Research Article - Allied Journal of Medical Research (2019) Volume 3, Issue 1
Expression of the CGRP family of peptides and their receptors in the rat retina
Karin Warfvingea1,2* and Lars Edvinsson1,2
1Department of Clinical Sciences, Division of Experimental Vascular Research, Lund University, Lund, Sweden
2Department of Clinical Experimental Research, Glostrup Research Institute, Glostrup Hospital, Glostrup, Denmark
- *Corresponding Author:
- Karin Warfvinge
Professor, Department of Clinical Experimental Research
Glostrup Research Institute Glostrup Hospital, Rigshospitalet
Denmark
E-mail: Karin.Warfvinge@med.lu.se
Received date: June 15, 2019
Abstract
The calcitonin gene-related peptide (CGRP) family of peptides includes CGRP itself, calcitonin, adrenomedullin and amylin. They are all related to each other vis-à-vis peptide sequence and receptor biology. Understanding expression of the peptides and their receptors lays the foundation for more deeply understanding their physiology and pathophysiology in eye diseases, and their plausible therapeutic possibility. We have designed an in-depth study of the expression of the CGRP family of peptides and their receptors in rat retina using single and double immunohistochemistry with antibodies against: CGRP, calcitonin, adrenomedullin, amylin, CLR, RAMP1, RAMP2, RAMP3 and CTR. We observed that CGRP was mainly seen in Müller cell end feet, adrenomedullin and calcitonin were expressed in the blood vessels, while amylin was widely distributed within the retina. CGRP receptors consist of CLR/RAMP1. These two receptor components are co-localized in the nerve fiber layer, RAMP2/3 are found in the nuclear layers, and CTR in the plexiform layers. The only co-localization ligand/ligand, receptor/receptor or ligand/receptor was CLR/RAMP1, and thereby suggesting a functional receptor. CTR is the only component that is needed for the calcitonin receptor. We conclude that in the normal rat retina, functional CGRP receptors and also calcitonin receptors (consisting of only CTR) exist, but not adrenomedullin or amylin receptors.Keywords
CGRP, Calcitonin, Adrenomedullin, Amylin, Receptors, Immunohistochemistry
Abbreviations
7TM: 7 Trans Membrane; AM: Adrenomedullin; AMY: Amylin; CGRP: Calcitonin Gene-Related Peptide; CLR: Calcitonin Receptor-Like Receptor; CT: Calcitonin; CTR: Calcitonin Receptor; DAPI: 4 ’ , 6-Diamino-2-Phenylindole; GPCR: G Protein-Coupled Receptor; PBS-T: Phosphate Buffer Saline-TritonX; RAMP: Receptor Activity-Modifying Protein.
Introduction
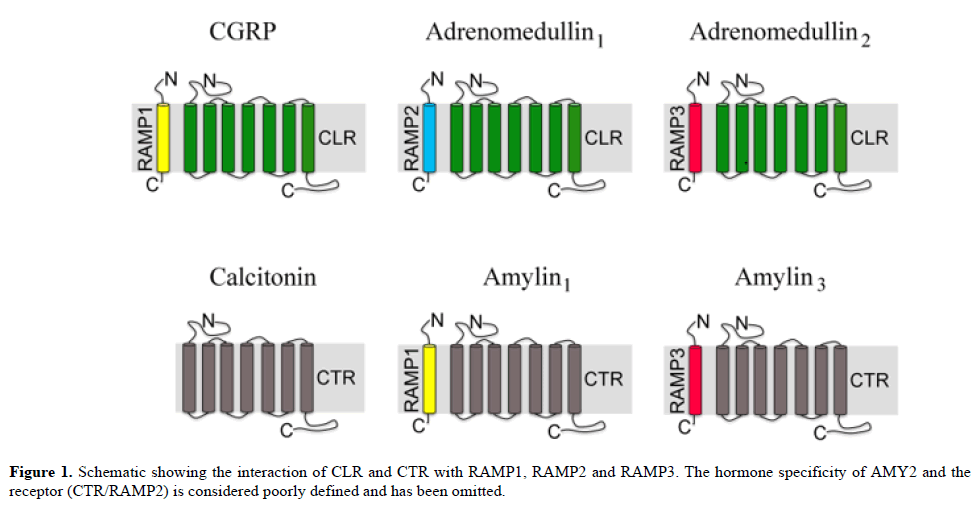
The calcitonin gene-related peptide (CGRP) family of peptides includes CGRP itself, calcitonin (CT), adrenomedullin (AM) and amylin (AMY). They are all related to each other vis-à-vis peptide sequence and receptor biology. All members of this family are clinically relevant drug targets due to their roles in the regulation of several critical homeostatic processes [1]. The peptides included in the CGRP family are ligands for family B type of G protein-coupled receptors (GPCRs). The peptides activate GPCRs, which can heterodimerize with accessory proteins called receptor activity-modifying proteins (RAMPs). RAMPs, a small family of three proteins, are single transmembrane-spanning proteins that alter the pharmacology, functionality and cell trafficking of specific GPCRs. Central to the CGRP or adrenomedullin receptor complex is a 7 transmembrane (TM) GPCR, the calcitonin receptor-like receptor (CLR). CLR is a required element in receptors for CGRP, AM1 and AM2. The AMY receptors comprise a core 7 TM family B GPCR, the CT receptor (CTR), which for completeness associates with any of the RAMPs (Figure 1). Due to the complexity of this peptide-receptor system, it is not yet clear which effects are physiological versus pharmacological or which receptors are responsible for the many effects of this group of peptides.
