- Biomedical Research (2016) Volume 27, Issue 3
Expression and correlation of HIF-1α, MIF, COX-2 and VEGF in psoriasis lesions.
Xingning Wang1#, Jianwen Ren2#, Jing Li3, Jingang An2*, Wei Liu4*1Clinical laboratory of Affiliated Hospital of Yan'an University, Shaanxi, Yan’an 716000, China
2Department of Dermatology, Second Hospital of Xi’an Jiaotong University, Xi’an Shaanxi, 710004, China
3Infection control department of Yan'an People's HospitalShaanxi, Yan’an 716000, ChinaShaanxi, Yan’an 716000, China
4Department of Geriatrics, Affiliated Hospital of Yan'an University, Shaanxi, Yan’an 716000, China
#These authors contributed equally to this work
*These authors contributed equally as corresponding authors
- *Corresponding Author:
- Jingang An
Department of Dermatology
Second Hospital of Xi’an Jiaotong University
China
Accepted date: February 24, 2016
Abstract
Psoriasis is a common chronic, inflammatory and proliferative skin disease that is widely associated with multiple factors under a polygenic background. The expression of hypoxia-inducible factor-1? (HIF-1?), macrophage migration inhibitory factor (MIF), cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) is associated with psoriasis; however, there have been no systematic studies analyzing the mRNA and protein levels in psoriasis vulgaris tissues. The aim of the present study was to investigate the mRNA and protein levels of these genes in psoriasis vulgaris tissues and assess their correlation. Tissue samples from 45 cases of psoriasis vulgaris lesions and 45 cases of normal skin were collected. The study used semi-quantitative polymerase chain reaction and western blot analysis to detect the mRNA and protein levels in the psoriatic lesions and normal skin tissue. The mRNA and protein levels of HIF-1?, MIF, COX-2 and VEGF were significantly higher in psoriasis vulgaris tissues compared with those in normal skin tissue, all P-values were <0.05. Additionally, the mRNA and protein levels in the psoriatic lesions were positively correlated with each other. In conclusion, these genes may have important roles in the development of psoriasis.
Keywords
Psoriasis lesions, Hypoxia-inducible factor-1α, Macrophage migration inhibitory factor, Cyclooxygenase-2, Vascular endothelial growth factor.
Introduction
Psoriasis is a common, chronic, relapsing and inflammatory skin disease characterized by skin lesions including scaly erythema [1]. The natural incidence rate of psoriasis is about 0.1%-3%. It is easy to relapse as it is usually aggravated in spring and winter, while it is usually alleviated in summer and autumn. While the pathogenesis of Psoriasis is not entirely clear, it is generally thought to be a polygenic disease which is triggered or influenced by a range of environmental factors. Factors known to induce or aggravate psoriasis include infection, stress and stressful events, trauma, surgery, smoking, and certain drugs [2].
Clinically, there are four main types of Psoriasis including psoriasis vulgaris, pustular psoriasis, arthritic psoriasis and erythrodermic psoriasis [3]. Psoriasis vulgaris is the main type of Psoriasis accounting for over 90% of all cases. The other three types usually derived from psoriasis vulgaris by inappropriate treatment (such as a sudden withdrawal during systemic use of corticosteroids or immunosuppressive agents), infection or stress. This study mainly focuses on the psoriasis vulgaris also known as plaque psoriasis. The lesions usually develop from erythematous macules or papules which extend peripherally, and coalesce to form plaques. The lesions are characterized by dry, sharply demarcated round/oval plaques with loosely adherent silvery white scales. The psoriasis vulgaris can appear in any part of the body; however, it mainly appears on the scalp or is symmetrically distributed over the elbows and knees. The main pathological change is the excessive proliferation of keratinocytes, hyperplasia and expansion of the microvasculature in the dermal layer as well as inflammatory cell infiltration.
