- Biomedical Research (2016) Volume 27, Issue 2
Expression of IL-8 level in lung squamous carcinogenesis of experimental rats.
| Fumei Zhang1, Lei Li2, Tingtong Yang3* 1Department of Pathology, Xinxiang Central Hospital , No.56 Jin Sui Road, Xinxiang 453002, Henan, PR. China 2Department of Pathology, the Affiliated Hospital of Jining Medical University, Jining 272067, Shandong, PR. China 3Department of Morphology Laboratory, Xinxiang Medical University, Xinxiang 453002, Henan, PR. China |
| Corresponding Author: Tingtong Yang, Department of Morphology Laboratory, Xinxiang Medical University, PR. China |
| Accepted: January 30, 2016 |
Abstract
This study aims to investigate the expression level of IL-8 during the rat lung squamous carcinogenesis, and to discuss the relationship with the lung cancer pathogenesis. 40 of 48 Wistar rats were instilled with the chemical agent into the left lobarbranchus to induce lung squamous cell carcinoma. The other 8 rats as in control group were instilled with iodized oil. Apical puncture haemospasia (4-6 ml) were performed before the rats being put to death in batch, the lung tissues of the perfusion parts were removed. The specimens of lung squamous cell carcinoma in each stage of the development process were obtained. Double-antibody ABC-enzyme-linked immunosorbent assay (ELISA) was used to detect serum IL-8 levels in 48 rats. In the study, 7 cases had epithelial hyperplasia, 15 cases had atypical hyperplasia, 6 cases had carcinoma in situ, 7 cases had invasive carcinoma, 6 cases had metastatic carcinoma, 7 cases were taken as normal controls. The expression levels of serum IL- the control group gradually increased with the progress of cancer. The expression levels of serum IL-8 among the hyperplasia, atypical hyperplasia and lung squamous cell groups had significant difference (P=0.018, P=0.000), while significant difference between the metastasis and the non- metastasis groups (P=0.002). This study suggested that serum IL-8 levels had close relationship with occurrence, invasion and metastasis of lung cancer, as well as great significance in the early diagnosis and prognosis.
Keywords |
||||
| Lung carcinoma, Recancerous lesion, Interleukin-8, Double-antibody ABC-enzyme-linked immunosorbent assay (ELISA). | ||||
Introduction |
||||
| In recent years, due to the increased environmental pollution, the lung cancer incidence annually increased to be one of the most common and one of the ten most seriously dangerous cancers, also the cancer with the highest mortality. One of the important reasons for the lung cancer patients (about 40%) was that the distant metastasis (IV period) have been occurred when found [1]. Therefore, the study of lung cancer development and metastasis mechanisms had important practical significance. Interleukin-8 (IL-8) was one of the multi-derived chemotactic cytokines first discovered by Yoshimura et al. in 1987 [2]. IL-8 upregulated the paracrine through autocrine of tumor cells and related chemokine cytokines in the inflammatory microenvironment, activated the relevant signal transduction pathways, and induced tumor vascular proliferation, tumor growth and metastasis [3-6]. Lung cancer, stomach cancer, breast cancer and other malignancies were closely related with IL-8 [7-10]. Because of its biological role diversity, IL-8 caught attention from researchers. So rat lung cancer models were prepared to simulate oncogenic pathways of human lung cancer designed by referring to Tian et al. [11], in order to perform dynamic analysis of the IL-8 expression characteristics of lung lesions in different stages and provide some new ideas for early diagnosis and clinical treatment of lung cancers. | ||||
Methods |
||||
Experimental animals |
||||
| 48 wistar rats belonged to healthy and clean class (Scxk YU 2005-0001) with weight of 180 ± 20g, aged from 4 to 6 months, half for male and half for female, were purchased from Experimental Animal Center of Henan Province. Conditions of the experimental animals were as follows: 4°C-25°C temperature, 40-60% humidity; 12 hours of light and 12 hours of darkness, free water (drinking water), perfect compound feed; 5-8 rats per box, sawdust packing, changed twice a week, cleaning 1 to 2 times per week. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Xinxiang Medical University. | ||||