CGRP is located in numerous sites throughout the central and peripheral nervous systems. Studies of the trigeminovascular system have provided much of what is known about the role of CGRP in the cranial sensory nerves, a key component of the pain pathway for headache [2,3]. The first study of CGRP distribution in the retina was performed in chickens in 1985 [4]. We have recently revisited this field and reported on the distribution of CGRP, CLR and RAMP1 in the rat retina [5]. Collectively it was concluded that almost all RAMP1 immunoreactive cells co-expressed CLR, and therefore we proposed that RAMP1 expression in the retina demonstrates functional CGRP receptor.
CT is a hormone produced by C cells of the thyroid, whose role is to reduce plasma calcium and promote bone formation [6]. CT is used clinically in the treatment of bone disorders characterized by increased bone resorption, osteoporosis and hypercalcemia due to malignancy [6]. However, there is still yet much to learn about the actions and role of CT outside the thyroid; recent data suggest that CT is abundantly expressed in many tissues in the body (Warfvinge, unpublished). In addition, CTR does not require a RAMP to function.
AM is mainly found in endothelial cells, it is important both in vascular homeostasis as well as in angiogenesis and lymphangiogenesis. Evidence for a functional AM signaling pathway has been described in the mouse retina. These results were the first to show the expression of AM in retina and some aspects on functional AM receptors. Since AM is increased in eyes with various ocular pathologies, the AM signaling pathway may provide a new target for ameliorating of such retinal pathologies [7].
AMY is produced by the pancreas and functions as a hormone, regulating nutrient intake, but may also have other roles. Research of neuronal deposition of AMY has mainly focused on a role for AMY in Alzheimer's disease. Recently, it was shown that AMY alters human brain pericyte viability. Specific AMY receptors or the possible physiological role of AMY in the retina have not hitherto been reported [8-10].
Understanding expression of the peptides and their receptors lays the foundation for more deeply understanding their physiology and pathophysiology in eye diseases, and their plausible therapeutic possibility [1]. In order to further grasp these aspects, we have now designed an in-depth study of the expression of the CGRP family of peptides and their receptors in rat retina.
Materials and Methods
The study was performed under the rules and regulations of the Regional Ethical Review Board in Lund, Sweden (M1715).
Male Sprague-Dawley rats (n=10, 250-300 g) were incapacitated using dry ice (CO2). Shortly after, the rats were decapitated, and the eyes carefully dissected out. During the dissection each eye was marked in the inner angle using a diathermy burner (FIAB SpA, Italy). This was made in order to be able to orientate each eye in a uniform fashion during embedding and subsequently allowing for vertical cryostat sectioning through the optic nerve. Then, the eyes were briefly put in 4% formaldehyde in phosphate buffer saline (PBS) to stabilize, allowing for the removal of the cornea and lens. The eyes were thereafter submerged in 4% formaldehyde in PBS buffer for 2-4 hours. Subsequently, each eye was washed in rising concentration of 10% and 25% of sucrose in Sorensen’s phosphate buffer (pH 7.2) to ensure cryo-protection. Finally, the eyes were embedded in a gelatin medium (30% egg albumin, 3% gelatin) and stored at -20°C (Dreisig, Blixt, and Warfvinge, 2018).
