Research Article - Biomedical Research (2017) Volume 28, Issue 6
Ex vivo expansion of EPCs derived from human peripheral blood mononuclear cells by Iron-Quercetin complex
Jiraporn Kantapan1,2, Songrak Dejphirattanamongkhol1,2, Krai Daowtak1,2, Sittiruk Roytrakul3, Padchanee Sangthong4 and Nathupakorn Dechsupa1,2*1Laboratory of Physical Chemistry, Molecular and Cellular Biology and Center of Excellence for Molecular Imaging, Thailand
2Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand
3Proteomics Research Laboratory, Genome Institute, National Science and Technology Development Agency, Pathumthani, Thailand
4Department of Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- *Corresponding Author:
- Nathupakorn Dechsupa
Department of Radiologic Technology
Faculty of Associated Medical Sciences
Chiang Mai University, Thailand
Accepted on November 10, 2016
Abstract
Cell-based therapy has emerged as a promising new treatment for cardiovascular diseases. One of the main obstacles to this approach is a limited number of endothelial progenitor cells (EPCs) obtained from the patient and lack of an effective culture-expanded techniques. Thus, we aimed to demonstrate the potential use of paramagnetic agent called “IronQ” (complexation of iron and quercetin) to expand progenitor cells derived from peripheral blood mononuclear cells (PBMCs) fraction and evaluate it as cell labeling probe using magnetic resonance imaging (MRI). PBMCs were cultured in the presence of IronQ complex compared to conventional culture medium. After expansion, cells were characterized by immunostaining, tube formation assay, intracellular uptake of IronQ and visualizing of magnetically labeled cells by in vitro MRI. Our data show that IronQ exerted an effective expansion of the cells by maintaining the progenitor content and increasing the population of angiogenic cells. Intracellular uptake of IronQ increased as time dependent and reached the maximum at 153 ± 95 pg IronQ/cells after labeled for 120 h. The labeled cells demonstrated an increase of MRI signal intensity in T1-weighted image when compared to the signal of unlabeled cells. From these data, IronQ complex could promote the conditions that suitable for the growth of endothelial-specific differentiated cells derived from PBMC fraction, after expansion, the magnetically labeled cells can be visualized and localized by MRI. Our study demonstrated that ex vivo expansion of EPCs fractions derived from PBMCs using the paramagnetic agent IronQ complex might be an alternative method for the treatment of cardiovascular disease.
Keywords
Ex vivo expansion, Endothelial progenitor cells, Peripheral blood mononuclear cells, IronQ complex, Stem cell tracking, MRI contrast agent.
Introduction
Stem cell therapy is emerging as a promising novel therapeutic strategy for cardiovascular regenerative medicine. Stem cells may participate in the tissue regeneration process via paracrine mechanisms or differentiation into native tissues. Bone marrow-derived endothelial progenitor cells (EPCs) that were first discovered in adult peripheral blood mononuclear cells (PBMCs) by Asahara et al. [1]. These EPCs play important role in neovascularization of ischemic tissues and in re-endothelialization of injured blood vessels [1,2]. Since then, accumulating evidence demonstrates that EPCs functionally incorporate in neovascularization in several models of tissue injury and remodeling [3,4]. Therefore, EPC-based cell therapy may be useful as a therapeutic approach for cardiovascular diseases. Autologous total mononuclear cells freshly isolated from bone marrow or peripheral blood have been applied to clinical vascular regenerative therapy in patients with a severe ischemic heart or limb diseases [5,6]. These studies indicated that cell-based therapy is safe, feasible, and effective.
Autologous stem cell transplantation using EPCs derived from peripheral blood or bone marrow has several impacts. However, the numbers of EPC cells found in bone marrow and peripheral blood is not high enough for use in a clinical setting [7]. In addition, patients with cardiovascular risk factors have been shown to have a lower of EPCs and a lower level of function [8-10]. Thus, the number and function of EPCs seem to be significant factors for effective therapy for those patients.
Accordingly, ex vivo culture and expansion of EPCs may be an encouraging strategy to resolve the problem of limited cell numbers. Several different ex vivo expansion EPCs methods have been developed. Mostly, EPCs have been cultured in fibronectin-coated plate in endothelial basal medium-2 supplemented with growth factor cocktails [11-13]. However, expansion of EPCs using growth factors requires high costs and is unsafe for the clinical application. The development of a method to expand EPCs without the need for growth factors is a promising approach to simplify clinical translation. Interestingly, several studies have shown the beneficial effects of polyphenols on EPCs, including increase in both number and functional activity of EPCs [14-16]. Quercetin a natural flavonoid compound is found in common foods and vegetables. It exerts numerous beneficial effects on human health, including anti-inflammation, anti-ischemia, cardiovascular protection and neuroprotective effects [17,18]. Recently, several studies have successfully shown that quercetin has beneficial effects on EPCs, including increased number and functional activity of EPCs and it protects against high glucose-induced damage to EPCs by inducing Sirt1- dependent eNOS up-regulation in EPCs [19,20]. All studies of these indicate that quercetin might be an attractive agent to expand EPCs.