Preparation of lung squamous cell carcinoma rat model |
||||
| 48 Wistar rats were successfully performed perfusion and randomly divided into experimental group (40) and control group (8). The rat lung squamous cancer model was prepared by one-time infusion of iodized oil (carcinogenic substance) with improvement via the left lung lobe bronchus [11]. Anesthetized rats were naturally supine on the adjustable operating stand, fixed limbs, maxillary incisors were caught by surgical sutures. Gently pulled the tongue with a duckbill tweezers, adjusted the angle of the stand to expose the larynx. Under the direct vision of the frontal mirror, the perfusion needle of 1ml syringe was inserted to tracheal bifurcation through the glottis in the instant of rats inhaling, then gently inserted obliquely to the left lower lobe, injected slowly cancer-induced agent (experimental group) or lipiodol for 0.1 ml (control group). The induced cancer agent perfusion fluid of the experimental group was 3-Methylcholanthrene (MCA) for 5~7 mg and Diethylinitrosamine (DEN) iodized oil suspension for 0.02 ml. The two kinds of cancer-induced agents were purchased from Beijing Shubowei Company (Sigma, USA). The control group was perfused by iodized oil solution. Antiinfection treatment was performed after perfusion. The animals were randomly divided into four groups, and male and female rats were raised separately with free-feeding, ventilation and sanitation were paid attention to. | ||||
Specimens of rat squamous cell carcinoma in various stages of development |
||||
| 30, 60, 90, 180 days after perfusion carcinogenic lipiodol (experimental group) or lipiodol (control group), 8 to 10 rats in the experimental group and 2 rats in the control group were randomly put to death (the animals with natural death during the experiment were also be taken into account). Lesion tissues and systemic organs of the infusion site (the lower left lobe) were obtained, autopsy specimens were immediately fixed in 4% neutral buffered formalin, conventionally dehydrated, paraffin embedded, sliced, the specimens were performed HE staining for review diagnosis and histological grading. | ||||
Serum IL-8 concentration measurement |
||||
| Before each batch of rats were put to death, the chest was cut to expose the heart, apical puncture was used to obtain 4~6 ml blood and placed in numbered sterile sample tubes, stood at room temperature for about 1.5 h, centrifuged with 3000 rpm (low speed) for 10 min. The supernatant was obtained to freeze at -20°C, determined the IL-8 concentration at end of the experiment. Double-antibody ABC-ELISA (enzyme-linked immunosorbent assay) was used for determination methods: 1) established a standard curve: 8 standard wells were set, 100 μl sample dilution was added to each well, 100 μl standard for the first well, sucked out 100 μl after mixing, moved to the second well, and so forth to the 7th well dilution, sucked out 100 μl from the 7th well to discard, so that the volumes were all 100 μl, the 8th well was taken as the blank control; 2) sample loading: 100 μl tested samples were added to each tested well; 3) thoroughly mixed the reaction plate and placed in the incubator at 37°C for 120 minutes; 4) washed plate: fully washed the react plates with washing liquid for 4-6 times, dried on the filter paper; 5) 50 μl first antibody working solution was added to each well, incubated at 37°C for 60 min; 6) washed plate: performed procedure was the same with the former; 7) added 100 μl enzyme labeled antibody working solution to each well, the reaction plate was incubated at 37°C 60 min; 8) washed plate: performed procedure was the same with the former; 9) 100 μl substrate working solution was added to each well, reacted in the dark at 37°C for 5-10 min; 10) a drop of stop solution was added to each well and mixed, the optical density (OD) at 492nm was read, the IL-8 concentrations were in proportional to the OD values, the concentration of the specimen IL-8 could be determined by plotting the standard curve. | ||||
Statistical methods |
||||