Hypoxia-inducible factor-1α (HIF-1α) is an active subunit of the oxygen-dependent transcription activator [4]. It has been reported that the activated HIF-1α can regulate angiogenesis, apoptosis, proliferation, erythropoiesis, and energy metabolism. It has also been reported that HIF-1α is highly expressed in psoriatic lesions [5,6]. Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine with complex biological activities produced by multiple cell types, including macrophages, dendritic cells and T lymphocytes [7]. It has been reported that the anti-MIF antibody inhibits T cell proliferation and IL-2 production in vitro and inhibits antigenactivated T-cell activation and antibody production in vivo. Recent research has revealed that MIF can promote tumorigenesis, angiogenesis and wound healing and participates in anti-tumor immunity. It is also involved in skin diseases such as psoriasis, skin photo aging, atopic dermatitis, contact dermatitis, systemic lupus erythematosus, scleroderma, and pityriasisrosea [8]. Cyclooxygenase-2 (COX-2) is an inducible cyclooxygenase and over-expression of COX-2 can promote cell proliferation and angiogenesis [9]. It has also been demonstrated by immunohistochemistry that the expression of COX-2 is up-regulated in the psoriatic lesions [10]. Vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen which does not only promotes endothelial cell proliferation, but can also effectively increase the capillary permeability [11]. Currently, there is increasing interest in the role of VEGF in the pathogenesis of psoriasis [10].
Although HIF-1α, MIF, COX-2 and VEGF have been implicated in psoriasis lesions, there are limited studies demonstrating the definitive expression and correlation of HIF-1α, MIF, COX-2 and VEGF in psoriasis lesions. In this study, we conducted semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) and western blot analysis to detect the mRNA and protein levels of HIF-1α, MIF, COX-2 and VEGF in Psoriasis vulgaris tissues compared to normal skin tissue to confirm the role of these mediators in psoriasis lesions.
Materials and Methods
Subjects
After obtaining informed consent, 45 adults with chronic psoriasis vulgaris diagnosed by clinical and pathological examination in our hospital were selected as the experimental group. All the patients are from Shaanxi province, China. The 45 patients comprised 22 males and 23 females aged from 22 to 66 years old with the average age being 36.13 ± 12.8 years old. The course of psoriasis vulgaris ranged from 2 weeks to 24 years. Among the 45 patients, 9 patients had early onset psoriasis vulgaris; while the other 36 patients had been not received systemic therapy and topical treatment within 1 month; and all the patients showed typical psoriasis vulgaris clinical appearance including rash colour bright red, cuticle thickening, and obvious itching [12]. The control group of 45 subjects comprised 24 males and 21 females with the average age being 38.13 ± 9.8 years old. There were no significant differences between the two groups in terms of the average age and gender ratio (P>0.05) by statistical analysis. Skin biopsy specimens (5 mm) were obtained from all the participants in both groups. The specimens were divided into two groups (one used for RNA isolation and semi-quantitative RT-PCR detection; and the other piece for protein isolation and western blot detection) then stored in liquid nitrogen until analysis.
RNA isolation
The skin biopsy specimens were processed using the QIAGENR Neasy kit. The frozen specimens were homogenized in buffer (RLT; QIAGEN) for 30-60s by using a rotor-stator tissue homogenizer. Then, the homogenate was micro centrifuged for 3 min at room temperature to pellet the debris. The supernatant was loaded onto the QIAGEN mini column and spun for 15 s at high speed. A series of washes and DNase digestion was followed by a final elution of RNA from the column using 30μLRNase-free water (Sigma-Aldrich) according to the manufacturer’s instructions. RNA was quantitated by UV spectrophotometry (NanoDrop2000).