Ten μm cryo-sections (vertical sectioning through the optic nerve) were washed in PBS with 0.25% Triton (PBS-T) for 15 min. Next, primary antibodies were applied (Table 1). The sections were incubated in incubation chambers at +8°C overnight and, during the following day, the glass slides were submerged in PBS-T 2 × 15 minutes. The remaining experiment was completed in a dark room, in order to preserve the fluorescence of the secondary antibodies. Appropriate secondary antibodies were diluted according to manufacturer’s instructions and incubated for one hour at room temperature (Table 1). Next, the sections were washed in PBS-T for 2 × 15 minutes and mounted with Vectashield mounting medium containing 4 ’ , 6-diamino-2-phenylindole (DAPI, Vector Laboratories, Burlingame CA, USA). Each procedure was repeated a minimum of three times to validate the results and minimize any experimental errors (Dreisig, Blixt, and Warfvinge, 2018).
| Primary antibodies | |||
|---|---|---|---|
| Antigen (species) | Dilution | Detects | Supplier |
| CGRP PA1-36017 (guinea pig) | 1:500 | Human and rat CGRP | Thermo Scientific, IL, USA |
| Calcitonin NBP1-30051 (mouse) | 1:50 | Calcitonin | Novus Biologicals, CO, USA |
| Adrenomedullin sc-80462 (mouse) | 1:100 | Adrenomeddulin of human origin | Santa Cruz Biotechnology, CA, USA |
| Amylin PA5-32261 (rabbit) | 1:100 | Amylin | Thermo Scientific, IL, USA |
| CLR 3155 (rabbit) | 1:500 | C-terminal of rat CLR | Merck & Co, Inc., USA |
| RAMP1 844 (goat) | 1:100 | C-terminal of human RAMP1 | Merck & Co, Inc., USA |
| RAMP2 GTX108524 (rabbit) | 1:100 | RAMP2 protein | GeneTex, USA |
| RAMP3 sc-365313 (mouse) | 1:100 | RAMP3 of mouse, rat and human | Santa Cruz Biotechnology, CA, USA |
| CTR, CAU24308 (rabbit) | 1:100 | Calcitonin receptor of rat | BioMatik, Delaware, USA |
| Secondary antibodies | |||
| Name | Dilution | Against | Supplier |
| Alexa 488, A1 1073 | 1:100 | Anti-guinea pig | Invitrogen, La Jolla, CA |
| Cy3, 715-165-150 | 1:400 | Anti-mouse | Jackson Immunoresearch, PA, USA |
| FITC, 715-095-151 | 1:100 | Anti-mouse | Jackson Immunoresearch, PA, USA |
| Cy3, 711-165-152 | 1:400 | Anti-rabbit | Jackson Immunoresearch, PA, USA |
| Cy2, 711-225-152 | 1:100 | Anti-rabbit | Jackson Immunoresearch, PA, USA |
| Cy3, 705-165-003 | 1:400 | Anti-goat | Jackson Immunoresearch, PA, USA |
Table 1. Details on primary and secondary antibodies.
In order to determine which combination of antibodies resulted in the most clear and unambiguous outcome, a series of different peptide and receptor primary antibodies were matched with various secondary antibodies, and their respective combinations were investigated. The present procedure will only include the antibodies used to produce our results. Antibodies are summarized and distinguished in Table 1.
For double immunohistochemistry the procedure was repeated two times. The first primary antibody was matched with its appropriate secondary antibody before the second round of primary and secondary antibodies was applied and finally mounted. Negative controls were performed for each set by omitting the primary antibody. Any resulting immunofluorescence would suggest unspecific binding of the secondary antibodies.
In addition, cryosections were Hematoxylin-Eosin stained (Figure 2) using following protocol: Htx (4 min), tap water, Eosin (1 min), distilled water, alcohol (70%, 95%, 99.5%), xylene and mounted with xylene based Pertex (HistoLab, Gothenburg, Sweden).
