Original Article - International Journal of Respiratory Medicine (2017) International Journal of Respiratory Medicine (Special Issue 1-2017)
Evolution of uncontrolled severe asthma under Omalizumab and specialized care unit treatment.
Alberto Levy-Nahon1-4, Lorena Piñel-Jiménez1*, Jesus Ramirez-Rodrigo4, Lucia Prieto4, Victoria Hidalgo-Sanjuan M1 and Pedro Valdivielso2-3
1Pneumology Service, University Hospital ‘Virgen de la Victoria’, Málaga, Spain
2Internal Medicine Service, University Hospital ‘Virgen de la Victoria’ Málaga, Spain
3Department of Medicine and Dermatology, University of Málaga, Spain
4Department of Nursing, Faculty of Health Sciences, University of Granada (in Ceuta), Spain
- *Corresponding Author:
- Lorena Piñel Jiménez
Pneumology Service University Hospital
‘Virgen de la Victoria’ Málaga, Spain.
Tel: +34622200763
E-mail: lorenapinel@gmail.com
Accepted date: June 15, 2017
Citation: Levy-Nahon A, Piñel-Jiménez L, Ramirez-Rodrigo J, et al. Evolution of uncontrolled severe asthma under Omalizumab and specialized care unit treatment. Int J Respir Med. 2017; 2(1): 1-5
Abstract
Severe uncontrolled asthma is a disabling and life-threatening disorder. This article evaluates the effectiveness of combining a specialized care clinic treatment with the prescription of Omalizumab (OML) when treating these patients. We analyzed 45 patients with severe uncontrolled asthma followed for 12 months, of which 30 had elevated levels of IgE and received omalizumab dose adjusted for weight and IgE levels. The overall effectiveness was measured through parameters such as emergency room visits (ER), the evolution of forced expiratory volume in one second (FEV1), the need for high-dose corticosteroids; Fractional exhaled nitric oxide (FENO) and a quality of life survey. At three, six and twelve months, all patients improved significantly in all measured parameters (p<0.05), except FENO, as a result of their treatment in the specialist office. The changes were even more favorable for patients who received OML in addition to the visits to the specialized care clinic, showing a significant interaction between drug use and clinical visit factors. Our results confirm that the benefits obtained in randomized clinical trials with OML and the specialist care of patients with severe asthma result in a significant clinical benefit in the context of everyday clinical practice.
Introduction
Bronchial asthma is one of the most frequent respiratory disorders, affecting 5% of the adult Spanish population [1], although its prevalence appears to be levelling off among adolescents [2]. The management of asthmatic patients is widespread among general practitioners, allergists and pneumologists, although the most severe forms prove challenging for professionals due to the associated disability, reduced quality of life and treatment costs.
The prevalence of severe uncontrolled asthma is not negligible; a recent study that recorded information from 164 hospital units in Spain over 6 months, showed that 666 (65.9%) patients among 36,649 adult asthmatics, met criteria for uncontrolled severe chronic asthma according to GINA criteria [3]. For this and other reasons, the creation of specific asthma units has led to improved assistance, a rational use of medical resources and the use of specific biologic therapies for this disease [4,5]. In this sense, the humanized monoclonal anti-IgE antibody Omalizumab (OML) is marketed in Europe since October 2005 and is indicated for the treatment of uncontrolled severe persistent asthma with high IgE levels. The use of OML in this type of patients has resulted in a reduced number of exacerbations while decreasing steroids use and improving the quality of life when compared to placebo (as shown in a recent systematic review of 6 randomized clinical trials [6]).
The aim of this study was to evaluate retrospectively the efficacy of an asthma specialist office in the clinical management of patients with uncontrolled severe persistent asthma and the potential benefit of treatment with OML.
Materials and Methods
We retrospectively analyzed medical records of patients with uncontrolled severe asthma treated between January 2008 and October 2009 at the Asthma office of the University Hospital "Virgen de la Victoria" in Malaga and selected those that met the following inclusion criteria: age over 14 years, clinical and functional diagnosis of uncontrolled severe persistent asthma [7] with more than 1 year follow-up and having completed at least the follow-up visits at 3, 6 and 12 months. We excluded smokers or patients with >1 year smoking history, pregnancy, comorbidities such as intercurrent infections or active neoplasia, and the presence of chronic obstructive pulmonary disease (COPD) or overlap syndrome according to GOLD criteria [8].
