Research Article - Biomedical Research (2017) Volume 28, Issue 5
Expression of NF-kappaB and p38 under intervention of rutin in lung cancer therapy
Xiao-hua Li1, Zhi-yi Liu2, Yue Gu3, Zhe Lv2, Yan Chen2 and Hai-cheng Gao2*1Department of Gynaecology and Obstetrics, Second Hospital of Jilin University, Changchun, PR China
2Department of Pharmacology, School of Pharmaceutical Sciences, Jilin University, Changchun, PR China
3Department of Surgery, Jilin University, First Hospital, Changchun, China
- *Corresponding Author:
- Hai-cheng Gao
Department of Pharmacology
School of Pharmaceutical Sciences
Jilin University, PR China
Accepted date: October 31, 2016
Abstract
Purpose: Rutin has been widely used in clinic for treatment of cardiovascular diseases in China. But rutin was rarely used in prevention and treatment of cancer. This study investigated the expression of NF-κB and p38 under intervention of rutin in lung cancer.
Methods: In this experiment, cells were divided into control group, cisplatin group, and rutin groups (low, middle and high). The MTT, flow cytometry, Western blot and immunofluorescence staining methods were used to examine cell cycle and the expression of NF-κB and P38, respectively.
Results: Rutin could regulate the cell cycle. In addition, rutin reduced the expression of NF-κB and P38.
Conclusion: These novel findings indicate that rutin inhibits NF-κB and P38 activity and regulates the development of lung cancer.
Keywords
NF-κB, p38, Lung cancer, Rutin
Introduction
Lung cancer is the second leading cause of death in the worldwide, it is a serious threat to human health [1,2]. With the increase of environmental pollution and smoking, the incidence and mortality of lung cancer also showed a trend of getting younger [3]. Therefore, prevention of lung cancer has become an urgent problem.
The activation of p38 MAPK can directly influence gene transcription, as a growing number of transcription factors are known to be direct targets of p38 [4]. Important target of the p38 MAPK include STAT1, NF-κB and c/EBPs [5]. According to reports show that the activation of NF-κB can induce the apoptosis of endothelial cells, which mainly involved C-myc, TNF-α and IL-1 gene [6]. Recent studies have found that NF- κB pathway play an important role in many-diseases [7].
Rutin as an old drug has been used for many years. Rutin is a phenolic compound and flavonoid glycoside that is found in flowers and fruits as a major source. Rutin can be broadly extracted from nature sources, including buckwheat, oranges, grapes, lemons, limes, peaches and berries. It has been widely recognized for many patients, including diabetic cardiomyopathy. The compound has been reported to possess antitumor functions [8]. Rutin may inhibit NF-κB expression and regulate the development of lung cancer. In this study, the effect of rutin on inhibiting NF-κB and p38 expression was investigated by analysing the development of lung cancer.
Materials and Methods
Materials
NF-κB antibody was purchased from Boster Biological Technology Co. Ltd (Wuhan, China). Other reagents were obtained from Sigma (America). The MCO- 5 AC CO2 thermostat incubator was obtained from Sanyo (Japan).
Cell culture
Lung carcinoma cells (cell lines) were provided by Jilin University School of Pharmaceutical Sciences. Lung carcinoma cells were prepared for NF-κB and p38 expression after treatment with 10-8, 2 × 10-8, 4 × 10-8 mol/L rutin for 48 h.
Cell cycle analysis
Human lung cancer cells were cultured in 50 cm2 plates (1 × 105 cells//well). Cells were released by digestion with trypsin and harvested. After centrifugation, the cells were washed twice with precooled PBS and fixed with precooled 70% ethanol overnight at 4°C. Then the cells were re-suspended in PBS containing 10% foetal bovine serum, filtered through a 400 mesh sieve, and stained with propidium iodide solution (PI, 50 μg/ml, 100 μg/ml RNase in PBS) at 37°C in the dark for 30 minutes. Finally, the cells were analysed for the cell cycle phase by FACScan (Beckman Coulter, Pasa-dena, CA, USA).
Western blot
Cells were homogenized in RIPA buffer (Sigma). Total protein concentration of the homogenates was measured with the BCA reagent. Equal amounts of protein were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes by electroblotting. After blocking, the membranes were incubated with antibodies directed against human NF-κB, and β-actin.