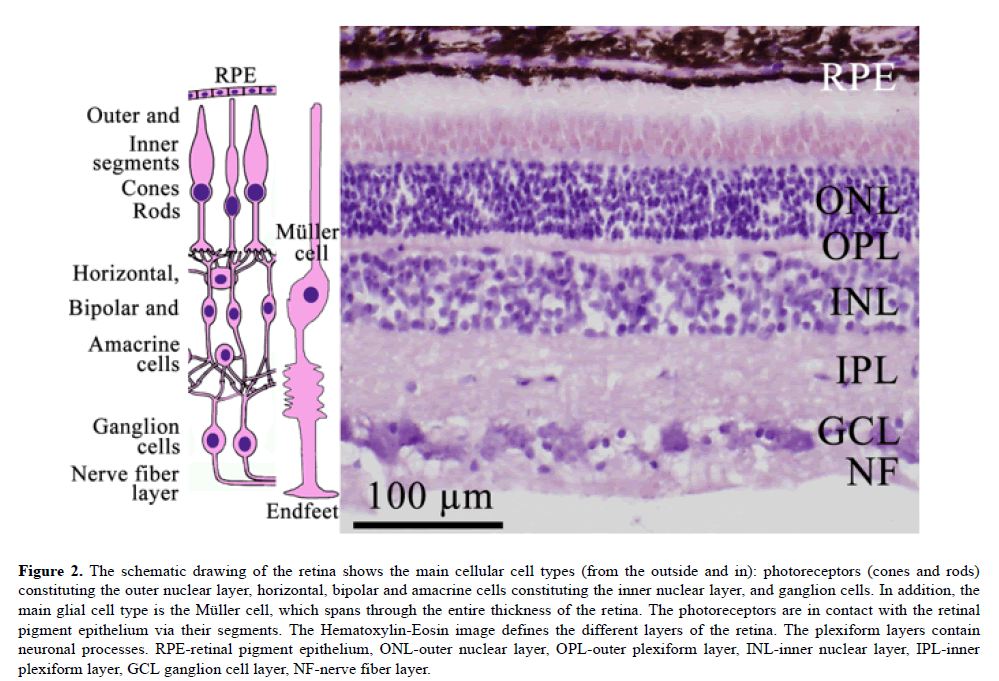
Figure 2: The schematic drawing of the retina shows the main cellular cell types (from the outside and in): photoreceptors (cones and rods) constituting the outer nuclear layer, horizontal, bipolar and amacrine cells constituting the inner nuclear layer, and ganglion cells. In addition, the main glial cell type is the Müller cell, which spans through the entire thickness of the retina. The photoreceptors are in contact with the retinal pigment epithelium via their segments. The Hematoxylin-Eosin image defines the different layers of the retina. The plexiform layers contain neuronal processes. RPE-retinal pigment epithelium, ONL-outer nuclear layer, OPL-outer plexiform layer, INL-inner nuclear layer, IPL-inner plexiform layer, GCL ganglion cell layer, NF-nerve fiber layer.
The stainings were examined using a light/epifluorescence microscope (Nikon 80i, Tokyo, Japan) combined with a Nikon DS-2MV camera. Images were analyzed and examined in Adobe Photoshop CS3 (v10.0 Adobe Systems, Mountain View, CA) and images taken with different filters were superimposed in order to determine potential co-localization.
Results
The CGRP family (CGRP, AM, CT, AMY) are ligands for this family of B type of GPCRs. The peptides activate GPCRs, which can heterodimerize with accessory proteins i.e. RAMP1, RAMP2 and RAMP3 in different combinations: CGRP receptor CLR/RAMP1, AM1 receptor CLR/RAMP2, AM2 receptor CLR/RAMP3, CT receptor CTR, AMY1 receptor CTR/RAMP1, and AMY3 receptor CTR/RAMP3 (Figure 1). As pointed out in functional studies the ligands may activate the different receptor subtypes, however the specificity of activation is recorded as maximum effect and in potency in the responses [11].
Hematoxylin-Eosin staining (Figure 2).
The neuro-retina consists of different layers with 5 classes of retinal neurons: the outer nuclear layer (photoreceptors), outer plexiform layer, inner nuclear (horizontal, bipolar and amacrine cells), inner plexiform layer, ganglion cell layer and the nerve fiber layer. There are two circulations to the retina; both are supplied by the ophthalmic artery. Thus, the outer part of the retina with the photoreceptors, receives its blood supply via the chorio-capillaries outside of the retinal pigment epithelium. The inner retinal layers from the outer plexiform layer to the ganglion cell layer, are nourished by the central retinal artery.
Ligands expression (Figure 3).
