Research Article - Biomedical Research (2017) Volume 28, Issue 7
Expression of inflammatory cytokines in retina ischemia-reperfusion injury rats
Qinglei Song1,2#, Dan Liu3#, Haijiang Zhang4*, Liqun Yi1 and Ming Huo4*1Department of Ophthalmology, the Second People’s Hospital, China Three Gorges University, PR China
2Department of Ophthalmology, the Second People’s Hospital of Yichang, Yichang, PR China
3Department of Ultrasound, the First Affiliated Hospital of Nanchang University, Nanchang, PR China
4Department of Ophthalmology, the First College of Clinical Medical Science, China Three Gorges University, PR China
#These authors contributed equally
- *Corresponding Author:
- Ming Huo
Department of Ophthalmology
The First College of Clinical Medical Science
China Three Gorges University, PR China
- Haijiang Zhang
Department of Ophthalmology
The First College of Clinical Medical Science
China Three Gorges University, PR China
Accepted on December 06, 2016
Abstract
Background: Through establishing a retina ischemia-reperfusion injury (RIRI) model, we observed the expression of inflammatory cytokines in retina to explore its possible role in the process of RIRI.
Methods: 75 healthy SD rats without eye disease were randomly divided into normal control group (NCG) and model group (MG).
Results: Protein expression of tumor necrosis factor-α (TNF-α), interleukin-23 (IL-23) and interleukin-17 (IL-17) enhanced obviously in RIRI retina. The TNF-α and IL-23 increased the most at 24 h after modeling, while IL-17 reached the peak at 72 h after modeling. RT-qPCR results showed that 12, 24, 72 and 144 hours after modeling, mRNA expression of TNF-α, IL-23 and IL-17 increased obviously in MG. The mRNA expression of TNF-α and IL-23 increased the most at 24 h after modeling, while IL-17 reached the peak at 72 h after modeling. The difference was significant (P<0.01).
Conclusions: Therefore, expression of TNF-α, IL-23 and IL-17 in retina enhanced obviously in RIRI SD rats. TNF-α, IL-23 and IL-17 can be viewed as pathogenic factors, involving in the pathological damage of RIRI through increasing retinal inflammation.
Keywords
Retina ischemia-reperfusion injury, Tumor necrosis factor-α, Interleukin-23, Interleukin-17.
Introduction
Retina ischemia-reperfusion injury (RIRI) has been found in a variety of fundus diseases, as well as all kinds of eye surgery that during the procedure, the retinal blood flow can be influenced. It seriously affects the visual function. Mechanism of RIRI has not been fully elucidated. In addition to those classic mechanisms such as the oxygen free radicals, calcium overload, nitric oxide concentration change, etc., effect on inflammation in the pathogenesis of RIRI has been popularly discussed in recent years, and the inflammatory cytokines mediated immune inflammatory response has drawn much attention [1,2]. At the subacute phase of retina ischemia, hypoxia inflammatory gene is activated. The inflammatory cascade reaction is the main cause of neurons delayed injury. It has been reported that in the acute high intraocular pressure model, retinal ganglion cell survival rate is higher and the retinal damage is less in T and B-lymphocytes combined immunodeficiency mice than in wild type mice [1]. Clinical test on ischemic optic neuropathy, central retinal artery embolism, and aqueous humor and serum in patients with diabetic retinopathy has also confirmed that the expression of inflammatory factors such as interleukin-8 (IL-8) is significantly higher than in the normal [3,4], suggesting immune inflammatory response plays a very important role in RIRI. Recent studies have found that a new kind of CD4+ effective cell, T help 17 (Th17), which is different from Th1 and Th2.