At the first visit, information on age, sex, anthropometric parameters, smoking habits, comorbidities, other clinical forms of atopy, and medications in used were collected, as well as a chest X-ray and an ECG. Additionally, a blood sample was performed for measuring blood count and total serum IgE by radioallergosorbent test (RAST). Moreover, during the first and subsequent visits (3, 6 and 12 months), data was collected on percentage of predicted forced expiratory volume in 1 second (FEV1 %), the number of visits to the emergency room (ER) in the previous three months, the inflammatory activity of the airways estimated as fractional expiratory nitric oxide (FENO), the anti-inflammatory treatment needs and a test of quality of life. Spirometry was performed with a portable spirometer (ESPIROBANK 2, Medical International Research, Waukerna, WI, USA), following the recommendations for this purpose by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and using the recognized normal range for the Spanish population [9]. The FENO was measured by chemiluminescence, using a portable device (MIOX-MIN, Aerocrine, Solna, Sweden). For the quality of life survey we used the Mini asthma Quality of Life Questionnaire (mQLQ) [10], generously made available by Dr. E. Juniper. We defined as "clinically important difference" the minimum score difference that the patient perceives as decisive and which, in the absence of side effects and / or exacerbation, would justify a change in the clinical management of the patient. A change of 0.5 in total questionnaire score is equivalent to a "clinically important change." Differences of 1.0 represent a moderate change and >1.5 represent large changes [11].
The anti-inflammatory treatment needs were analyzed by assigning the following scores: 1=inhaled corticosteroids (ICS) at medium doses; 2=high-dose ICS; 3=long-acting β2-receptor agonists (LABAs) plus medium-dose ICS; 4=LABAs + highdose ICS; and 5=LABAs + high-dose ICS + oral corticosteroids. Patients were treated following the recommendations of the GINA 2015; thus, patients with total IgE levels >100 IU were treated with OML, administered subcutaneously at a dose adjusted for weight and IgE levels, as indicated [12]. Statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc, Michigan, USA). Variables are shown as mean ± SD or as a percentage (%). For each group, the variation along the follow-up period was estimated by repeating the analysis of variance. Significant differences between groups at each visit and interaction between the 'visit' and the 'OML' factor variables were tested using respectively the Mann-Whitney test and the Friedman test.
As this is a retrospective analysis of clinical practice and the data was anonymized once recorded, informed consent was not required. The study received approval from the ethics committee of the center.
Results
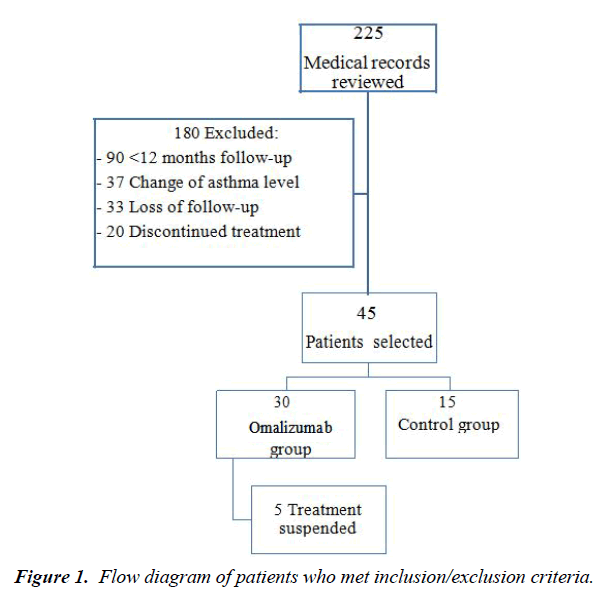
Of 225 patients medical records reviewed, 180 were excluded from further analysis according to the previous determined criteria: 90 had less than 12 months of follow-up, 37 changes the asthma level at visit 2, 33 were lost during the follow-up and 20 because of treatment discontinuation. The final group therefore consists of 45 patients, of whom 30 had been treated with Omalizumab due to basal levels of total IgE >100 IU. Only 5 patients treated with OML were rejected from the analysis; 3 due to treatment non adherence and 2 as a result of adverse effects (diarrhea in one case and alopecia in one case) (Figure 1).