RT-PCR detection
After quantifying the concentration of the total RNA isolated, 1μg of total RNA was used for the cDNA synthesis using the Prime Script™ RT Reagent Kit (Perfect Real Time; Takara). All primers were synthesized by Shanghai SANGON (China). Semiquantitative RT-PCR reactions were performed according to the manufacturer’s instructions (TaKaRa Ex Taq).We used an Applied Biosystems Bio-rad Thermal Cycler (MyCycler) for 5 min at 95°C for pre-denaturation, and 30 cycles for 30 s at 94°C, 30 s at 56°C, 30 s at 72°C, followed by 10 min at 72°C.The PCR products were analyzed on 1% agarose gel with ethidium bromide staining. And the image was obtained by the gel imaging system (Bio-rad, GelDocXRSystem). The relative expression level was quantitated by measuring the gray scale value using image J software. The human glyceraldehyde-3- phosphate dehydrogenase (GADPH) gene, a house keeping gene, was used to normalize each sample and each gene. The primers used are shown in table 1.
| Genes | Forward primer | Reverse primer | Product size |
|---|---|---|---|
| HIF-1α | GATGGAAGCACTAGACAAAGTTCA | ATCAGTGGTGGCAGTGGTAGTG | 360 bp |
| MIF | AGAACCGCTCCTACAGCAAG | ATTTCTCCCCACCAGAAGGT | 243 bp |
| COX-2 | AAGAAGAAAGTTCATTCCTGATCC | TGACTGTGGGAGGATACATCTC | 251 bp |
| VEGF | GGGAACGCTCCAGGACTTAT | GGGAACGCTCCAGGACTTAT | 250 bp |
| GAPDH | GGTCATCCATGACAACTTTGG | CCAAATTCGTTGTCATACCAGG | 450 bp |
Table 1. The primers used in the study
Protein extraction
Tissues from psoriasis vulgaris and normal skin were prepared by homogenization in extraction buffer (0.05 M Tris-HCl, pH 7.5, 2 mm phenylmethylsulphonyl fluoride, 1 m Methylenediaminetetraacetic acid, 10 μg/ml of pepstatin A, antipain, leupeptin, and chymostatin) supplemented with 1.5% sodium dodecyl sulphate (SDS), sonicated and centrifuged. The tissue extracts were then mixed with 1/3 volume of 4 × sample buffer (1 M sucrose, 8% SDS, 0.25 M Tris-HCl buffer, pH 6.8, 0.01% bromophenol blue), heated in the presence of 5% of total volume 2-mercaptoethanol for 5 min at 95°C and stored at −80°C. The concentration of total protein was determined with Bicinchoninic Acid assay (BCA) (Beyotime biotechnology, China).
Western blot analysis
Aliquots containing 15 μg of total protein extracted from psoriasis vulgaris and normal skin were size-fractionated by SDS-polyacrylamide gel electrophoresis using a 10% gel (Mini Protean II, Bio-Rad) and transferred to a PVDF membrane (Whatman, USA). After washing five times (for 5 min every time) in Tris-buffered saline (TBS), pH 7.5, blots were blocked with 5% skim milk/TBS at room temperature for 1 hour and subsequently incubated with Rabbit anti-human polyclonal antibody at a dilution of 1:250 over light at 4°C. This was followed by incubation with peroxidase-conjugated antimouse/ anti-rabbit immunoglobulin and then detected using the Western blot Kit (EasySee® Western Blot Kit, China).Rabbit anti human polyclonal antibodiesforHIF-1α, MIF, COX-2 and VEGF were bought from NeoMarkers Company (USA). The relative protein level was quantitated by measuring the gray scale value using image J software. The human β-tubulin, a house keeping gene, was used to normalize the protein levels of each sample.
Statistical analysis
The gray scale of RT-PCR and Western blot values were measured for the mean ± SD. Data were analyzed for statistical significance using the independent sample t test to compare the mRNA and protein levels in psoriasis vulgaris and normal skin. A value of p<0.05 was considered significantly different and the correlation analysis was conducted by SPSS15.0 software.