Th17 is differentiated from natural T cell precursors. It possesses the ability of independent differentiation and regulation, and specifically produce interleukin-17 (IL-17), playing a key role in the process of inflammation, infection and autoimmune disease [5]. IL-17, as a powerful preinflammatory factor, can promote the expression of a variety of inflammatory factors, adhesion molecules and chemokines, and adjust the raise of neutrophils and other inflammatory cells, thus playing an important role in host defense, inflammation and autoimmune disease [6-9]. In ischemia-reperfusion model of heart, liver, kidney, brain and intestine, etc., IL-17 can increase tissue damage. It is the new focus of ischemia reperfusion model [7,10-12]. At the same time, tumor necrosis factor-α (TNF-α) and interleukin-23 (IL-23), as promoting factors of IL-17, promote the secretion of IL-17, and involve in a variety of pathophysiological process mediated by IL-17 [6,13]. In the present study, we established RIRI model in Sprague-Dawley (SD) rats using anterior chamber perfusion of saline to elevate intraocular pressure. Aiming to explore whether TNF-α, IL-23 and IL-17 involve in the pathological damage of RIRI process as a pathogenic factor, to provide experimental basis for further in-depth study.
Materials and Methods
Animals and grouping
75 SPF SD male rats (quality certificate: 42010200000111; license: SYXK E 2011-0061), with weight at 250-300 g and without eye diseases were provided by Animal Center of Sanxia University. Rats were divided into normal control group (NCG) and model group (MG) using the random number method. According to the time point, rats in MG were divided into four groups (12, 24, 72 and 144 h), with a total of 5 groups and each group of 15 SD rats.
Model preparation
The right eye was selected as experiment eye. No operation was conducted in eyes in normal group. Tropicamide compound eyedrops were applied in the right eye to enlarge pupil for three times with 5 minutes each. Propyl hydrochloride eyedrops were used for conjunctival surface anesthesia for three times with 5 minutes each. Intraperitoneal injection of sodium pentobarbital (20 g/L, 40 mg/kg) was used to induce anesthesia. RIRI model was established using methods described by Buchi et al. [14] with modifications: number 4.5 needle, which was connected to saline infusion bag, was pierced into the anterior chamber from lateral temporal corneal limbus, and was fixed. The infusion bag was hang above 150 cm from rats to induce 110 mmHg intraocular pressure within the eye. When the eyelid and conjunctiva was pale, and fundus retinal was pale and vascular flow was stopped under direct ophthalmoscope, the ischemic change was induced. After one hour, the height of infusion bag was slowly reduced down to the animal level. The anterior chamber puncture infusion needle was pulled out. When there was flush on the eye, congestion on eyelid and conjunctival, restoration of retinal vascular blood flow, a successful retina ischemia-reperfusion injury model was built. Rats with cornea injury or traumatic cataract were excluded. Rats in MG were put to death using intraperitoneal injection of sodium pentobarbital at four time points (12, 24, 72 and 144 h) after RIRI, separately. The experiment followed the state administrative regulations on protection of experimental animals.
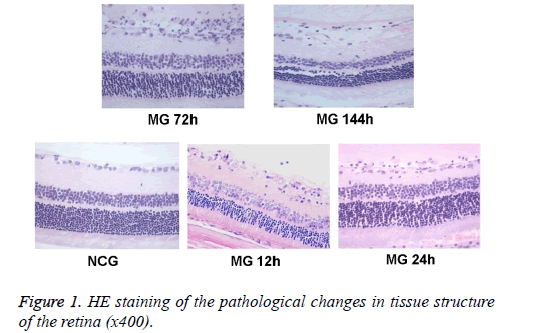
HE staining of retina
After the successful establishment of model, four rats from each group were randomly taken for anesthesia using intraperitoneal injection of sodium pentobarbital. The eyeball was removed and fixed in 4% paraformaldehyde solution to make retinal paraffin sections that were paralleled with the optic sagittal axis. Four sections were serially made at 2 mm within the scope of the optic nerve section, with the thickness of about 5 microns, and then were stained with HE.