To effectively utilize EPCs cell-based therapy in the clinical setting, two critical factors are involved: generating sufficient numbers of therapeutic relevant cells (ex vivo expand) and developing a technique to monitoring the in vivo biodistribution of transplanted cells. Magnetic resonance imaging (MRI) is the most powerful imaging technique available for in vivo diagnostic with a good resolution and localization. To allow MRI detection, the cells should be labeled with MRI contrast agent. The most commonly used MR contrast agent is commercial SPIO nanoparticles [21-23]. However, the low efficiency of loading these particles into cells and the cytotoxicity of these particles limit their usage as a tracking stem cells probe [24].
In our laboratory, we have developed a paramagnetic agent called “IronQ” (a complexation of iron and quercetin) for use as a MRI contrast agent. As mentioned above quercetin has beneficial effects on EPCs together with the paramagnetic properties of iron. We hypothesized that IronQ complex may be able to enrich contained EPCs during in vitro expansion as well as able to visualize labeled EPCs with MR imaging. Thus, peripheral blood mononuclear cells were cultured with IronQ complex and compared to those cultures in basic culture medium without a cytokine additive. Expanded cells were characterized with flow cytometric analyses, and their angiogenic potentials were evaluated in vitro with tube formation assay. Moreover, intracellular uptake of IronQ was determined by Prussian blue stain and spectrometric technique.
Visualization of IronQ labeled EPCs was also investigated by in vitro MR imaging.
Materials and Methods
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy human peripheral blood. All donors signed the consent form, and all procedures were approved by the Human Research Ethics Committee of the Faculty of Associated Medical Sciences, Chiang Mai University (ref. no. AMSEC-58EM-005). Whole blood (60 mL) was collected in heparinized tubes (1430 USP units). Fifteen milliliters of whole blood was transferred to a new 50 mL sterile tube, then 15 mL phosphate buffer saline (PBS) was added and gently mixed. Fifteen milliliters of Ficoll-hypaque (Lymphoprep™) was carefully injected into the bottom of the tube prior to centrifugation at 1500 rpm for 30 min. The PBMCs were isolated and washed once with sterile PBS. Then the cells pellets were resuspended in RBC lysing solution for 5 min. After that, the cells were washed twice with sterile PBS and then resuspended in an RPMI1640 medium with L-glutamine supplemented with 10% fetal bovine serum (FBS; Sigma- Aldrich) and 1% penicillin/streptomycin (BioMedia). After that, the flask was placed in an incubator at 37°C in 5% CO2 and 95% humidity.
Cells culture with IronQ
PBMCs (106 cells/mL) were seeded on 6-well plates in 4 mL RPMI 1640 with 10% FBS and 1% penicillin/streptomycin. For the control group, PBMCs were culture in culture medium alone and compared to the treated group. PBMCs were cultured in the presence of 500 μg/mL IronQ complex, and the morphology of PBMCs was monitors under inverted microscope. The fresh medium was changed every 3 days.
Cell proliferation assay
The effect of IronQ on cell proliferation was determined by 3- (4, 5-dimethylthiazol-2-yl)-2, 5-diphynyl-2H-tetrazolium bromide (MTT) assay. Cells (1×106/ml) were seed on 24-well plates (Sigma-Aldrich) in the presence of various concentrations of IronQ for 72 h. To determine the reaction time-dependent, cells were incubated with 500 μg/mL IronQ for 1, 3, 5, 7, and 10 days. At the time point 200 μL MTT (5 mg/mL) was added to each well and cells were further incubated for 4 h. The supernatant were removed and 500 μL of dimethyl sulfoxide (DMSO) was added. When the formazan crystals were dissolved evenly in the DMSO, the light absorption value was measured with a spectrometer (Agilent 8453 UV-visible spectroscopy) using a 550 nm wavelength.
Flow cytometry analysis
For characterization of the EPCs population from IronQ culture, cells were analyzed for endothelial progenitor cell markers (CD34 and CD133). Cells (1 × 106) were briefly centrifuged for 1 min at 7000 rpm at room temperature, resuspended, and washed once with 1 mL ice-cold PBS. The cells were then centrifuged for 1 min at 7000 rpm. The cell pellets were collected and resuspended in 100 μL of ice-cold PBS containing 0.5% BSA and 2 mM EDTA; thereafter, 10 μL of the antibody specific to the membrane proteins labeled with fluorophores, mouse anti-human anti CD34 conjugated FITC (anti-CD34-FITC), and mouse anti-human anti-CD133 conjugated PE (anti-CD133-PE) (Miltenyi Biotec) were added and gently mixed. The cells were then incubated for 10 min at 4°C in darkness; afterward, 400 μL of PBS was added and analyzed using a flow cytometer (Coulter Counter, Epics). Isotype controls were used for all the analyses.