Results
Semiquantitative RT-PCR analysis
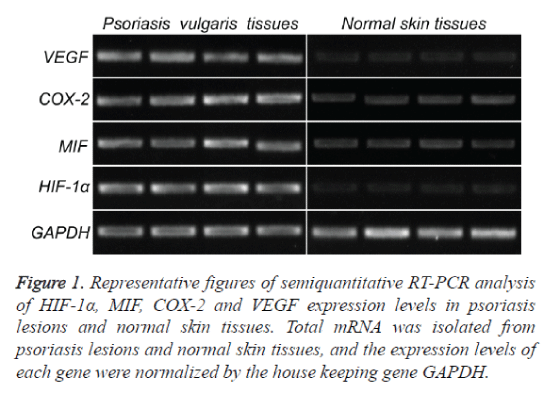
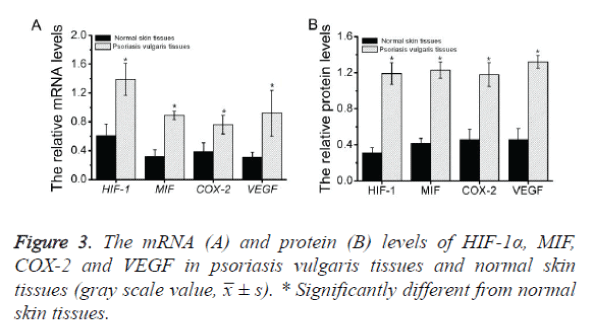
To investigate the expression of HIF-1α, MIF, COX-2 and VEGF mRNA in psoriatic lesional skin and normal skin, semiquantitative RT-PCR was performed as described above (Figure 1). HIF-1α, MIF, COX-2 and VEGF mRNA were present in all specimens from both psoriatic skin (Figure 1, left) and normal skin (Figure 1, right). Amplified PCR signals for HIF-1α, MIF, COX-2 and VEGF in psoriatic skin specimens showed relatively stronger intensity compared with those in normal skin. The house keeping gene, GADPH, was used to normalize each sample and each gene. By measuring the gray scale value using image J software, it concluded that the expression levels of all the four genes in psoriatic skin specimens are significance higher than those in normal skin (p<0.05) (Figure 3A and Table 2).
Figure 1. Representative figures of semiquantitative RT-PCR analysis of HIF-1α, MIF, COX-2 and VEGF expression levels in psoriasis lesions and normal skin tissues. Total mRNA was isolated from psoriasis lesions and normal skin tissues, and the expression levels of each gene were normalized by the house keeping gene GAPDH.
| Psoriasis vulgaris tissues (n=45) | Normal skin tissues (n=45) | t value | P value | |
|---|---|---|---|---|
| HIF-1α | 1.19 ± 0.12 | 0.31 ± 0.06 | 83.12 | <0.05 |
| MIF | 1.23 ± 0.09 | 0.42 ± 0.05 | 69.13 | <0.05 |
| COX-2 | 1.18 ± 0.13 | 0.46 ± 0.11 | 75.11 | <0.05 |
| VEGF | 1.32 ± 0.07 | 0.36 ± 0.09 | 89.22 | <0.05 |
Table 2. The expression levels of HIF-1α, MIF, COX-2 and VEGF in psoriasis vulgaris tissues and normal skin tissues (gray scale value, x̅ ± s).
Western blot analysis
The results of semi quantitative RT-PCR clearly revealed that the mRNA levels of HIF-1α, MIF, COX-2 and VEGF in psoriasis vulgaris tissues were higher than those in normal skin tissues. Thus, we next measured the protein levels of these genes in the corresponding tissues by western blot analysis as described above (Figure 2).The results demonstrated that all four proteins were present in all specimens from both psoriatic skin (Figure 2, left) and normal skin (Figure 2, right). As shown in the figure, the gray scale of these proteins in psoriasis vulgaris tissues demonstrated relatively stronger intensity compared with those in normal skin. The house keeping gene, β-tubulin, was used to normalize each sample and each gene. By measuring the gray scale value using image J software, it concluded that the all the protein levels in psoriatic skin specimens are significance higher than those in normal skin (p<0.05) (Figure 3B and Table 3).