Real-time fluorescence quantitative (RT-qPCR) detection of mRNA expression of TNF-α, IL-23 and IL-17
mRNA expression of TNF-α, IL-23 and IL-17 was detected using RT-qPCR. At 12, 24, 72 and 144 h after modeling, 5 SD rats were randomly selected to execute. Eyeball was removed, and retinal tissue was separated under the microscope with dry ice. Cell lysis buffer was added with mechanical homogenate, and Trizol was used to extract total RNA. Real-time RT-PCR kit was used, and template cDNA and specific primers were added for amplification. IL-17: upstream primer 5’CCTCA GACTA CCTCA ACCGT-3’ and downstream primer 5’- ATGTG GTGGT CCAAC TTCCC-3’; TNF-α: upstream primer 5’- CCAGG TTCTC TTCAA GGGAC AA-3’ and downstream primer 5’-GGTAT GAAAT GGCAA ATCGG CT-3’; IL-23: upstream primer 5’-TCATG GCTGT TTCTG GCTGT TGCT-3’and downstream primer 5’-GACTC CTTTT CGGCT TCGTGA-3’. According to the real-time RT-PCR amplification and dissolve curve, CT values were calculated, and the mean CT values were taken to analyze.
Western blot detection of protein expression of TNF- α, IL-23 and IL-17
Five SD rats were randomly selected to execute. Eyeball was removed, and retinal tissue was separated under the microscope. Cell lysis buffer (20 mol/L HEPES, 10 g/L Triton X-100, 1.0 mol/L EDTA, 0.1 mol/L NaCl, pH=7.5) was added with mechanical homogenate. After centrifugal, the supernatant was extracted for determination of protein content using Bradford method. SDS polyacrylamide gel was prepared. Protein electrophoresis samples were added in sample grooves respectively, and then transferred to nitrocellulose membrane. Then skim milk was used to close nitrocellulose membrane. Rabbit polyclonal antibody IL-23 or IL-17 (1:1000) was used for incubation at 4°? overnight. The horseradish peroxidase labeled secondary antibody (1:3000) was used for incubation at room temperature for 1 h. Chemiluminescence chromogenic reagent was used for imaging. AlphaEase FC software was used for grey value analysis on the film protein bands. Gray ratio of target protein and β-actin was used as the relative expression of target protein.
Statistics process
SPSS16.0 software was used to analyze the data. All experimental data were expressed as a mean ± standard deviation (x? ± s). Wilcoxon test was used to compare between groups, and P<0.05 was considered statistically significant.
Results
HE staining
The tissue structure of the retina was observed under optical microscope. Hierarchy of retina structure in the NCG group was clear. The ganglion cells, and cells in the inner and outer nuclear layer were lined up. The ganglion cells were monolayer, and the cell body was large. The thickness of nerve fiber layer was normal. Cells in inner and outer nuclear layer were closely arranged and in order, without dead tissue. While in MG group, retinal tissue was loose, the arrangement was disordered, and nucleus concentration was observed in ganglion cell layer and inner nuclear layer. Changes in retinal organization structure were most obvious at 24 h and 72 h (Figure 1).
RT-qPCR detection of mRNA expression of TNF-α, IL-23 and IL-17
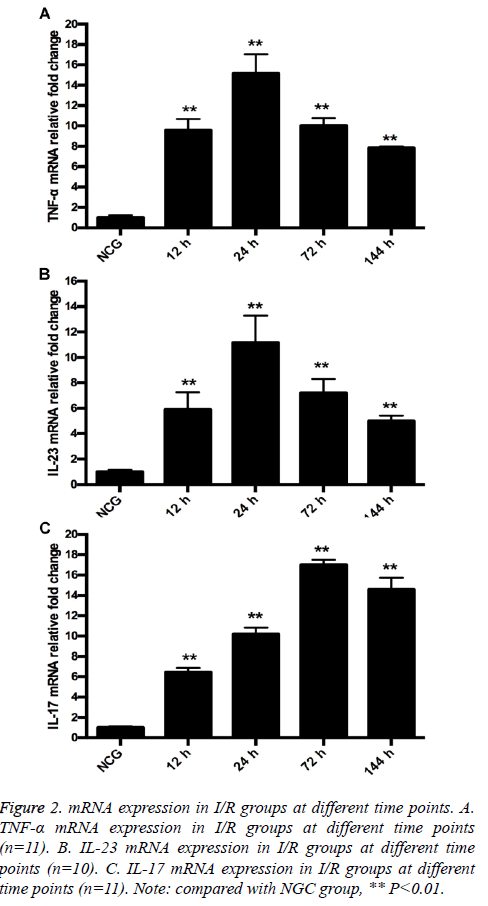
RT-qPCR results showed that mRNA expression of TNF-α and IL-23 was low. After modeling, mRNA expression of TNF-α and IL-23 increased, and reached the peak at 24 h. At 12, 24, 72 and 144 h after modeling, mRNA expression of TNF-α and IL-23 increased in MG compared with in NCG (P=0.000). The difference was significant (P=0.000; Figures 2A and 2B). mRNA expression of IL-17 in retina tissue was low. After modeling, mRNA expression of IL-17 increased, and reached the peak at 72 h. At 12, 24, 72 and 144 h after modeling, mRNA expression of IL-17 increased in MG compared with in NCG (12 h, P=0.001; 24 h, 72 h, 144 h, P=0.000) (Figure 2C).