Immunofluorescence staining
IronQ-treated cells were analyzed by immunocytochemistry for the expression of endothelial progenitor cell markers. The specific antibody, anti-CD34 conjugated FITC was used. PBMCs incubated with IronQ for 14 days, the adherent cells were fixed in 4% formaldehyde for 15 min, washed with PBS, and then blocked with 1% BSA in PBS for 1 h. Cells were then incubated anti-CD34-FITC or with anti-CD133-PE 1:100 (Miltenyi Biotec) in 1% BSA in PBS for 1 h. Cells were washed with PBS and further nuclear stain with 4',6- diamidino-2-phenylindole (DAPI). Cells were analyzed by fluorescent microscopy.
In vitro angiogenesis assay
The angiogenic potential was tested in the presence of basement membrane matrix using in vitro angiogenesis assay kit (Abcam) according to the manufacturer’s instructions. Briefly, extracellular matrix solution was placed in a 96-well plate at 37°C for 1 h to allow the matrix solution to solidify.
The PBMCs control and PBMCs treated with IronQ for 14 days were harvested and re-plated (5 × 104 cells/well) on the solidified matrix solution. Cells seeded on Matrigel were incubated in RPMI 1640 medium with L-glutamine supplemented with 10% fetal bovine serum and 1% penicillin/ streptomycin at 37°C and the tubule-like formation was then inspected under an inverted light microscope.
Intracellular uptake of IronQ
To verify the intracellular uptake of IronQ, potassium hexacyanoferate(II) trihydrate (Prussian blue; Merck) was used to stain the iron component in the EPCs. PBMCs were incubated with 500 μg/mL IronQ for 14 days. Then, free IronQ outside cells were removed and cells were washed with PBS for three times. Cells were continuously incubated for 20 min with 2% potassium ferrocyanide in 6% hydrochloric acid, and the iron component was investigated under inverted microscope. The quantification of intracellular iron concentration within the labeled EPCs was performed as previously descripted [25], after labeling with IronQ, the cells were harvested and washed with PBS for three times. Then, cell pellets were incubated at 110°C overnight. The next day, the iron was dissolved by adding 1 mL of 5 M hydrochloric acid, and the samples were further incubated at 60°C for 4 h. Then 0.5 mL of acid solution was transferred to a new tube and 0.5 mL of 2% potassium ferrocyanide was added. After that, the samples were analyzed with spectrometer (Agilent 8453 UV-visible spectroscopy). The analysis process was performed triplicate and the mean value and standard deviation was obtained. IronQ concentration was determined using the standard curve.
In vitro MR imaging
PBMCs were treated with 500 μg/mL IronQ for 14 days, and then cells were detached with 1 mM EDTA in PBS and washed with PBS for three times. Then, IronQ-labeled EPCs cells (2,000 and 20,000 cells/μL) were resuspended in 0.25% agar phantom. In two other tubes, 2,000 cells/μL unlabeled cells were added to one, while the remaining tube to which no cells were added which was designated as the control. MRIs were conducted on a 1.5 T MR scanner (Achieva 1.5T, Philips Medical Systems) with a Knee receiver coils. T1-wieghted image of IronQ-labeled EPC was performed using a spin echo sequence with TE/TR=15/1000 msec. The matrix size was 336 × 269, field of view=210 mm, slice thickness=4 mm, and number of averages=4. The T1-wieghted signal intensity of region of interest (ROI) was readout using Philips DICOM viewer R3.0 SP3. Three ROI were randomly selected and measured in each tube for a mean value.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The statistical analysis was performed by Student’s t-test. Values of p ≤ 0.05 were considered statistically significant at the 5% significance level.
Results
Morphological observation on PBMCs treated IronQ complex
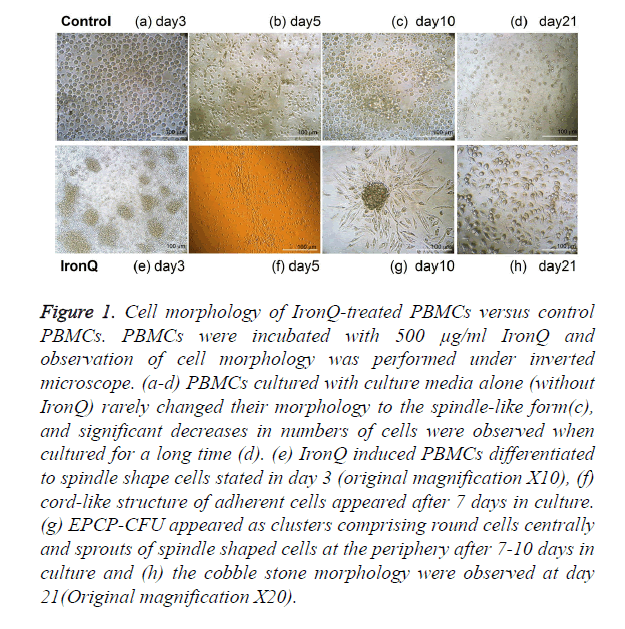
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy human peripheral blood by gradient centrifugation. These populations consisted of endothelial progenitor cells (EPCs), along with leukocytes and monocytes. Upon isolation, cells were expanded under two conditions and compared. One condition was that the PBMCs were cultured under the condition of IronQ complex and the other one was that PBMCs were cultured in RPMI-1640 medium with 10% FBS and 1% penicillin/streptomycin without adding any specific growth factors. The PBMCs cultured in the presence of IronQ complex appeared rapidly proliferation and increased number of attaching cells. After 5-9 days of cultivation, the majority of PBMCs were attached and the cells gradually developed a spindle shape and tended to form cord-like structures (Figure 1f) that lined resembling the first stages of vasculogenesis [1]. The spindle shape cells appeared either as colonies composed of round cells centrally with sprouts of spindle-shape cells at the periphery or as single spindle-shape cells (Figure 1g). On day 21 in the culture, the cells displayed cobblestone-like morphology (Figure 1h) and the cells could be maintained in the culture for up to 30 days. On the other hand, PBMCs cultured with culture media alone (without IronQ) rarely changed their morphology to the spindle-like form (Figure 1c), and significant decreases in numbers of cells were observed when cultured for a long time (Figure 1d).