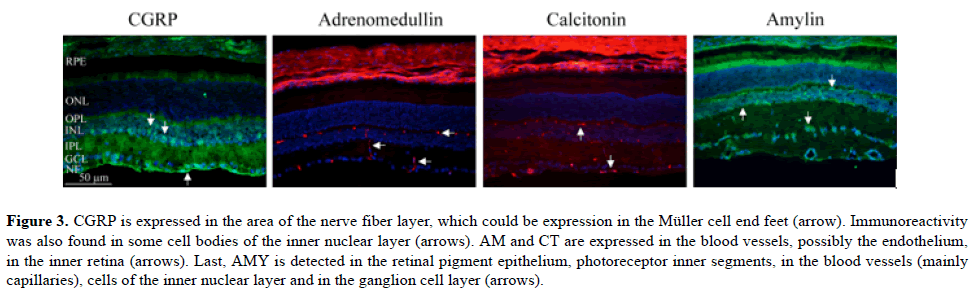
Figure 3: CGRP is expressed in the area of the nerve fiber layer, which could be expression in the Müller cell end feet (arrow). Immunoreactivity was also found in some cell bodies of the inner nuclear layer (arrows). AM and CT are expressed in the blood vessels, possibly the endothelium, in the inner retina (arrows). Last, AMY is detected in the retinal pigment epithelium, photoreceptor inner segments, in the blood vessels (mainly capillaries), cells of the inner nuclear layer and in the ganglion cell layer (arrows).
We have recently reported that CGRP is mainly seen in Müller cell end feet spanning through the entire retina [5]. In the present paper, we show that CGRP is in addition expressed in the area of the nerve fiber layer, which could be an extension of the Müller cell end feet. Immunoreactivity was also found in some cell bodies of the inner nuclear layer. AM and CT were expressed in the blood vessels, mainly the endothelium of capillaries, in the inner retina. Last, AMY was detected in the retinal pigment epithelium, photoreceptor inner segments, in the blood vessels (mainly capillaries), cells of the inner nuclear layer and in the ganglion cell layer.
Taken together the study suggest that the expression of AM, CT and AMY in the blood vessels seems to be present in the endothelium.
Receptor elements expression (Figure 4).
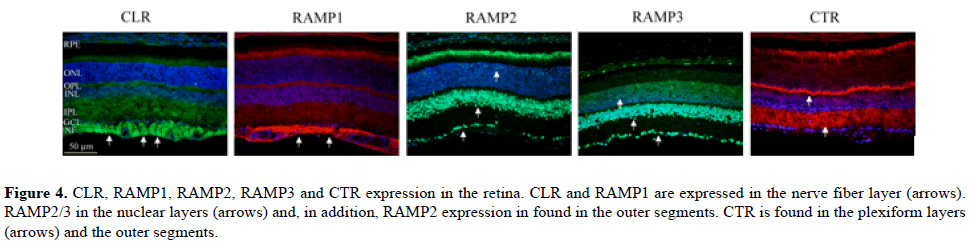
Figure 4: CLR, RAMP1, RAMP2, RAMP3 and CTR expression in the retina. CLR and RAMP1 are expressed in the nerve fiber layer (arrows). RAMP2/3 in the nuclear layers (arrows) and, in addition, RAMP2 expression in found in the outer segments. CTR is found in the plexiform layers (arrows) and the outer segments.
CLR and RAMP1, forming the classical CGRP receptor; were expressed in the nerve fiber layer. For the AM receptors, in addition to CLR expression in the nerve fiber layer, the RAMP2/3 immunoreactivities were seen in the retinal pigment epithelium and in nuclear layers. In addition, RAMP2 expression was seen in the photoreceptor outer segments.
CTR was seen in the photoreceptor outer segments and the plexiform layers. For the AMY receptors, CTR and RAMP1 or RAMP3 expression is required to form a functional receptor. Thus, CTR expression in the photoreceptor outer segments and the plexiform layers, and RAMP1 in the nerve fiber layer or RAMP3 in the retinal pigment epithelium and in nuclear layers were found.