Figure 2: mRNA expression in I/R groups at different time points. A. TNF-α mRNA expression in I/R groups at different time points (n=11). B. IL-23 mRNA expression in I/R groups at different time points (n=10). C. IL-17 mRNA expression in I/R groups at different time points (n=11). Note: compared with NGC group, ** P<0.01.
Western blot detection of protein expression of TNF- α, IL-23 and IL-17
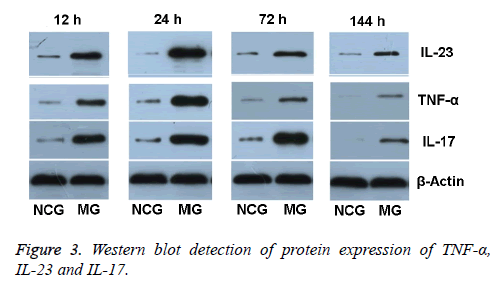
TNF-α protein expression in retinal tissue in NCG was low (0.13 ± 0.03). After modeling, TNF-α protein expression in retinal tissue in MG gradually increased at 12 h, reached peak at 24 h, and then decreased. At 12 h after modeling, TNF-α protein expression was (0.63 ± 0.13), and was 4.85 times higher compared with that in NCG at the same time. At 24 h after modeling, TNF-α protein expression level was obviously higher (1.10 ± 0.07). Compared with that in NCG, it increased 8.46 times. At 72 h after modeling, the expression of TNF-α started to fall (0.59 ± 0.07), but was still 4.54 times higher than in NCG. At 144 h after modeling, the TNF-α expression was (0.17 ± 0.03), and was 1.31 times higher than in NCG.
IL-23 protein expression in retinal tissue in NCG was low (0.10 ± 0.01). After modeling, IL-23 protein expression in retinal tissue in MG gradually increased at 12 h, reached peak at 24 h, and then decreased. At 12 h after modeling, IL-23 protein expression was (0.46 ± 0.03). Compared with that in NCG at the same time, it increased 3.6 times. At 24 h after modeling, IL-23 protein expression was obviously higher (1.25 ± 0.02). Compared with that in NCG, it increased 7.3 times. At 72 h after modeling, the expression of IL-23 protein started to fall (0.80 ± 0.04), but was still 4.7 times higher than in NCG. At 144 h after modeling, the IL-23 protein expression was (0.63 ± 0.03), but was still 2.9 times higher than in NCG.
IL-17 protein expression in retinal tissue in NCG was low (0.15 ± 0.02). At 12 h after modeling, IL-17 protein expression gradually increased at 12 h (0.87 ± 0.01), and was 5.80 times higher than in NCG. At 24 h after modeling, IL-17 protein expression level was (0.98 ± 0.07), and was 6.53 times higher than in NCG. At 72 h after modeling, the protein expression of IL-17 increased obviously and reached the peak (1.74 ± 0.16), and was 11.6 times higher than in NCG. At 144 h after modeling, the IL-17 protein expression started to fall (0.86 ± 0.08), but was still 5.73 times higher than in NCG (Figure 3).