Figure 1. Cell morphology of IronQ-treated PBMCs versus control PBMCs. PBMCs were incubated with 500 μg/ml IronQ and observation of cell morphology was performed under inverted microscope. (a-d) PBMCs cultured with culture media alone (without IronQ) rarely changed their morphology to the spindle-like form(c), and significant decreases in numbers of cells were observed when cultured for a long time (d). (e) IronQ induced PBMCs differentiated to spindle shape cells stated in day 3 (original magnification X10), (f) cord-like structure of adherent cells appeared after 7 days in culture. (g) EPCP-CFU appeared as clusters comprising round cells centrally and sprouts of spindle shaped cells at the periphery after 7-10 days in culture and (h) the cobble stone morphology were observed at day 21(Original magnification X20).
Characterization of PBMCs treated IronQ complex
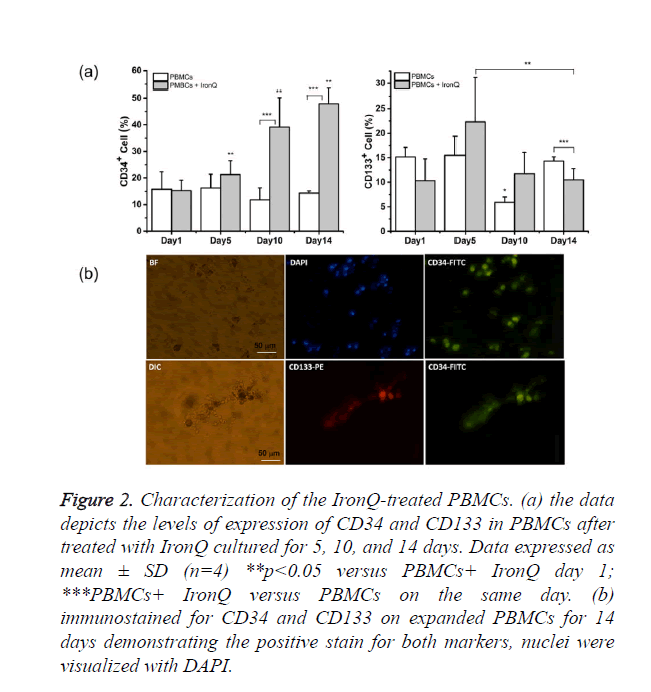
PBMCs pre- and post-treated with IronQ complex were characterized by means of flow cytometric analysis of endothelial progenitor markers; EPCs were characterized as adherent cells expression of CD133, CD34, and VEGF receptor (VEGFR-2) as characterized by others [1,3]. The results were shown in Figure 2a. The expression of CD34 of PBMCs culture with IronQ complex was 15.29 ± 3.3% on day zero and significantly increased within 10 days, representing 39.2 ± 10.9%. There was still an increase of CD34+ expression to 47.9 ± 5.6% after 14 days. CD133 was expressed in 10.5 ± 4.4% of freshly isolated PBMCs. Incubation of PBMCs with IronQ complex for 5 days increased expression of CD133 on PBMCs to 22.3 ± 8.8%, and the expression of CD133 decreased with the increased culture time due to the differentiation of stem/progenitor cells to mature endothelial cells. On the other hand, in non-treated PBMCs, a decreased number of CD34 and CD133 positive cells was observed when the cells were maintained in the culture for 14 days. We further confirmed the expression of CD34/CD133 on the adherent cells after PBMCs treated with IronQ for 14 days by immunocytochemistry. The results showed that cells were strongly positive for CD34 (Figure 2b), while around 5% of the cells population was positive for CD133. These were in line with the flow cytometric analysis that showed the decreased expression of CD133 with increased the culture time.
Figure 2. Characterization of the IronQ-treated PBMCs. (a) the data depicts the levels of expression of CD34 and CD133 in PBMCs after treated with IronQ cultured for 5, 10, and 14 days. Data expressed as mean ± SD (n=4) **p<0.05 versus PBMCs+ IronQ day 1; ***PBMCs+ IronQ versus PBMCs on the same day. (b) immunostained for CD34 and CD133 on expanded PBMCs for 14 days demonstrating the positive stain for both markers, nuclei were visualized with DAPI.