Figure 2. Representative figures of western blot analysis of HIF-1α, MIF, COX-2 and VEGF protein levels in psoriasis lesions and normal skin tissues. Total protein was extracted from psoriasis lesions and normal skin tissues, and 15μg of total protein was used for western blotting. The expression levels of each protein were normalized by the house keeping gene β-tubulin.
| Psoriasis vulgaris tissues (n=45) | Normal skin tissues (n=45) | t value | P value | |
|---|---|---|---|---|
| HIF-1α | 1.39 ± 0.22 | 0.61 ± 0.16 | 79.32 | <0.05 |
| MIF | 0.89 ± 0.06 | 0.32 ± 0.09 | 81.13 | <0.05 |
| COX-2 | 0.76 ± 0.13 | 0.39 ± 0.12 | 79.16 | <0.05 |
| VEGF | 0.92 ± 0.32 | 0.31 ± 0.07 | 92.12 | <0.05 |
Table 3. The protein levels of HIF-1α, MIF, COX-2 and VEGF in psoriasis vulgaris tissues and normal skin tissues (x̅ ± s).
Correlation analysis
The SPSS15.0 software was used to analyze the correlation of mRNA and protein levels of HIF-1α, MIF, COX-2 and VEGF in psoriasis vulgaris tissues. The results showed that in psoriasis vulgaris tissues, the mRNA levels forHIF-1α, MIF, COX-2 and VEGF were positively correlated with each other (HIF-1α vs. MIF, r=0.653, P<0.05; HIF-1α vs. COX-2, r=0.664, P<0.05; HIF-1α vs. VEGF, r=0.731, P<0.05; MIF vs. COX-2, r=0.681, P<0.05; MIF vs. VEGF, r=0.681, P<0.05; COX-2 vs. VEGF, r=0.691, P<0.05). The protein levels are consistent with the mRNA levels (HIF-1α vs. MIF, r=0.653, P<0.05; HIF-1α vs. COX-2, r=0.664, P<0.05; HIF-1α vs. VEGF, r=0.731, P<0.05; MIF vs. COX-2, r=0.681, P<0.05; MIF vs. VEGF, r=0.681, P<0.05; COX-2 vs. VEGF, r=0.691, P<0.05).
Discussion
Psoriasis vulgaris is the most common type of psoriasis with the main pathological manifestations including the excessive proliferation of epidermal keratinocytes, inflammatory cell infiltration, microvascular proliferxation and expansion of the dermal papilla layer. Angiogenesis is closely related to the development, continuation and recurrence of psoriasis and plays an important role in the pathogenesis of psoriasis [1,13]. Psoriatic epidermal keratinocytes not only involve excessive proliferation, but also involves abnormal apoptosis. In tumor tissue, HIF-1α induced tumor angiogenesis provides energy for tumor cell metabolism, inhibits tumor cell apoptosis and promotes tumor cell proliferation, invasion and metastasis [14,15]. Both psoriasis and cancer have shown certain similarities in cell proliferation, local hypoxia and angiogenesis. Recent studies have reported that HIF-1α and its downstream factors are over-expressed in psoriasis lesions. Our results also found that the mRNA and protein levels of HIF-1α, COX-2 and VEGF in psoriatic skin are higher than the normal control group. These results are consistent with what has been reported in the literature, confirming that HIF-1α, COX-2 and VEGF are likely involved in the development of psoriasis.
MIF has been considered an inflammatory mediator which plays a key role in inflammation and immune regulation. It can inhibit macrophage migration, promote macrophage and T lymphocyte infiltration in the inflammatory response, aggregation, proliferation and activation and enhances adhesion and phagocytosis capability. It can also promote the generation of a variety of inflammatory cytokines, including IL-1, IL-6, IL-8, MCP-1, TNF-α, IFN-γ, COX-2, and matrix metalloproteinase contributing to the development of inflammation. MIF is also implicated in lupus, psoriasis, pityriasis rosea, contact dermatitis, systemic scleroderma, cancer, chronic nephritis, coronary sclerosis and other inflammatory and autoimmune diseases. It has been reported that the expression level of MIF is up-regulated in psoriasis lesions as demonstrated by immunohistochemistry, western blot and RT-PCR methods [16]. Shimizu et al. found that the MIF levels were elevated in the serum and peripheral blood mononuclear cells of patients with psoriasis vulgaris [17,18].