Immunofluorescence staining
The cells were seeded in cell crawling on the sheet until the addition was complete conditioned with 4% paraformaldehyde for 15 min. Cells were washed in PBS 3 times. The cell dropped 1: 200 antibody concentrations at 4°C overnight, washed in PBS three times for 5 min, added into 1: 500 concentration of fluorescence-labelled secondary antibody for 2 h. The cells applied DAPI, p38 and NF-κB staining solution for 5 min. the slices were mounted with 10% glycerol and observed with a microscope.
Statistical analysis
All the data obtained from at least three independent experiments were shown as the mean ± SEM. Statistical comparison was made with Students P-Values of less than 0.05 were considered to be of statistical significance. Statistical analysis of the data was performed via SPSS for Windows Version 11 (SPSS, Chicago, II, USA). *p <0.05 cisplatin and rutin vs. control.
Results
Cell cycle
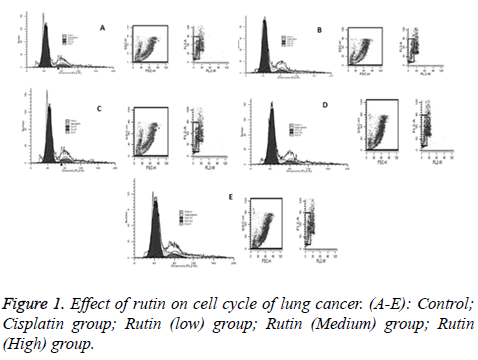
In order to further evaluate the effects of rutin, flow cytometry assay was performed with 10-8, 2 × 10-8, 4 × 10-8 mol/L acting upon cells for 48 h. In cells treated with rutin and cisplatin, a subpopulation of cells in sub-G1 phase of the cycle was increased. The cells in G2 phase of the cycle were reduced. Excepted cisplatin group, the cells in G1 phase of the cycle was also reduced (Figure 1 and Table 1). Therefore, rutin should be considered as an effective therapeutic drug for controlling the growth of cells.
| Group | G1% | S% | G2% | CV% |
| control | 73.18 | 12.52 | 14.30 | 14.24 |
| cisplatin | 76.23 | 13.31 | 10.46 | 12.76 |
| Rutin(Low) | 70.84 | 21.34 | 7.82 | 12.54 |
| Rutin(Medium) | 72.93 | 19.97 | 9.10 | 12.76 |
| Rutin(High) | 71.04 | 20.26 | 8.7 | 14.79 |
Table 1: Effect of rutin on cell cycle of lung cancer.
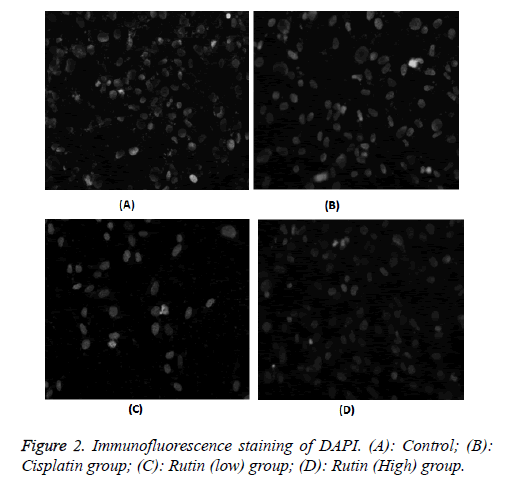
Immunofluorescence staining of DAPI
As shown in Figure 2, the experimental results showed that these were uniform chromatin, large nucleus, integrity of the nuclear envelope. Cell morphology has been significantly improved by adding rutin; the results indicated that rutin had a role of regulation on tumor cell morphology.
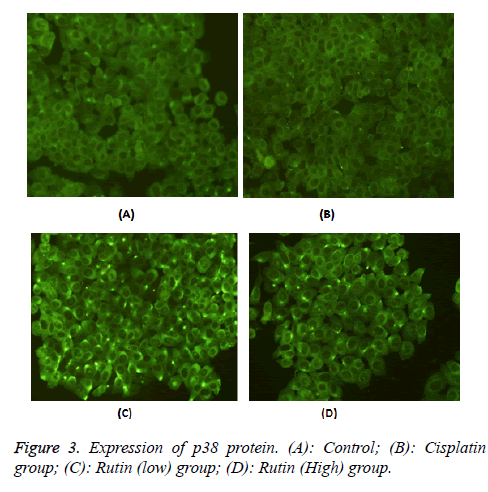
Expression of p38 protein
To validate the expression pattern of p38 of lung cancer, we performed p38 specific fluorescent staining in Lung carcinoma cells. The experimental results of Figure 3 showed that the expression of p38 was 5.16 ± 0.16. The expression of p38 protein reduced obviously in cisplatin group (2.84 ± 0.22*, p<0.05). In addition, the expression of p38 protein also reduced obviously in rutin group (2.94.5 ± 0.15*, 3.36.1 ± 0.23* and 3.43 ± 0.16*, p<0.05).