Proliferation of IronQ treated PBMCs
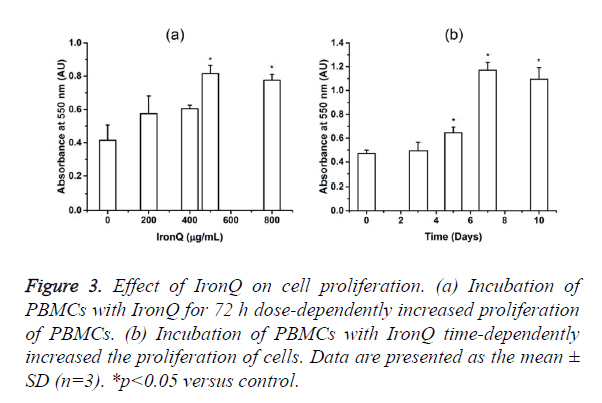
The incubation of PBMCs with IronQ complex increased the number of a spindle-shaped endothelial cell-like morphology and increased the number of CD34/CD133 positive cells. The effect of IronQ complex on EPCs proliferation was investigated using MTT assay. As expected, IronQ complex dose-dependently improved EPC proliferative potential. This effect that was maximal at a dose of 500 μg/mL of IronQ complex was observed. In time-dependent experiments performed with 500 μg/mL of IronQ, EPC proliferative potential increased throughout the 10 days in culture (Figure 3).
Figure 3. Effect of IronQ on cell proliferation. (a) Incubation of PBMCs with IronQ for 72 h dose-dependently increased proliferation of PBMCs. (b) Incubation of PBMCs with IronQ time-dependently increased the proliferation of cells. Data are presented as the mean ± SD (n=3). *p<0.05 versus control.
In vitro angiogenic potential
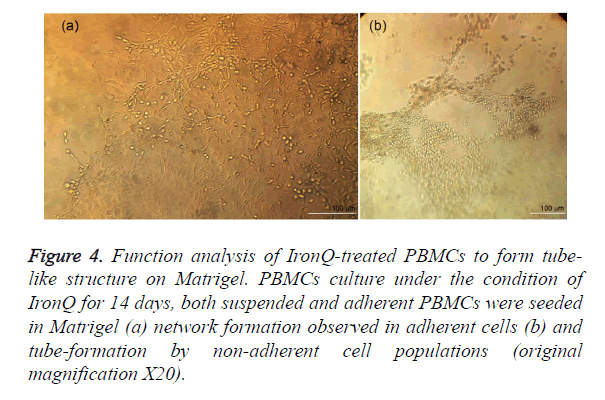
To access the angiogenic potential of the treated PBMC-derived cells. Matrigel tube-forming assay was performed on the PBMCs after incubated with IronQ 500 μg/mL for 14 days. The treated PBMC-derived cells gave rise to tightly adherent cords and capillary-like structure, assembled in branching reticular networks (Figure 4a). While the non-adherent mononuclear cells formed networks of tube (Figure 4b). However, the PBMCs control group was distributed within the extracellular matrix without network creation.
Figure 4. Function analysis of IronQ-treated PBMCs to form tube-like structure on Matrigel. PBMCs culture under the condition of IronQ for 14 days, both suspended and adherent PBMCs were seeded in Matrigel (a) network formation observed in adherent cells (b) and tube-formation by non-adherent cell populations (original magnification X20).
Intracellular iron content determination of IronQ-labeled EPCs
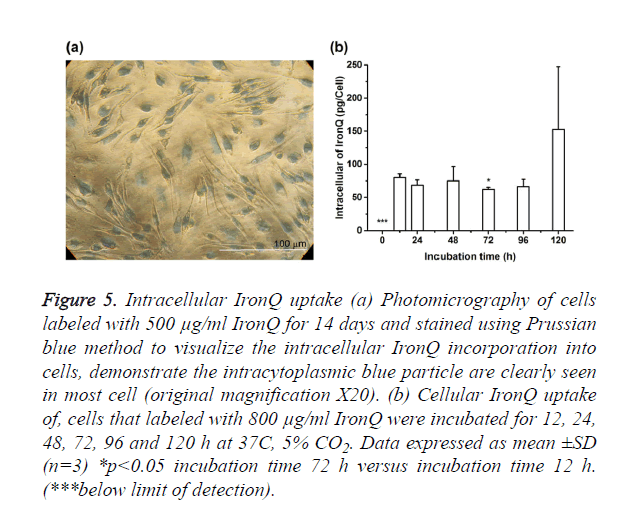
Qualitative analysis intracellular uptake of IronQ was performed on microscopic of labeled cells that were stained with Prussian blue. Figure 5a showed the representative photomicrographs of PBMCs incubated with 500 μg/mL of IronQ for 14 days. Labeled cells exhibited abundant uptake of IronQ complex that showed as blue particles inside the cells on Prussian blue stained slides. Labeling efficiency analysis revealed that more than 99% of the cells were positive for Prussian blue. Furthermore, quantification of cellular IronQ uptake was performed using spectroscopic technique. The spectroscopic measured IronQ content reported per cells as showed in Figure 5b. IronQ content in unlabeled cells was below the detection limit. There was significant uptake of IronQ for an incubation interval of 12 h. The highest uptake was achieved with a longer incubation interval of 120 h with the mean iron concentration about 153 ± 95 pg IronQ/mL/cells.