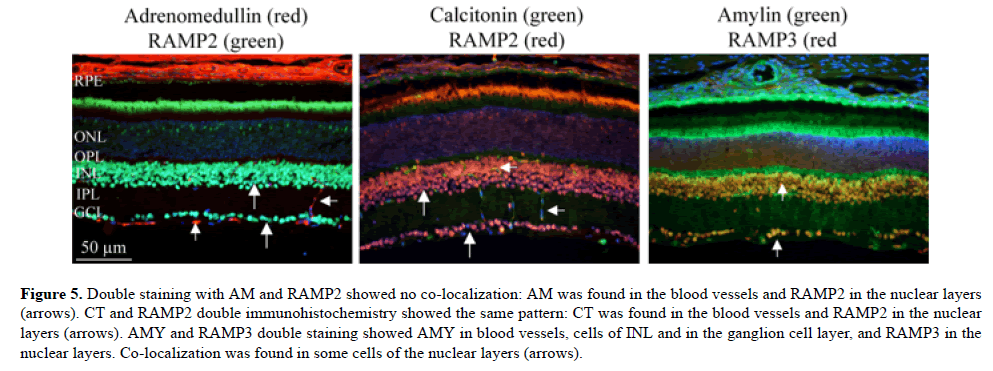
Ligand/receptor double immunohistochemistry (Figure 5)
Figure 5: Double staining with AM and RAMP2 showed no co-localization: AM was found in the blood vessels and RAMP2 in the nuclear layers (arrows). CT and RAMP2 double immunohistochemistry showed the same pattern: CT was found in the blood vessels and RAMP2 in the nuclear layers (arrows). AMY and RAMP3 double staining showed AMY in blood vessels, cells of INL and in the ganglion cell layer, and RAMP3 in the nuclear layers. Co-localization was found in some cells of the nuclear layers (arrows).
In order to provide morphological clues for functionality of the CGRP family of peptides in the retina, we performed a series of double immunohistochemistry.
CGRP did not co-localize with or come close to its receptor components (CLR, RAMP1) in the retina. CGRP was found mainly in Müller cell end feet spanning on the inside of the retina, while CLR and RAMP1 were co-expressed in the nerve fiber layer. We have earlier shown that CGRP co-expressed with vimentin, which suggested that CGRP expression was found in Müller glial cells. CLR and RAMP1 were coexpressed in the nerve fiber layer [5]. We confirm these results in the present study.
Double staining with AM and RAMP2 showed no colocalization: AM was found in the blood vessels and RAMP2 in the nuclear layers. Double staining with AM and RAMP3 could not be performed, since both antibodies were made in mouse. However, both RAMP2 and RAMP3 were expressed in the nuclear layers, and consequently the double staining with AM and RAMP3 could show the same pattern as for RAMP2, i.e. no co-localization.
CT and RAMP2 double immunohistochemistry showed the same pattern: CT was found in the blood vessels and RAMP2 in the nuclear layers. Since CT and RAMP3 both are made in mouse, the same reasoning as for AM and RAMP3 would suggest the same results as for CT and RAMP2, suggesting no co-localization.
AMY and RAMP3 double staining showed AMY in blood vessels, cells of INL and in the ganglion cell layer, and RAMP3 in the nuclear layers. Co-localization was found in some cells of the nuclear layers. AMY and RAMP2 double immunohistochemistry could not be performed because AMY and RAMP2 are both made in rabbit.
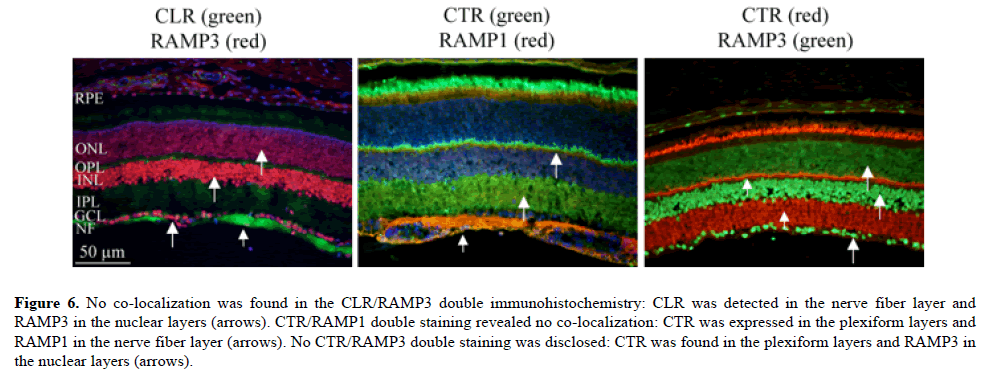
Receptor/receptor double immunohistochemistry (Figure 6).
Figure 6: No co-localization was found in the CLR/RAMP3 double immunohistochemistry: CLR was detected in the nerve fiber layer and RAMP3 in the nuclear layers (arrows). CTR/RAMP1 double staining revealed no co-localization: CTR was expressed in the plexiform layers and RAMP1 in the nerve fiber layer (arrows). No CTR/RAMP3 double staining was disclosed: CTR was found in the plexiform layers and RAMP3 in the nuclear layers (arrows).