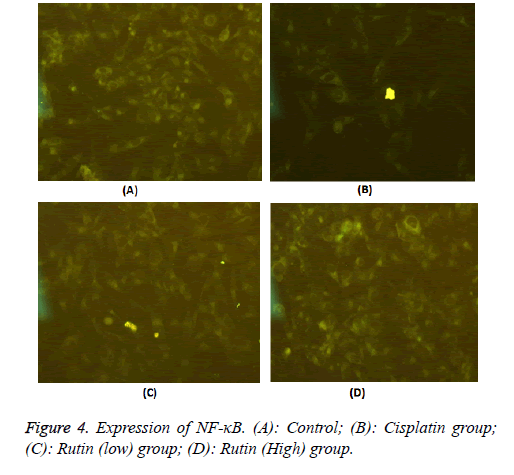
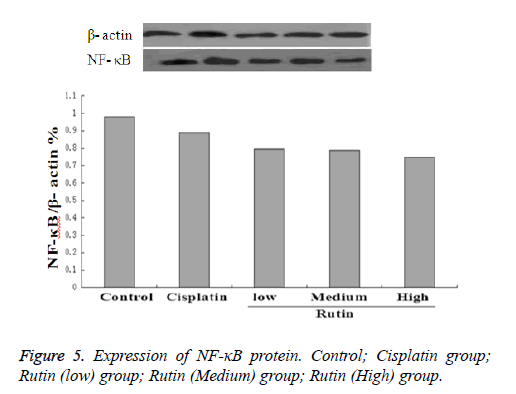
Expression of NF-κB protein
To validate the expression pattern of NF-κB of lung cancer, we performed NF-κB specific fluorescent staining in Lung carcinoma cells. As shown in Figure 4, the experimental results showed that these were uniform chromatin; large nucleus in normal group, the expression of NF-κB was 17.5 ± 1.21. The expression of NF-κB protein reduced obviously in cisplatin group (7.53 ± 0.90*, p<0.05). In addition, the expression of NF-κB protein also reduced obviously in rutin group (9.38.5 ± 0.56*, 10.2 ± 0.32* and 11.3 ± 1.16*, p<0.05).
Western blot
Western blotting was used to investigate the expression of NF- κB. The results were analysed semi-quantitatively according to the grayscale value. As shown in Figure 5, the expression of NF-κB in cisplatin groups increased significantly as compared with control groups. In addition, the expression of NF-κB in the rutin groups (Low, Medium and High) also increased obviously compared with control groups.
Discussions
Lung cancer is one of the morbidity and mortality of the fastest growing [9]. Its incidence continues to increase at a rate of approximately 0.5% per year, and the number of cancer deaths caused by this disease expected to rise to 50% by 2020 [10]. In spite recent progress in diagnosis and multimodality therapies for lung, the prognosis still remain unsatisfactory with 5 year survival rates of less than 15% [11]. They highlight the fact that new strategies based on molecular mechanisms are highly required for lung cancer treatment. Cell Proliferation is a journal devoted to studies into all aspects of cell proliferation and differentiation in normal and abnormal states. In this expression, the results showed Rutin promoted the proliferation of lung cancer cells at 48 h, these results indicated that rutin might inhibit to the lung cancer cells. In addition, different concentrations of rutin could regulate the cell cycle and promote the apoptosis of lung cancer. It is further suggesting that rutin can inhibit the effect on the development of lung cancer cells in vitro.