Figure 5. Intracellular IronQ uptake (a) Photomicrography of cells labeled with 500 μg/ml IronQ for 14 days and stained using Prussian blue method to visualize the intracellular IronQ incorporation into cells, demonstrate the intracytoplasmic blue particle are clearly seen in most cell (original magnification X20). (b) Cellular IronQ uptake of, cells that labeled with 800 μg/ml IronQ were incubated for 12, 24, 48, 72, 96 and 120 h at 37C, 5% CO2. Data expressed as mean ±SD (n=3) *p<0.05 incubation time 72 h versus incubation time 12 h. (***below limit of detection).
MRI detection of labeled EPCs
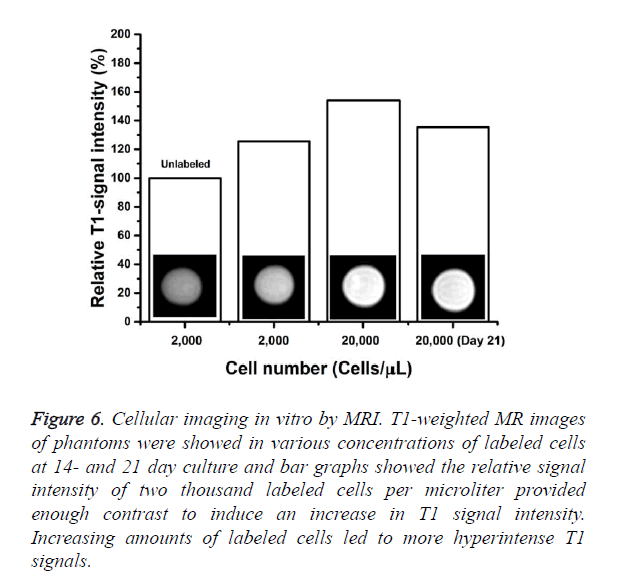
Agar phantoms containing EPCs labeled under the various conditions were studied by MRI to determine detectability limits. The PBMCs were cultured with 500 μg/mL of IronQ complex for 14 and 21 days. The minimum cells density that was still detectable under those conditions was determine in MRI experiments where phantoms were loaded with decreasing numbers of labeled cells (2,000 and 20,000 cells/ μL). The results were shown in Figure 6 the hyperintense contrast in T1-wieghted MR image was markedly modulated compared to unlabeled controls. Increasing the amount of labeled cells resulted in a further increase in signal intensity. Even after continued proliferation for 21 days and subsequent intracellular dilution of the IronQ complex, those magnetically labeled cells were still sufficient for significant increase in MRI signal intensity when compared to unlabeled cells (Figure 6). The relative signal intensities in the T1-weighted images were calculated and plotted. The relative signal intensities of 2,000 cells/μL labeled cells was 1.25 folds and that of the 20,000 labeled cells was 1.54 folds higher than in unlabeled cells. It should be noted that the contrast image became visible when studied with a clinical 1.5 T MR scanner.
Figure 6. Cellular imaging in vitro by MRI. T1-weighted MR images of phantoms were showed in various concentrations of labeled cells at 14- and 21 day culture and bar graphs showed the relative signal intensity of two thousand labeled cells per microliter provided enough contrast to induce an increase in T1 signal intensity. Increasing amounts of labeled cells led to more hyperintense T1 signals.
Discussion
The main obstacle of the usage of autologous EPCs in regenerative cardiovascular medicine is the low number of cells that can be obtained from the peripheral blood or bone marrow. More importantly, the number and functionality of circulating EPCs decline as patients age [8] and in patients with cardiovascular risk factors [9,10]. Thus, the problem caused by the limited number of EPCs must be overcome for success and effectiveness in EPC-based cell therapy in patients with cardiovascular diseases. Here, we demonstrated the feasibility of increasing the limited number of endothelial progenitor cells obtained from non-mobilized peripheral blood mononuclear cells, using a simplified culture method without any cytokine cocktail additive. Using our home-synthesized MR contrast agent “IronQ complex”, PBMCs culture with IronQ complex showed differentiation of cells into spindle-shaped cells with a cell cluster consisting of round cells with radiating elongated spindle-like cells at peripheries and finally turned into cobblestone-like cells. These observations are consistent with the morphological signs of early and late EPCs [26]. According to the PBMCs culture with basic culture medium without IronQ complex, cells rarely developed into spindle shape cells and no colonies were observed. The results indicate that IronQ complex can promote conditions favoring endothelial-specific differentiation. For a better characterization of the expanded cells, the specific surface markers of endothelial progenitor cells were used to stain the IronQ-treated PBMCs, and the percentages of expressing cells to the non-treated PBMCs were compared. The results showed that PBMCs culture under the condition of IronQ complex experienced an increase in number of cells that expressed CD34 and CD133 as compared to PBMCs culture in the basic culture medium (control group).