Discussion
Retina ischemia-reperfusion injury (RIRI) is an obvious visual dysfunction caused by the recovery of retinal blood flow in patients with ischemic eye disease. Instead of alleviating the damage of retina, reperfusion aggravates retinal neuronal cell death or apoptosis. It is a relatively rare clinical pathological physiological damage. Its pathological characteristic is the thinning of retinal cortical nerve and apoptosis of ganglion cells and the inner nuclear layer cells, leading to the decline or loss of retina function. Inflammatory factors have different characteristics in ischemic pathological damage at different stages. Usually, the pathophysiological process of ischemic damage is divided into three different stages, the acute phase: a few hours within vascular damage; the subacute phage: a few hours to a few days (generally about 7 days); the chronic phase: a few days to a few months. Inflammatory response in subacute phase is particularly important in the retina ischemiareperfusion injury. Previous researches suggested that the inflammatory cascade reaction caused by retina ischemia was a major cause of retinal neurons delayed damage. Its main characteristic was the activation of inflammatory genes, local up-expression of inflammatory factors and infiltration of inflammatory cells in retinal tissue [15,16].
Multiple organ tissue ischemia-reperfusion injury models have confirmed that the expression of TNF-α, IL-23 and IL-17 increased in infarction tissue and participated in the procedure of local inflammation [7,10-12]. As the main pathogenic inflammatory factors in the subacute stage, they play very important roles in the process of histopathological injury in ischemia reperfusion, especially IL-17. In the immune response procedure, the T help (Th) cells act on various periods of immune response. It has recently been found that a new effective CD4+ cell, the T help cell 17 (Th17), has its own unique differentiation and regulating mechanism. It mainly produces inflammation factor IL-17. Further studies have found that IL-17 is not only produced by Th17 cells, but also by epithelial cells, glial cells, endothelial cells, NK cells, γδT cells, neutrophils and acidophilic granulocytes, thus playing a key role in the process of inflammation, infection and autoimmune diseases. IL-17, as a powerful pre-inflammatory factor, acts on local tissue inflammation by releasing inflammatory factors and raising neutrophils. On one hand, it can promote the activation of T cells and stimulate endothelial cells and macrophages, etc. to produce a variety of inflammatory factors such as TNF-α, metalloproteinases and chemokines, etc. to amplify inflammatory response [6,17,18]. Its main signaling pathway is: through the combination of IL-17 and its receptor, the mitogen activated protein kinase (MAPK) and the NF-κB signaling pathway is activated, and the ratio of Bax/Bcl-2 increases. The MAPK activation leads to up-expression of nitric oxide synthase and cyclooxygenase 2 [19], and the activated NF-κB signal pathway can stimulate the secretion of inflammatory factors such as IL-6, TNF-α and cell adhesion molecule-1, etc. [20], thus improving the expression of Bax protein, increasing the Bax/Bcl-2 ratio, inducing cell apoptosis and increasing tissue damage [10].
TNF-α and IL-23, as promoting factors of IL-17, participate in the process of IL-17 mediated inflammatory response. TNF-α promotes the secretion of IL-17, enhances the function of IL-17, and has good synergistic effect with IL-17, thus amplifying inflammatory response. TNF-α promotes the secretion of IL-17, which induces the secretion of a variety of inflammatory cytokines such as IL-6 and macrophage inflammatory protein (MIP), etc. [21]. NF-kB and MAPK-p38 are the co-signal pathways through which TNF-α and IL-17 play an important role in inflammation. They collaboratively promote the expression of inflammation factors (IL-1β, IL-6, etc.), adhesion molecules (ICAM-1, etc.), chemokines (MCP1 and MCP2, etc.), nitric oxide, and free radical, etc. [6,21-24]. IL-23 is a bridge connecting natural immune and acquired immune, and mainly composed of dendritic cells, phagocytes, and microglia antigen presenting cells that can not only promote the differentiation of Th cells to Th17 cells, but also stimulate T cells to secrete IL-17. Thus, IL-23 is the positive adjustment upstream of IL-17. In intestinal ischemia reperfusion mice model, the expression of IL-23 and IL-17 significantly increased, while in the IL-23p19-/- mice, IL-17 expression decreased significantly, and tissue damage also significantly reduced [25]. It has also been confirmed that in heart ischemia-reperfusion injury model, IL-17 mediated the rat cardiac tissue damage. Administration of IL-17 antibody for intervention can reduce the level of serum troponin and cardiac infarction area [26]. Studies on cerebral ischemia-reperfusion injury model has also showed that the aggregation and activation of lymphocytes plays a very important role in the brain tissue damage, and IL-23 and IL-17 were obviously higher in the cerebral infarction group than that in normal brain tissue [21]. Early invasive tissue macrophages secreting large amounts of IL-23 were detected by flow cytometry, thereby promoting γδT cells infiltration in the subacute stage, thus inducing the secretion of IL-17, eventually leading to brain injury [27]. For the IL-23p19-/- and IL-17-/- mice, number of apoptotic neurons, infarction area and mortality in mice brain after ischemia reperfusion significantly reduced [28].