Co-localization between CLR and RAMP1 (CGRP receptor) in the nerve fiber layer has previously been described [5]. No colocalization was found in the CLR/RAMP3 (AM2 receptor) double immunohistochemistry; CLR was detected in the nerve fiber layer and RAMP3 in the nuclear layers. This means that no anatomical support for functional receptor of AM2 could be detected. However, it is not known whether CLR and RAMP2 (both made in rabbit) immunohistochemistry would show the same pattern, and consequently show lack of functional AM1 receptor. CTR/RAMP1 (AMY1 receptor) double staining revealed no co-localization; CTR alone was expressed in the plexiform layers and RAMP1 in the nerve fiber layer. No CTR/ RAMP3 (AMY3 receptor) double staining was disclosed; CTR was found in the plexiform layers and RAMP3 in the nuclear layers. It should be pointed out that the CT receptor consists of CTR only, which was expressed in the photoreceptor outer segments and the plexiform layers.
Discussion
This is the first in detail study of the expression of the CGRP family of peptides and their receptor elements in the rat retina. The similarities between the molecular compositions of the CGRP family peptides cause an overlap in their ability to activate the different receptor subtypes. AMY and CGRP are the most closely related peptides in terms of amino acid sequence. Also, the receptors themselves are related and share components (Figure 1). Therefore, the work is more challenging because of their cross-reactivity [1,11]. This might be one of the reasons for the lack of some expected findings in the retina. However, examinations, like the present study, describing qualitatively the distribution of the markers might define potential targets in different pathological conditions. The purpose of this study was to unravel the differential expression of the CGRP family of peptides and the relation to the expression of their various receptors.
Migraine is associated with visual phenomena such as aura with photophobia. In addition, it seems that CGRP may have a role in photophobia [12]. However, the function of CGRP within the retina has not been well studied. Nevertheless, some experiments designed to find out details on relation to the CGRP family of peptides and receptors have appeared [5]. This has resulted in experiments designed to unravel their possible relation to the visual phenomena [13]. The authors concluded that ocular structures are richly innervated by trigeminal sensory neurons containing CGRP and, given that CGRP plays a fundamental role in migraine pathophysiology; its contribution to photophobia is beyond doubt. Moreover, we have described that CGRP immunoreactivity is located in the Müller cells and CLR/RAMP1 in the nerve fiber layer [5], which we confirm in the present paper. Interestingly, the level of RAMP1 affects the severity of photophobia, which often accompanies migraine attacks [14]. Recent reports have demonstrated protective properties of CGRP on retinal cells after various ischemic conditions [15,16]. In addition, intracerebroventricular administration of CGRP caused a significant increase in light aversion, a response that is prevented with simultaneous treatment with the human CGRP receptor antagonist olcegepant [17]. CGRP injection into control mice caused the development of an aversion to strong light, and this response was attenuated by a triptan, indicating that activation of endogenous CGRP receptors can drive this hypersensitive response [18].Together these studies demonstrate that CGRP, which is released in migraine, might contribute to photophobia in migraineurs [12].
The most abundant AM source in the human body is the endothelial cells of the vascular walls [19]. In the eye, AM was first described in the retinal pigment epithelium [20]. In addition, evidence for a functional AM signaling pathway has been described in the mouse retina [7]. AM participates in the pathophysiology of diabetic retinopathy and age-related macular degeneration, both of which are leading causes of blindness [20]. In the present study, we show that AM is mainly expressed in the blood vessels in the inner retina of healthy rats, which is in agreement with the early findings of Sugo et al. [19] but not that of Blom et al. [7] who demonstrates AM throughout the mouse retina. Our findings might give a hint how to explore the role of AM in the pathology of various diseases. The significance of the expression of the receptor components is unclear: AM is expressed in the vascular walls, CLR in the nerve fiber layer and RAMP2/3 in the nuclear layers. Collectively this does not support the presence of AM receptors. However, findings on thymic epithelial cells using both immunofluorescence and immunogold stainings demonstrated RAMP2 localization in the nucleus, CLR both intracellularly and in the plasma membrane and AM in the cytoplasm, results which is in agreement with our results. The authors suggest that AM activates its receptor in the nucleus to modulate transcription [21].
CT does not appear to be expressed in the central nervous system, although binding sites for CT are found in many brain structures. In the present study we show that CT is expressed in the blood vessels in the inner retina. Moreover, the CT receptor CTR is widely expressed within the nervous system [1]. In the present study we show that CTR is expressed in the plexiform layers, which constitutes the neuronal processes. In conclusion, we confirm the results from brain studies; little CT but wide CTR expression. It is also for this peptide and its receptor a challenge for future studies to understand why this differential expression of ligand and receptor exist.