The increase in number of CD34/CD133 positive cells indicated the expanded population of immature cells. Interestingly, the number of CD34 positive increased as a function of culturing time, while the cells that expressed CD133 reduced in number with the increased time in culture. These results were consistent with a previous study that demonstrated the reduction of CD133 positive cells in more mature endothelial precursor cells [27]. Immunocytochemistry revealed that CD34 and CD133 were positive in PBMCs expanded under IronQ complex conditions. Nevertheless, the expanded cells were more strongly positive to CD34 than CD133. This finding is in line with the results of the flow cytometric analysis. The ability of PBMCs to expand under the condition of IronQ was investigated using MTT assay. The results demonstrated that PBMCs cultured under the condition of IronQ doubled their population number in 7 days of culturing and still increased until days 10, while the number of PBMCs in the control group progressively decreased with the increased time in culture. Moreover, we observed the proliferation of IronQ treated-PBMCs cells remaining in culture for up to 30 days. On the other hand, control PBMCs appeared almost dead (data not shown). We also showed that the expanded cells under condition of IronQ displayed an in vitro angiogenic potential compared with the control group, demonstrated by the tube formation capacity performed on Matrigel. This result suggested the angiogenic defense that the EPCs-IronQ treated cells acquires through the expansion process.
The mechanisms involved in the ability to expand and enrich the progenitor cell populations of IronQ complex are still under investigated. Several studies have reported the effect of quercetin on number and functionality EPCs. Recently, Zhou et al. showed that quercetin enhanced cell proliferation, osteogenic differentiation, and VEGF secretion of bone marrow mesenchymal stem cells via activation of the extracellular signal-regulated protein kinases (ERK) and p38 pathways [28]. In addition, quercetin is known to be able to protect against high glucose-induced damage by inducing Sirt1-dependent eNOS upregulation in EPCs [20]. There are several possible mechanisms by which IronQ complex could increase the number and functionality of EPCs. IronQ may enhance the secretion of VEGF by EPCs, which could regulate EPC differentiation and contribute to the pro-angiogenic effects of these cells. The exact mechanism of IronQ complex should be studied further. In addition, in our culture condition the mix populations of mononuclear cells other than EPCs contained in the PBMCs such as natural killer (NK) cells and lymphocytes can contribute, as well, to ischemic neovascularization by secreting angiogenic cytokine. Thus, the microenvironment may affect to number of EPCs in our culture condition [29,30].
After the transplantation of stem cells, an important question need to be address in preclinical and also clinical research is the localization, migration, and observation of the fate of the transplanted cells. In order to monitor transplant cells, several non-invasive techniques have been investigated [21,31,32]. MR imaging has been proven to be effective in tracking transplanted stem cells by which cells should undergo in vitro tracking with MR contrast agent prior to transplantation. At present, commercial SPIO nanoparticles are widely used for cell labeling. However, the use of SPIO nanoparticles still has some limitations, such as the low efficiency of loading these particles into the cells. Moreover, the cytotoxicity and side effects for cells biological of these particles are still debated [24]. Although MRI cell tracking using SPIOs has now been widely used for tracking transplanted cells in various organs but SPIO-labeled cells cannot be distinguished from other hypointense regions, such as hemorrhage and blood clots, which are common in many lesions. Therefore, alternative tracking methods using “positive” contrast agents have been explored, i.e. gadolinium (Gd)-based complexes that can generate hyperintense regions as a result of their predominant effects on the longitudinal (T1) relaxation time of water protons in tissue [33,34]. Unfortunately, gadolinium-based contrast agents are now associated with nephrogenic systemic fibrosis (NSF), which makes them less favorable agents [35].
In our study, we have established a home-synthesized paramagnetic agent IronQ complex as a positive contrast for T1-wieghted MR image. The prominent characteristic of IronQ was high efficiency of loading into the cells, as shown in the results of Prussian blue stain slide; IronQ-labeled EPCs have an efficiency of loading of more than 99%, and intracellular IronQ content per cell increased with the increase in labeling time. In addition, magnetically labeled EPCs were visualized by a clinical 1.5 T MR scanner when the cell quantity was more than 2,000 cells/μL. Interestingly, the effectiveness to visualize the IronQ labeled cells was still detectable when the labeled cells were in the culture for 21 days. Moreover, IronQ complex exhibited no cytotoxicity. In contrast, IronQ has the effect on increase the proliferation of progenitor cells and induce differentiation of PBMCs to be the spindle shape endothelial-like cells as already mention above. The novel technique which was investigated in this study could be an alternative method for used EPCs in treating cardiovascular diseases. It has the advantage of using paramagnetic agents both to expand the limited number of EPCs from nonmobilized PBMCs without the addition of growth factors and to serve as the magnetic label for MRI in the same time. However, for a better understanding of the mechanism under the effect of IronQ on EPCs, further study is warranted.