As a continuation of the brain, retina and brain tissue has high homology. Whether inflammatory factors such as TNF-α, IL-23 and IL-17 involve in RIRI, and how their expression changes are the main content of this study. In this study, through the establishment of RIRI SD rat model, immunohistochemical staining method was used to detect TNF-α, IL-23 and IL-17. Although TNF-α, IL-23 and IL-17 in NCG only expressed a little in the retina, their expression in MG was high. They mainly expressed in the cytoplasm of retinal ganglion cells and the inner nuclear layer cells. RTqPCR detection of TNF-α and IL-23 showed that at 24 h after modeling, the expression reached the peak, while IL-17 reached peak at 72 h after modeling. The time difference indicates that TNF-α and IL-23 may cause cascading effect of IL-17 mediated inflammatory response, and their high expression is the main reason for retinal structure damage and the decline in function. In addition, we found that at 144 h after ischemia reperfusion, TNF-α maintained in a higher level, which was different from the previous studies [29,30]. Therefore, we speculated that with longer duration of ischemia reperfusion, the increased expression of IL-17 promoted the expression of TNF-α. It may also provide a new clue in the inflammatory response. As for cell source of IL-17, Edgerton et al. reported that in intestinal ischemia reperfusion injury model, IL-17 was produced by infiltrating CD4+ T cells [25]. While Shichita et al. pointed out that in the cerebral ischemic reperfusion model, γδT cells were the main source of IL-17 [27], which may be related to the local microenvironment of different organizations. However, in RIRI SD rat model, we observed that TNF-α, IL-23 and IL-17 positive cells mainly existed in retinal ganglion cells and the inner nuclear layer. But the specific cell, which secretes IL-17, requires further study.
Results of this study showed that the TNF-α, IL-23 and IL-17 protein expression in RIRI SD rat model was high, indicating TNF-α, IL-23 and IL-17 may increase retinal inflammation and participate in the pathological damage of RIRI. At the same time, results showed that the expression of TNF-α and IL-23 reached peak at 24 h after RIRI, while IL-17 reached peak at 72 h. These results are basically consistent with the results of cerebral ischemic reperfusion injury [6], suggesting TNF-α, IL-23 and IL-17 play a key role in mediating RIRI retinal inflammation at subacute phase. Inhibiting the TNF-α, IL-23 and IL-17 expression may provide a good time window for prevention and treatment of RIRI. According to the expression change pattern, we can formulate RIRI interventions in clinical, to save the retinal function to the greatest extent. RIRI itself, however, is a complex pathological process. It is a result of the combined actions of a variety of factors and multiple pathways. The secretion, signal pathway, and mediating inflammatory reaction mechanism of TNF-α, IL-23 and IL-17 still require further exploration.
References
- Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis 2010; 16: 2882-2890.
- Andreeva K, Zhang M, Fan W, Li X, Chen Y, Rebolledo-Mendez JD, Cooper NG. Time-dependent Gene Profiling Indicates the Presence of Different Phases for Ischemia/Reperfusion Injury in Retina. Ophthalmol Eye Dis 2014; 6: 43-54.
- Goldenberg-Cohen N, Kramer M, Bahar I, Monselise Y, Weinberger D. Elevated plasma levels of interleukin 8 in patients with acute anterior ischaemic optic neuropathy. Br J Ophthalmol 2004; 88: 1538-1540.
- Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, Karasawa Y, Ito M. Elevated Levels of Cytokines Associated with Th2 and Th17 Cells in Vitreous Fluid of Proliferative Diabetic Retinopathy Patients. PLoS One 2015; 10: e0137358.
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 2010; 10: 479-489.
- Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, Suzumura A. Production and functions of IL-17 in microglia. J Neuroimmunol 2008; 194: 54-61.
- Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect 2009; 11: 625-630.
- Shi PQ, Zhu S, Qian YC. Research of IL-17 function and signal transduction. Chin Cell Biol J 2011; 33: 345-357.
- Liu J, Cao XT. 2012 annual immunology research important progress. Chin J Immunol 2013; 29: 3-13.
- Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, Yuan J, Jevallee H, Wei F, Shi GP, Cheng X. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol 2012; 59: 420-429.
- Park SW, Kim M, Brown KM, D'Agati VD, Lee HT. Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology 2011; 53: 1662-1675.
- Li HL, Kostulas N, Huang YM, Xiao BG, van der Meide P, Kostulas V, Giedraitas V, Link H. IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol 2001; 116: 5-14.
- McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol 2006; 27: 17-23.
- Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica 1991; 203: 138-147.
- Huo YJ, Huang P, Zhang SM, Zhang C. Influence of T- and B-cell-deficiency on retinal neurocytes of mice with acute ocular hypertension. Chin Ophthal Res 2010; 28: 193-197.
- Suto A, Nakajima H, Ikeda K, Kubo S, Nakayama T, Taniguchi M, Saito Y, Iwamoto I. CD4(+)CD25(+) T-cell development is regulated by at least 2 distinct mechanisms. Blood 2002; 99: 555-560.
- Chang HL, Yang XG, Yu JN, Qi Z. Effect of Crocin on structure and the expression of tumor necrosis factor-α and interleukin-1β in rat retina after injury by ischemia-reperfusion. Chin J Ocular Fund Dis 2013; 29: 300-304.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27: 485-517.
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol 2002; 71: 1-8.
- Chen Y, Kijlstra A, Chen Y, Yang P. IL-17A stimulates the production of inflammatory mediators via Erk1/2, p38 MAPK, PI3K/Akt, and NF-kappaB pathways in ARPE-19 cells. Mol Vis 2011; 17: 3072-3077.
- Li GZ, Zhong D, Yang LM, Sun B, Zhong ZH, Yin YH, Cheng J, Yan BB, Li HL. Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol 2005; 62: 481-486.
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol 2007; 184: 53-68.
- Agardh CD, Agardh E, Obrosova IG, Smith ML. The aldose reductase inhibitor fidarestat suppresses ischemia-reperfusion-induced inflammatory response in rat retina. Pharmacology 2009; 84: 257-263.
- Li YZ, Min H, Liu C, Wu H. The combination of IL-17 and TNF-α in adjusting nitric oxide synthetase in osteoarthritis disease. J Clin Rehab Tiss Eng Res 2012; 16: 4393-4397.
- Edgerton C, Crispin JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang YT, Tsokos GC. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol 2009; 130: 313-321.
- Xia N, Tang TT, Liu Y, Zhou SF, Yan XX, Zhu ZF, Nie SF, Liu J, Zhang WC, Yang Y, Liao YH, Cheng X. Preliminary research on the effect of interleukin-17 in myocardial ischemia reperfusion injury. J Clin Cardiol 2010; 26: 130-134.
- Shichita T, Sakaguchi R, Suzuki M, Yoshimura A. Post-ischemic inflammation in the brain. Front Immunol 2012; 3: 132.
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gamma-delta T cells in the delayed phase of ischemic brain injury. Nat Med 2009; 15: 946-950.
- Husain S, Liou GI, Crosson CE. Opioid receptor activation: suppression of ischemia/reperfusion-induced production of TNF-alpha in the retina. Invest Ophthalmol Vis Sci 2011; 52: 2577-2583.
- Berger S, Savitz SI, Nijhawan S, Singh M, David J, Rosenbaum PS, Rosenbaum DM. Deleterious role of TNF-alpha in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci 2008; 49: 3605-3610.