NF-κB is a transcription factor that regulates the expression of a large number of genes including those involved in inflammation. NF-κB also plays an essential role in innate immune response and many signalling pathways are associated with NF-κB [12-16]. NF-κB belongs to downstream signalling molecule of p38 MAPK. NF-κB has five subtypes: P65, P50, P52, Rel B and c-Rel, different subtypes can be combined with each other and form different dimers; the most common is the p65/p50 [17]. NF-κB activation can modulate the corresponding target genes, including cytokines, chemokines, and cell adhesion molecule expression, thereby regulating cell proliferation, migration and apoptosis [18]. In this expression, the expression of NF-κB and p38 decreased in rutin group (p<0.05). These results indicate that NF-κB and p38 play an important role in lung cancer. In addition, rutin can regulate the expression of NF-κB and p38, and thus to inhibit the development of lung cancer.
Acknowledgments
The authors gratefully acknowledge the assistance of College of Pharmacy.
Funding
This study was supported by the Science and Technology Department of Jilin Province (Grant no. 20140312002ZG).
Conflict of Interest Statement
None declared
References
- Gridelli C, Besse B, Brahmer JR, Crino L, Felip E, de Marinis F. The evolving role ofnivolumab in non-small-cell lung cancer for second-line treatment: a new corner stone for our treatment algorithms. Results from an International Experts Panel Meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer 2016; 17: 161-168.
- Cheng L, Huang FZ, Cheng LF, Zhu YQ, Hu Q. GE11-modified liposomes for non-small cell lung cancer targeting: preparation, ex vitro and in vivo evaluation. Int J Nanomedicine 2014; 9: 921-935.
- Schikowski T, Sugiri D, Reimann V, Pesch B, Ranft U. Contribution of smoking and air pollution exposure in urban areas to social differences in respiratory health. BMC Public Health 2008; 8: 179.
- Huang P, Zhang Y, Jiang T, Zhang N. Effects of p38 MAPK signalling pathway and aldose reductase on transforming growth factor-ß1 induced expression of fibronectin in cultured human mesangial cells. Zhonghua Bing Li XueZaZhi 2015; 44: 778-782.
- Carter AB, Tephly LA, Hunninghake GW. The absence of activator protein 1-dependent gene expression in THP-1 macrophages stimulated with phorbol esters is due to lack of p38 mitogen-activated protein kinase activation. J BiolChem 2001; 276: 33826-33832.
- Staiger K, Staiger H, Weigert C, Haas C, Häring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes 2006; 55: 3121-3126.
- Lai HS, Lin WH, Lai SL, Lin HY, Hsu WM. Interleukin-6 mediates angiotensinogen gene expression during liver regeneration. PLoS One 2013; 8: e67868.
- Wang YB, Ge ZM, Kang WQ, Lian ZX, Yao J. Rutin alleviates diabetic cardiomyopathy in a rat model of type 2 diabetes. ExpTher Med 2015; 9: 451-455.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90.
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100: 1672-1694.
- Li LF, Liao SK, Huang CC, Hung MJ, Quinn DA. Serine/threonine kinase-protein kinase B and extracellular signal-regulated kinase regulate ventilator-induced pulmonary fibrosis after bleomycin-induced acute lung injury: a prospective, controlled animal experiment. Crit Care 2008; 12: R103.
- Hu Y, Cong X, Chen L, Qi J, Wu X, Zhou M. Synergy of TLR3 and 7 ligands significantly enhances function of DCs to present inactivated PRRSV antigen through TRIF/MyD88-NF-κB signaling pathway. Sci Rep 2016; 6: 23977.
- Duan D, Zhang S, Li X, Guo H, Chen M, Zhang Y. Activation of the TLR/MyD88/NF-κB signal pathway contributes to changes in IL-4 and IL-12 production in piglet lymphocytes infected with porcine circovirus type 2 in vitro. PLoS One 2014; 9: e97653.
- Nguyen TT, Niloofar R, Rubbo PA, Nils K, Bollore K, Ducos J. Cytokine response associated with hepatitis c virus clearance in HIV coinfected patients initiating peg interferon-a based therapy. Mediterr J Hematol Infect Dis 2016; 8: e2016003.
- Caamano J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. ClinMicrobiol Rev 2002; 15: 414-429.
- Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-κB signalling pathway. Sci Rep 2015; 5: 18038.
- Loy B, Apostolova G, Dorn R, McGuire VA, Arthur JS, Dechant G. p38a and p38ß mitogen-activated protein kinases determine cholinergic trans-differentiation of sympathetic neurons. J Neurosci 2011; 31: 12059-12067.
- Sun S, Wang X, Wu X, Zhao Y, Wang F. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit Vectors 2011; 4: 186.