It has also been proposed that the infiltration of mononuclear cells such as T lymphocytes increased in psoriasis lesions leading to increased MIF levels which in turn promote immune disorders, persistent inflammation and keratinocyte proliferation. Our experimental results showed that MIF and its downstream regulatory factor, COX-2, was significantly higher than control for both mRNA and protein levels. Thus, we conclude that keratinocytes and endothelial cells released by MIF may play an important role in the inflammatory response in psoriasis.
We also did correlational analysis of HIF-1α, MIF, COX-2 and VEGF in psoriasis lesions and found a positive correlation between the four genes. It has been reported that hypoxia can induce HIF-1α expression and stabilize HIF-1α protein, causing the up-regulation of downstream factors including COX-2, VEGF, inducible nitric oxide synthase (iNOS), matrix metalloproteinase-2 (MMP-2) glucose transporter protein-1/-3 (GLUT-1/-3), erythropoietin (EPO) and insulin-like growth factor-2 (IGF-2). This leads to inflammation, angiogenesis, cell apoptosis, proliferation, promoting erythropoiesis regulating oxygen transport, energy metabolism and other reactions.
Studies have found that MIF can induce human dermal microvascular endothelial cell migration and tube formation in vitro, which is similar to the function of Bfgf [19]. MIF was expressed significantly higher in tumor cells, and its mRNA level has a strong positive correlation with VEGF mRNA level [20]. Studies have found that during the period of psoriasis vulgaris, the expression of MIF and VEGF are positively correlated indicating that both genes may play important roles in the occurrence and development of psoriasis. There may exist a positive regulatory relationship between the two genes which may promote the secretion of either genes leading to vascular endothelial cell proliferation, angiogenesis and cell infiltration at psoriasis lesions, promoting the development of psoriasis. However, the mechanism for the interaction between these two factors still needs to be studied in further depth. Kyzas et al have found that the expression of COX-2is related to VEGF expression where the over-expression of COX-2 can promote angiogenesis by stimulating VEGF up-regulation in head and neck squamous cell carcinoma [21]. It has also been reported that COX-2 and VEGF are highly expressed in gastric cancer and prostate cancer, and their expression was significantly correlated [22,23]. Our results showed that the expression of COX-2 and VEGF are positively correlated, leading to the speculation that COX-2 stimulates VEGF expression to play an important role in psoriasis angiogenesis and inflammation. In psoriasis lesions, the expression of MIF and COX-2 were positively correlated indicating that MIF and COX-2 may have a synergistic effect in psoriasis lesions. MIF as a pro-inflammatory cytokine may induce the expression of COX-2 however the mechanism of the interaction still needs further in-depth studies. HIF-1α can induce the downstream expression of the COX-2 and VEGF gene. It has also been proven that the stability of HIF-1α is related to MIF as a MIF knock out can lead to the destabilization of HIF-1α under hypoxic induction. Further MIF transcription is directly affected by HIF-1α found that an excess of MIF improved the stability of hypoxia-induced HIF-1α, and HIF-1α combines with HRE to promote the expression of the downstream gene involved in promoting the process of cell proliferation and angiogenesis, together with MIF [24-26]. While hypoxiainduced VEGF expression increases in the context of overexpression of MIF, in the MIF knock-out background, its expression is significantly reduced.
References
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. The Lancet 2007; 370: 263-271.
- Sabat R, Wolk K. Pathogenesis of psoriasis In: Psoriasis: Diagnosis and Management Sterry W, Sabat R, Philipp S, 2014; 28-48.
- Ortonne J. Recent developments in the understanding of the pathogenesis of psoriasis. British J Dermatol 1999; 140: s1-s7.