AMY is an endocrine hormone that signals to the brain and is expressed mainly in the Langerhans islets of the pancreas [8]. In the circulation there are very high levels. Immunohistochemical examinations have shown AMY expression in for example the trigeminal ganglion and dorsal root ganglion [22]. AMY and CGRP are the most closely related peptides in terms of amino acid sequence, which makes it challenging to define the results when considering potential overlap. Moreover, CGRP and AMY have both affinity for the CLR/RAMP1 receptor but CGRP is more potent. By comparing the distribution of the peptides and their receptors could explain potential overlap.
Conclusion
In the present study, we did not detect overlaying immunoreactivity between the peptides and their receptor components, or surprisingly not between the receptor components except for CLR and RAMP1. This indicates that in the normal rat retina, functional CGRP receptors exist, but not morphological evidence for AM or AMY receptors. However, recent observations on AM and its receptor distribution strengthens our results and indicates that the absence of colocalization does not necessarily mean absence of functional receptors [21]. It is possible that the expressional profile might differ in disease processes and hence future studies using disease models might unravel unexpected expressional profiles and indicate avenues for novel therapy in eye diseases.
As Hendrikse et al. [1] pointed out; qualitative descriptions are highly subjective processes. However, careful and thorough immunohistochemical analysis will clearly contribute to further characterization of the distribution of CGRP family peptides and their receptors in the retina.
Acknowledgement
The Lundbeck Foundation, Denmark, is acknowledged.
References
- Hendrikse ER, Bower RL, Hay DL, et al. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia. 2019;39:403-413.
- Warfvinge K, Edvinsson L. Distribution of CGRP and CGRP receptor components in the rat brain. Cephalalgia. 2019;39:343-353.
- Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies: successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350.
- Kiyama H, Katayama Y, Hillyard CJ, et al. Occurrence of calcitonin gene-related peptide in the chicken amacrine cells. Brain Res. 1985;18:367-369.
- Blixt FW, Radziwon-Balicka A, Edvinsson L, et al. Distribution of CGRP and its receptor components CLR and RAMP1 in the rat retina. Exp Eye Res. 2017;161:124-131.
- Findlay DM, Sexton PM. Calcitonin. Growth Factors. 2004;22:217-224.
- Blom J, Giove TJ, Pong WW, et al. Evidence for a functional adrenomedullin signaling pathway in the mouse retina. Mol Vis. 2012;18:1339-1353.
- Hay DL. Amylin. Headache. 2017;57:89-96.
- Mietlicki-Baase EG. Amylin in Alzheimer's disease: Pathological peptide or potential treatment? Neuropharmacology. 2018;136:287-297.
- Schultz N, Byman E, Fex M, et al. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab. 2017;37:1470-1482.
- Hay DL, Garelja ML, Poyner DR, et al. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol. 2018;175:3-17.
- Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97:553-622.
- Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia. 2018;1.
- Recober A, Kuburas A, Zhang Z, et al. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798-8804.
- Yang JH, Zhang YQ, Guo Z. Endogenous CGRP protects retinal cells against stress induced apoptosis in rats. Neurosci Lett. 2011;501:83-85.
- Sakamoto K, Kuroki T, Okuno Y, et al. Activation of the TRPV1 channel attenuates N-methyl-D-aspartic acid-induced neuronal injury in the rat retina. Eur J Pharmacol. 2014;733:13-22.
- Russo AF, Kuburas A, Kaiser EA, et al. A Potential Preclinical Migraine Model: CGRP-Sensitized Mice. Mol Cell Pharmacol. 2009;1:264-270.
- Kaiser EA, Kuburas A, Recober A, et al. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012;32:15439-15449.
- Sugo S, Minamino N, Kangawa K, et al. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160-1166.
- Iesato Y, Yuda K, Chong KT, et al. Adrenomedullin: A potential therapeutic target for retinochoroidal disease. Prog Retin Eye Res. 2016;52:112-129.
- Castellani G, Paliuri G, Orso G, et al. An intracellular adrenomedullin system reduces IL-6 release via a NF-kB-mediated, cAMP-independent transcriptional mechanism in rat thymic epithelial cells. Cytokine. 2016;88:136-143.
- Edvinsson L, Goadsby PJ, Uddman R. Amylin: localization, effects on cerebral arteries and on local cerebral blood flow in the cat. ScientificWorldJournal. 2001;1:168-180.