| Method for calculating the concentration of IL-8: regression equation was obtained by curve fitting from the data of standard well. The data were expressed with mean and standard deviation ( |
||||
Results |
||||
Pathological and morphological changes of lung squamous cell carcinogenesis in rats |
||||
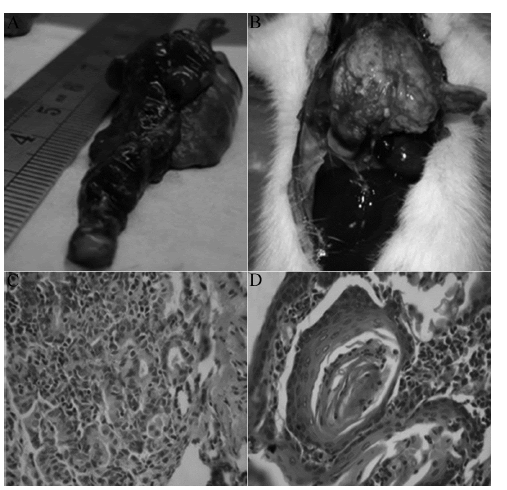
| On the 30th, 60th, 90th, 180th days of the experimental process, the rats were put to death. Dronchial epithelial hyperplasia, squamous metaplasia, dysplasia, carcinoma in situ, invasive cancer and other stage diseases were observed on the tissues of the left lung. Liver, kidney metastasis and pleural planting were observed in rats with lung squamous cell carcinoma on the 180thday. Under the light microscope, induced squamous cell carcinoma were found in a total of 19 experimental rats (six cases had carcinoma in situ, seven cases had invasive cervical cancer, six cases had metastatic cancer; Figure 1), 15 cases had precancerous lesions (including squamous metaplasia and atypical hyperplasia), 7 cases had proliferative phase. Of the 2 rats in the control group after infusion of iodized oil, hyperplasia was visible in pulmonary change of 1 case, which was included in the hyperplasia group. The rest seven cases were included in the normal group. | ||||
Serum IL-8 levels |
||||
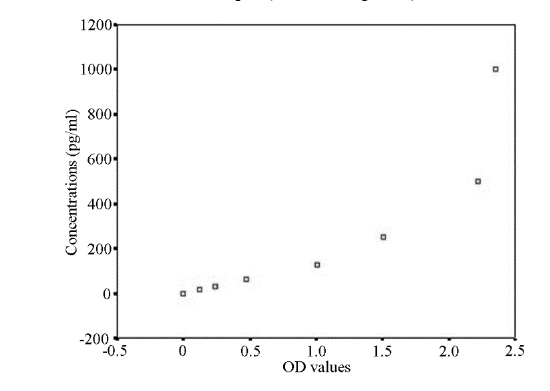
| Sample concentrations of IL-8 were calculated in strict accordance with the instructions. The standard concentration of IL-8 was first taken as the vertical axis (logarithmic scale), OD value as the abscissa (logarithmic scale), standard curve was plotted on a logarithmic graph paper. The regression equation of the standard curve was calculated through the concentration of standard and OD value. Because the standard IL-8 concentration was dilution in ratio, the span was large, the regression equation was obtained by curve fitting method: Y=101.312+0.694x, the sample OD value was put in the equation to calculate the concentration of the sample. | ||||
| The OD values of the serum sample were put into the regression equation to calculate the concentration of IL-8, multiplied by the dilution factor to obtain the actual concentration of the sample (Table 1, Figure 2). | ||||
Comparison of IL-8 levels between different groups |
||||
| With the progress of carcinogenesis in rats, the content of IL-8 also will be increased, and the difference was significant (P=0.000). In addition to no significant difference between normal and hyperplastic groups (P=0.491). The differences between normal and atypical hyperplasia groups, atypical hyperplasia and squamous cell carcinoma groups and among other groups were statistically significant (P<0.05). In rats with lung cancer, the incidence of serum IL-8 levels in the tumor metastasis group were significantly higher than that in the nonmetastasis group (P=0.000). | ||||
Discussion |
||||
| Serum IL-8 detection results for the different lesion stage of the lung squamous cell models showed that the serum IL-8 levels showed an upward trend in the process of the cancer with a statistically significant difference, suggesting that the occurrence of squamous cell carcinoma of the lung in rats was closely related to IL-8, especially significant differences in the comparison between normal and atypical hyperplasia groups, atypical hyperplasia and squamous cell carcinoma, which indicated that statistically significant differences between benign lesions and cancer groups, precancerous lesions and squamous cell carcinoma groups. The results were similar to the results of Yuan et al. [12] (using real-time quantitative PCR to detect the resected tissues of the cancers and expression of IL-8mRNA in the adjacent tissues of 58 patients with nonsmall cell lung cancer) and Sunaga et al. [13], except that the experiment focused on the dynamic changes of serum IL-8 levels in lung cancer with different stages of the occurrence and development process (including the normal control and benign hyperplasia, precancerous lesions or atypical hyperplasia stage, squamous cell carcinoma and concomitant metastasis stages). According to the results in this study, to detect the expression levels of IL-8 had important implications for screening and early diagnosis of lung cancer. Due to the limitations of the