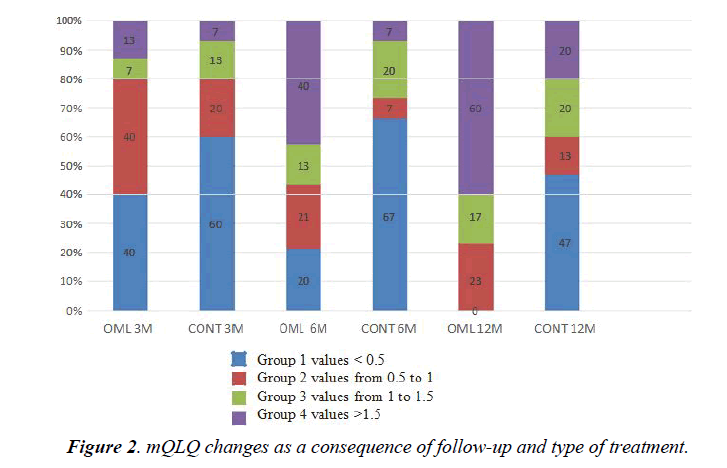
Patient baseline characteristics are shown in (Table 1), which highlights that at baseline the only difference between the groups was total IgE levels. (Table 2) shows the changes in FEV1, quality of life, FENO, anti-inflammatory treatment needs and number of visits to the ER in both groups during the followup. There was a statistically significant improvement (p<0.05) in both groups in all variables except the FENO. About the number of visits to the ER, both groups improved significantly, without differences between them (from 4.6 ± 4.5 in OML group and 3 ± 1.9 in control group to 0.2 after 12 months of followup). On the contrary, improvement in FEV1% and quality of life questionnaire was greater in the group receiving OML than in the control group, although it is noteworthy that there was a significant interaction between the variables “treatment group” and “specialized office effect”. When we categorized the changes in quality of life from 1 (change <0.5) to 4 (change >1.5), we observed that the group treated with OML had a higher percentage of patients in category 4 than the control group (Figure 2).
| Variables | Total | Omalizumab group | Control group | Statistical significance |
|---|---|---|---|---|
| N = 45 | N = 30 | N = 15 | ||
| Male sex No (%) | 31 -68.9 | 22 -71 | 9 (60) | |
| Age (years) | 54 ± 14 | 55 ± 12 | 54 ± 17 | NS |
| BMI (Kg/m2) mean ± SD |
31 ± 7 | 31 ± 7 | 32 ± 8 | NS |
| Asthma evolution (years) mean ± SD |
25 ± 17 | 26 ± 19 | 23 ± 14 | NS |
| Total IgE (IU) mean ± SD |
269 ± 274 | 378 ± 276 | 50 ± 32 | <0.05 |
| Clinical forms of Atopy No (%) | ||||
| - Rhinitis | - 26 (57.8) | - 18 (69.2) | - 8 (30.8) | |
| - Conjunctivitis | - 32 (71.1) | - 18 (56.3) | - 14 (43.7) | |
| - Dermatitis | - 1 (2.2) | - 1 (100) | - 0 | |
BMI: Body Mass Index; NS: No Statistically Significant; IU: International Unit; SD: Standard Deviation
Table 1. Characteristics of the patients at base-line visit.
| FEV1 (%)d | Baseline Mean ±SD | 3 Months Mean ±SD | 6 Months Mean ±SD | 12 Months Mean ±SD | Analysis of variance for repeated measurements | |
| OML | 54± 10 | 66± 17a | 72 ± 16ab | 75 ± 16abc | <0.05 | |
| CONT | 53± 9 | 62± 17a | 64 ± 14a | 64 ± 13a | <0.05 | |
| FENO (PPM) | OML | 40± 32 | 35 ± 33 | 30 ± 22 | 30 ± 22 | NS |
| CONT | 35± 43 | 29 ± 22 | 28 ± 20 | 27 ± 19 | NS | |
| Emergency room visits | OML | 4.6± 4.5 | 1.0 ± 1.9a | 0.2 ± 0.5ab | 0.2 ± 0.4ab | <0.05 |
| CONT | 3.0± 1.9 | 1.0 ± 1.2a | 0.5 ± 1.1a | 0.2 ± 0.6ab | <0.05 | |
| mQLQd | OML | 29 ± 8 | 38 ± 12a | 50 ± 15ab | 57 ± 14abc | <0.05 |
| CONT | 26 ± 7 | 33 ± 14a | 34 ± 15a | 39 ± 14ab | <0.05 | |
| Anti-inflammatory treatment needs | OML | 4.4± 1.4 | 4 ± 1.5 | 4 ± 1.5a | 4 ± 1.4ab | <0.05 |
| CONT | 4.9 ±1.9 | 4.7 ± 1.8 | 4.1 ± 1.5a | 4 ± 1.4ab | <0.05 |
Table 2. Parameters changes according to treatment group and follow-up time.
Discussion
Although most asthma patients receive treatment, a considerable proportion of them are only partially or poorly controlled [13]. The National Asthma Education and Prevention Program (NAEPP) recommends that patients who are receiving mediumdose ICS + LABA (i.e., step 4 of care or higher) require a consultation with an asthma specialist [14,15]. However, only 22% of asthma patients in the U.S. are treated by pneumonologists or allergists [16]. In Spain, 71% of patients with asthma treated by primary care physicians and 55% of patients treated at pneumonology and allergy specialist clinics, are poorly controlled [17,18]. In this context, our study shows that severe persistent asthma patients clearly benefit from inclusion in a specialized third-level asthma office. Thus, the control group, which did not receive biological therapy, reduced with statistical significance the number of ER visits as well as the need for anti-inflammatory medication. This was coupled with the also significant improvement of the FEV1% and the quality of life, assessed at the 6 and 12 months follow-up. The reasons behind why visiting a specialist is more beneficial than applying the usual care are related to better implementation of international guidelines, a reduction of the variance in clinical practice and application of techniques for disease control and cutting-edge treatments [5], as well as better patient treatment adherence [19].