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Rev Cancer 2004; 4: 437-447.
- Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neurooncol 2005; 7: 134-153.
- Tovar-Castillo LE, Cancino-Díaz JC, García-Vázquez F, Cancino-Gómez FG, León-Dorantes G. Under-expression of VHL and over-expression of HDAC-1, HIF-1α, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. IntJDermatol 2007; 46: 239-246.
- Baugh JA, Bucala R. Macrophage migration inhibitory factor. Critical care medicine 2002;30: 27-35.
- Donn RP, Plant D, Jury F, Richards HL, Worthington J, Ray DW, Griffiths CE. Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol 2004; 123: 484-487.
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009; 30: 377-386.
- Yalcin B, Tezel GG, Arda N, Erman M, Alli N. Vascular endothelial growth factor, vascular endothelial growth factor receptor-3 and cyclooxygenase-2 expression in psoriasis. Analytical and quantitative cytology and histology/the International Academy of Cytology [and] American Society of Cytology 2007; 29: 358-364.
- Ferrara N. Vascular endothelial growth factor. Euro J Cancer 1996; 32: 2413-2422.
- Marks R, Barton SP, Shuttleworth D, Finlay AY. Assessment of disease progress in psoriasis. Archives of Dermatology 1989; 125: 235-240.
- Lowes MA, Bowcock AM, Krueger JG. Pathogen Therapy Psoriasis. Nature 2007; 445: 866-873.
- Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends in molecular medicine 2002; 8: 62-67.
- Vaupel P. The role of hypoxia-induced factors in tumor progression. The Oncologist 2004; 9: 10-17.
- Steinhoff M, Meinhardt A, Steinhoff A, Gemsa D, Bucala R, Bacher M. Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. British J Dermatol 1999; 141: 1061-1066.
- Shimizu T, Nishihira J, Mizue Y, Nakamura H, Abe R, Watanabe H, Ohkawara A, Shimizu H. High macrophage Migration Inhibitory Factor (MIF) serum levels associated with extended psoriasis. J Invest Dermatol 2001; 11: 989-990.
- Nishihira J, Koyama Y, Mizue Y. Identification of Macrophage Migration Inhibitory Factor (Mif) in Human Vascular Enothelial Cells and Its Induction by Lipopolysaccharide. Cytokine 1998; 10: 199-205.
- Kats R, Metz CN, Akoum A. Macrophage migration inhibitory factor is markedly expressed in active and early-stage endometriotic lesions. The Journal of Clinical Endocrinology & Metabolism 2002; 87: 883-889.
- Shi X, Leng L, Wang T, Wang W, Du X, Li J,McDonald C, Chen Z, Murphy JW, Lolis E,Noble P,Knudson W,Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006; 25: 595-606.
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nature reviews immunology 2003, 3: 791-800.
- De Benedetti F, Meazza C, Vivarelli M, Rossi F, Pistorio A, Lamb R, Lunt M, Thomson W, Ravelli A, Donn R. Functional and prognostic relevance of the-173 polymorphism of the macrophage migration inhibitory factor gene in systemic‐onset juvenile idiopathic arthritis. Arthritis & Rheumatism 2003;48: 1398-1407.
- Piette C, Deprez M, Roger T, Noël A, Foidart JM, Munaut C. The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. Journal of biological chemistry 2009; 284: 32483-32492.
- Nguyen MT, Lue H, Kleemann R, Thiele M, Tolle G, Finkelmeier D,Wagner E,Braun A,BernhagenJ. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. The Journal of Immunology 2003; 170:3337-3347.
- Oda S, OdaT, Nishi K, Takabuchi S, Wakamatsu T, Tanaka T, Adachi T, Fukuda K, Semenza GL, Hirota K.Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner 2008. 3: e2215.
- Winner M, Koong AC, Rendon BE, Zundel W, Mitchell RA. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor–dependent hypoxia-inducible factor stabilization. Cancer research 2007; 67: 186-193.