number of experimental specimens, comparisons of tumor size and differentiation degrees of IL-8 level expression among groups cannot be statistically analyzed due to the small number of cases, which still needed further study on the basis of expanding the number of samples to explore the clinical significance of the early tumor serum IL-8 concentration and provide help for the early clinical screening and diagnosis for lung cancer. Chemokine IL-8 was not expressed in normal cells, but expressed under hypoxia, acidity and various stimuli. The IL-8 expression could be increased by autocrine of tumor cells and paracrine of fibroblasts, monocytes and epithelial cells to promote the tumor cell proliferation and growth [3,14]. When the cells were stimulated, IL-8 levels increased rapidly, NF-kB, AP-1 and other transcription factors were detected to play an important role in the regulation of IL-8 gene within 60 min [15]. On the other hand, studies showed that inflammation could not only lead to tumorigenesis, also associated with tumor development process. Inflammatory microenvironment consisted of main substrates such as tumor cells, tumor-associated fibroblasts, macrophages and extracellular matrix had close relationship with the occurrence and development, recurrence and metastasis of tumor [16]. In this experiment, despite the perfused rat model were performed anti-infection treatment, with the progress of disease, a large number of macrophages, plasma cells and alveolar epithelial cell proliferation can be observed around the tumor. Whether the results have intrinsically linked to tumorigenesis was pending further studies to explore. | ||||
| In this study, the serum IL-8 levels in the cancer distant metastasis of the squamous cell carcinoma group were significantly higher than that of non-metastasis group, the normal group and benign lesion group, which was similar with the study results of Liu et al. [17], Li et al. [18] used recombinant human IL-8 to act on the human umbilical vein endothelial cells and human dermal microvascular endothelial cells in order to induce endothelial cell proliferation and capillary formation, which confirmed that IL-8 played an important role in angiogenesis. Further studies suggested that epidermal growth factor receptor could promote the activation of vascular endothelial cells, induce epidermal growth factor receptor phosphorylation, regulate endothelial barrier permeability [19,20], so that the tumor cells had local invasion and entered into lymphangion or angiogenesis, then metastasis occurred and accelerated. The existing research not only described that IL-8 expression was positively correlated with microvessel density [13], but also promoted tumor metastasis through the upregulation of the matrix metalloproteinase-9 activity [16], which further validated the biological function of IL-8, that is, IL-8 had a positive regulatory role with the occurrence and development of tumor. IL-8 could be used as a new marker in determining tumor invasiveness. Moreover, gender has become an important factor in many studies, and we found that no significant difference is found between male and female groups (P=0.742) in this study. | ||||
| This study aims to detect the concentration of IL-8 in serum. We found that the concentration of IL-8 is gradually increased with the process of occurrence and development of tumor, although further experiments should be performed to identify the consistence of the concentration and expression level of IF-8. The study is relative simple and easy to perform to detect expression of IL-8 mRNA in different periods, and further studies will be performed in future. | ||||
Conclusion |
||||
| IL-8 was associated with the occurrence and development of lung squamous cell carcinoma, and may be one of the important link to contact inflammation and immunity with cancer; IL-8 serum levels increased with the development of squamous cell carcinoma, and had significant differences among the normal group, cancer group and pre-cancerous lesion group, which could be used as an important marker in early screening, diagnosis and prognosis of lung cancer. | ||||
Tables at a glance |
||||
|
||||
Figures at a glance |
||||
|
||||
References |
||||
|