Conclusion
In the present study, we prepared a paramagnetic agent IronQ complex and demonstrated that IronQ can promote conditions favoring endothelial-specific differentiation, resulting in an increase in the limited number of EPCs obtained from the PBMCs population. This low-cost, effective, and safe approach of enriching an unlimited source of cells simplifies their use in the clinical setting, especially for autologous use. Moreover, with the dual function of IronQ complex the magnetically labeled cells after being expanded in culture can be visualized and localized by MR imaging. Our study demonstrated that ex vivo expansion of EPCs fraction derived from PBMCs with paramagnetic agent, IronQ complex, might be an alternative method for applied used in cardiovascular medicine.
Acknowledgement
We would like to give thanks to the Office of Commission on Higher Education, Thailand for supporting this research under the Strategic Scholarships for Frontier Research Network of the Joint Ph.D. Research program (Grant no. CHE-PhD-SW 3/2550).
References
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964-967.
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5: 434-438.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85: 221-228.
- Kalka C, Masuda H, Takahashi T, Kalka-Moll W, Silver M. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000; 97: 3422-3427.
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002; 360: 427-435.
- Moriya J, Minamino T, Tateno K, Shimizu N, Kuwabara Y. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Intervent 2009; 2: 245-254.
- Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 2011; 29: 1650-1655.
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 2005; 45: 1441-1448.
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89: E1-E7.
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593-600.
- Kawamoto A, Gwon HC, Iwaguro H,Yamaguchi JI, Uchida S. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001; 103: 634-637.
- Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 2008; 111: 1302-1305.
- Lu Y, Gong Y, Lian J, Wang L, Kretlow JD. Expansion of endothelial progenitor cells in high density dot culture of rat bone marrow cells. PLoS One 2014; 9: e107127.
- Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol 2010; 30: 869-877.
- Wang XB, Zhu L, Huang J, Yin YG, Kong XQ. Resveratrol-induced augmentation of telomerase activity delays senescence of endothelial progenitor cells. Chin Med J 2011; 124: 4310-4315.
- Lucchesi D, Russo R, Gabriele M, Longo V, Del Prato S. Grain and bean lysates improve function of endothelial progenitor cells from human peripheral blood: involvement of the endogenous antioxidant defenses. PLoS One 2014; 9: e109298.
- Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer's disease. J Nutr Biochem 2009; 20: 269-275.
- Boydens C, Pauwels B, Vanden Daele L, Van de Voorde J. Protective effect of resveratrol and quercetin on in vitro-induced diabetic mouse corpus cavernosum. Cardiovasc Diabetol 2016; 15: 46.
- Sánchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, et al.. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens 2006; 24: 75-84.
- Zhao LR, Du YJ, Chen L, Liu ZG, Pan YH. Quercetin protects against high glucose-induced damage in bone marrow-derived endothelial progenitor cells. Int J Mol Med 2014; 34: 1025-1031.
- Hu SL, Lu PG, Zhang LJ, Li F, Chen Z. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem 2012; 113: 1005-1012.
- Mai XL, Ma ZL, Sun JH, Ju SH, Ma M. Assessments of proliferation capacity and viability of New Zealand rabbit peripheral blood endothelial progenitor cells labeled with superparamagnetic particles. Cell Transplan 2009; 18: 171-181.
- Janic B, Jafari-Khouzani K, Babajani-Feremi A, Iskander ASM, Verma NR. MRI tracking of FePro labeled fresh and cryopreserved long term in vitro expanded human cord blood AC133+ endothelial progenitor cells in rat glioma. PLoS ONE 2012; 7: e37577.
- Crabbe A, Vandeputte C, Dresselaers T, Sacido AA, Verdugo JM. Effects of MRI contrast agents on the stem cell phenotype. Cell Transplant 2010; 19: 919-936.
- Janic B, Rad AM, Jordan EK, Iskander AS, Ali MM, et al.. Optimization and validation of FePro cell labeling method. PLoS One 2009; 4: e5873.
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004; 24: 288-293.
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identi?es a population of functional endothelial precursors. Blood 2000; 95: 952-958.
- Zhou Y, Wu Y, Jiang X, Zhang X, Xia L. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal cells. PLoS ONE 2015; 10: e0129605.
- Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol 2007; 178: 6482-6494.
- Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K. Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro. Haematologica 2010; 95: 542-550.
- Wang X1, Rosol M, Ge S, Peterson D, McNamara G. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging. Blood 2003; 102: 3478-3482.
- Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 2003; 107: 2134-2139.
- Aime S, Delli Castelli D, Lawson D, Terreno E. Gd-loaded liposomes as T1, susceptibility, and CEST agents, all in one. J Am Chem Soc 2007; 129: 2430-2431.
- Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS. Novel MRI contrast agent for molecular imaging of fibrin implications for detecting vulnerable plaques. Circulation 2001; 104: 1280-1285.
- Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment--report of 33 cases. 2009; Radiology 250: 371-377.