As expected, patients with IgE-mediated asthma who received OML showed a marked improvement in FEV1%, number of emergency room visits, quality of life and number of antiinflammatory treatments. We want to emphasize that in the case of the FEV1% and the quality of life mini-survey, the improvement at 6 and 12 months was even greater in the control group which did not receive biological treatment. None of this is a surprise; some randomized clinical trials [20,21] have shown how the personalized follow-up of patients with uncontrolled persistent asthma leads to an improvement of their evolution [22,23], as was the case of our patients. The design of this study, to assess the monitoring of patients with and without biological treatment, allows us to highlight the relevance of the interaction between the follow-up in a specialist office and OML treatment. The main limitations of this study are its retrospective nature and small sample size due to it being limited to a single hospital and to the use of a number of exclusion criteria. We do not analyze the evolution of those two subjects who, although being eligible for OML treatment, did not tolerate it. In summary, our study confirms that the establishment of monographic asthma offices and Omalizumab treatment in selected patients are beneficial for patients with persistent uncontrolled asthma.
References
- Martinez-Moratalla J, Almar E, Sunyer J, et al. European Asthma Study. Identifying and treating young adults with epidemiological criteria for asthma in five areas of Spain. Spanish Group of the European Asthma Study. Arch Bronconeumol 1999;35:223-8.
- Garcia-Marcos L, Quiros AB, Hernandez GG, et al. Stabilization of asthma prevalence among adolescents and increase among schoolchildren (ISAAC phases I and III) in Spain. Allergy 2004;59:1301-7.
- Quirce S, Plaza V, Picado C, et al. Prevalence of uncontrolled severe persistent asthma in pneumology and allergy hospital units in Spain. J Investig Allergol Clin Immunol 2011;21:466-71.
- Cisneros C, Antón E, Casanova A, et al. Características clínico-funcionales de los pacientes asmáticos en una consulta monográfica de neumología. Revista de Patología Respiratoria 2006;9:5-9.
- Domingo Ribas C. Effectiveness and efficiency of an outpatient clinic for corticosteroiddependent asthmatics. Arch Bronconeumol 2001;37:274-80.
- Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 2011;139:28-35.
- GINA. Global Initiative for Asthma. Global strategy for asthma management and prevention – Update 2015. www.ginasthma.com
- Fabbri LM, Hurd SS. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
- Roca J, Sanchis J, Agusti-Vidal A, et al. Spirometric reference values from a Mediterranean population. Bull Eur Physiopathol Respir 1986;22:217-24.
- Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32-8.
- Ferrer M, Alonso I. Cuestionario de Calidad de Vida en Asma – Versión Reducida (Mini AQLQ). 2012.
- Marcus P. Incorporating Anti-IgE (Omalizumab) Therapy Into Pulmonary Medicine Practice. Chest 2006;129:466-74.
- Backer V, Bornemann M, Knudsen D, et al. Scheduled asthma management in general practice generally improves asthma control in those who attend. Respir Med 2012;106:635-41.
- National Asthma Education and Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120: S94-138.
- Urbano FL. Review of the NAEPP 2007 Expert Panel Report (EPR-3) on Asthma Diagnosis and Treatment Guidelines. J Manag Care Pharm 2008;14:41-9.
- Murphy KR, Meltzer EO, Blaiss MS, et al. Asthma management and control in the United States: Results of the 2009 Asthma insight and management survey. Allergy Asthma Proc 2012;33:54-64.
- Diez Jde M, Barcina C, Munoz M, et al. Control of persistent asthma in Spain: associated factors. J Asthma 2008;45:740-6.
- Hermosa JL, Sanchez CB, Rubio MC, et al. Factors associated with the control of severe asthma. J Asthma 2010;47:124-30.
- Weinstein AG. The potential of asthma adherence management to enhance asthma guidelines. Ann Allergy Asthma Immunol 2011;106:283-91.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16.
- Bardelas J, Figliomeni M, Kianifard F, et al. A 26-Week, Randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma 2012;49:144-52.
- Korn S, Schumann C, Kropf C, et al. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol 2010;105:313-9.
- Schumann C, Kropf C, Wibmer T, et al. Omalizumab in patients with severe asthma: The exclusive study. Clin Respir J 2012;6:215-27.