malignancy of the tumors. In condition of high expressing p27 protein, cells were still capable of highly proliferating might be due to the translocalization of the protein from the nucleus into the cytoplasm which have been observed via immunogold electron microscopy analysis. The translocalization was believed to inactivate the normal function of p27 as the tumor suppressor gene. However, current study only provides a basis of understanding about involvement of p27 and cyclin D1 in brain tumor. Further research is therefore necessary to gain further information about the underlying mechanism behind the translocalization and overexpression of the proteins in glioma.
Acknowledgement
This research is funded by MAKNA (National Cancer Registry)-USM (Universiti Sains Malaysia)-UPM (Universiti Putra Malaysia) grant.
References
- Bahnassy AA, Zekri ARN, Saleh M, Lotayef M, Moneir M, Shawki O: The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clinical Pathology 2007, 7: 1-11.
- Piva R, Cavalla P, Bortolotto S, Cordera S, Richiardi P, Schiffer D: p27/kip1 expression in human astrocytic gliomas. Neurosci Lett 1997, 234: 127-130.
- Zagzag D, Blanco C, Friedlander DR, Miller DC, Newcomb EW: Expression of p27KIP1 in human gliomas: relationship between tumor grade, proliferation index, and patient survival. Hum Pathol 2003, 34: 48-53.
- Kirla RM, Haapasalo HK, Kalimo H, Salminen EK: Low expression of p27 indicates a poor prognosis in patients with high-grade astrocytomas. Cancer 2003, 97: 644-648.
- Egozi D, Shapira M, Paor G, Ben-Izhak O, Skorecki K, Hershko DD: Regulation of the cell cycle inhibitor p27 and its ubiquitin ligase Skp2 in differentiation of human embryonic stem cells. Faseb J 2007, 21: 2807-2817.
- Ewen ME, Lamb J: The activities of cyclin D1 that drive tumorigenesis. TRENDS in Molecular Medicine 2004, 10: 158-162.
- Garrett MD: Cell cycle control and cancer. Current Science 2001, 81: 515-522.
- Nho RS, Sheaff RJ: p27kip1 contributions to cancer. Progress in Cell Cycle Research 2003, 5: 249-259.
- Sherr CJ, Roberts JM: CDK inhibitors: positive and negative regulators of G1- phase progression. Genes and Development 1999, 13: 1501-1512.
- Moignea VL, Robreaua G, Borotb C, Guesdonc J-L, Mahana W: Expression, immunochemical characterization and localization of the Mycobacterium tuberculosis protein p27. Tuberculosis 2005, 85: 213–219.
- Wu FY, Wang SE, Sanders ME, Shin I, Rojo F, Baselga J, Arteaga CL: Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res 2006, 66: 2162-2172.
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavanee WK: The WHO classification of tumors of the nervous system. Journal of Neuropathology and Experimental Neurology 2002, 61: 215-225.
- Viglietto G, Motti ML, Bruni P, Melillo RM, D’alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al: Cytoplasmic relocalization and inhibi-tion of the cyclindependent kinase inhibitor p27Kip1 by PKB/Akt- mediated phosphorylation in breast cancer. Nature Medicine 2002, 8: 1136-1144.
- Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW: p27kip1: A multifunctional cyclin -Dependent Kinase Inhibitor with prognostic significance in human cancer. American Journal of Pathology 1999, 154: 313-323
- Singh SP, Lipman J, Goldman H, Jr. FHE, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M: Loss or altered subcellular of p27 in Barrett’s associated adenocarcinoma. Cancer research 1998, 58: 1730-1735.
- Fuse T, Tanikawa M, Nakanishi M, Ikeda K, Tada T, Inagaki H, Asai K, Kato T, Yamada K: p27Kip1 expression by contact inhibition as a prognostic index of human glioma. J Neurochem 2000, 74: 1393-1399.
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG: Minire-view: Cyclin D1: Normal and Abnormal Functions. Endrocrinology 2004, 145: 5439-5447.
- Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Pestoni D, Hall PA, Hanby AM, et al: High Level Expression Of P27kip1 And Cyclin D1 In Some Human Breast Cancer Cells: Inverse Correlation Between The Expression Of P27kip1 And Degree Of Malignancy In Human Breast and Co- lorectal Cancers. Medical Sciences 1997, 94: 6380-6385.
- Masuda A, Osada H, Yatabe Y, Kozaki K-i, Tatematsu Y, Takahashi T, Hida T, Takahashi T, Takahash T: Protective Function of p27KIP1 against Apoptosis in Small Cell Lung Cancer Cells in Unfavorable Micro-environments. American Journal of Pathology 2001, 158: 87-96.
- Krtolica A, Krucher N, Ludlow J: Molecular analysis of selected cell cycle regulatory proteins during aerobic and hypoxic maintenance of human ovarian carcinoma cells. British Journal of Cancer 1999, 80: 1875-1883.
- Sui L, Tokuda M, Ohno M, Hatase O, Hando T: The Concurrent Expression of p27kip1 and Cyclin D1 in Epithelial Ovarian Tumors. Gynecologic Oncology 1999, 73: 202-209.
- Ding Q, Grammer JR, Nelson MA, Guan JL, Stewart JE, Jr., Gladson CL: p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J Biol Chem 205, 280: 6802-6815.
- Keles N, Erdamar B, Kaur A, Er KD: p21, p53, and p27 Kip1 alterations in benign and malignant tumors of sinonasal epithelium. Otolaryngology–Head and Neck Surgery 2003, 129: 77-84.
- Coqueret O: New roles for p27 and p21 cell cycle in-hibitors; a function for each cell compartment? . TRENDS in cell biology 2003, 13: 65-70.
- Ratschiller D, Heighway J, Gugger M, Kappeler A, Pirnia F, Schmid RA, Borner MM, Betticher DC: Cyclin D1 Overexpression in Bronchial Epithelia of Patients With Lung Cancer Is Associated With Smoking and Predicts Survival Journal of Clinical Oncology 2003, 21: 2085-2093.
- Mouriaux Fdr, Maurage C-A, Labalette P, Sablonnie`re B, Malecaze Fo, Darbon J-M: Cyclin-Dependent Kinase Inhibitory Protein Expression in Human Choroidal Melanoma Tumors. Investigative Ophthalmology and Visual Science 2000, 41: 2837-2843.