Research Article - Biomedical Research (2017) Volume 28, Issue 12
Evolution of cerebrovascular changes in mouse cortex during normal pregnancy by in vivo two-photon imaging
Changbo Jin1,2,3#, Xinjia Han1,2,3#, Jinying Yang2 and Huishu Liu1,2*1Guangzhou Medical University, Guangzhou, PR China
2Department of Obstetrics, Guangzhou Women and Children's Medical Center, PR China
3Guangzhou Institute of Pediatrics, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, PR China
#These authors contributed equally to this paper
- *Corresponding Author:
- Huishu Liu
Department of Obstetrics
Guangzhou Women and Children’s Medical Center, PR China
Accepted date: April 24, 2017
Abstract
Past studies have shown that cerebral autoregulation is a physiologic process that maintains blood flow at an appropriate level. Impairment of cerebral autoregulation during pregnancy may contribute to further diseases like preeclampsia and eclampsia. However, little is known about the normative cerebrovascular adaptation during pregnancy. Two photon microscopy has become an important tool for living brain function research due to its deeper detection, higher resolution, lower photodamage compared to wide-field and confocal microscopy. In the present study we observed adaptive changes in the blood flow and vessel diameter from prepregnancy to late pregnancy by in vivo two-photon imaging. 12 female C57 mice were randomly divided into four groups: non-pregnancy, early-pregnancy, middlepregnancy, late pregnancy. A thinned skull window was made over the motor cortex. One day later, Rhodamin B was injected intraperitoneally to label the vessels 15 minutes before beginning the imaging experiments. The animal was anesthetized and placed under two photon microscope to observe; blood flow signal pictures were taken using line-scan and analysed by blood flow analysis software programmed. The blood flow velocity of arteriovenous vessels and capillaries increased significantly in middle and late pregnancy compared to non-pregnancy and early-pregnancy; vascular diameters in middle and late pregnancy also became bigger than that in non-pregnancy and early-pregnancy. Both the blood flow velocity and diameter of cortical micro vessels increased significantly from nonpregnancy and early-pregnancy to middle and late pregnancy. Such results demonstrated a progressive cerebrovascular adaptation from prepregnancy to late pregnancy.
Keywords
Pregnancy, Blood flow velocity, Vascular diameters, Two photon.
Introduction
Normal pregnancy is a state of physiological adaptation [1]. Changes in cardiovascular, renal, immune, and endocrine systems during pregnancy are necessary and important for maternal health, normal growth and development of the fetalplacenta unit [2]. Systemically, pregnancy is a high volume, low resistance state characterized by a large increase in cardiac output response to high levels of circulating hormones during the course of gestation [3]; Plasma volume also increases 40%-50% during pregnancy [4]. In addition, organs like uterus, kidney and heart undergo substantial increases in blood flow during pregnancy [5]. However, the adaptation of the brain to pregnancy is distinct from other organs. The brain is an organ of high metabolic demand that consumes 20% of the body’s oxygen at rest, despite comprising only 2% of body weight [6]. Importantly, the brain has a relatively narrow capacity to tolerate changes in ion and water balance, and blood flow [6]. So the adaptation of the cerebral circulation during pregnancy appears to be to maintain a constant blood supply and the relative intolerance to increased blood volume. But the cerebral circulation is not unchanged during pregnancy.
Presently, the adaptations of the cerebral circulation to pregnancy and the related mechanism have not been understood clearly. It has been difficult to assess how CBF change during pregnancy in human. In recent studies, several non-invasive techniques such as Transcranial Doppler (TCD) ultrasound, Computed Tomography Perfusion (CTP), Magnetic Resonance Imaging (MRI), have been widely employed in patients to measure cerebral blood flow [7]. However, changes in blood flow calculated from TCD studies may not accurately reflect CBF due to the lack of information about vessel diameter [8]. In addition, all the above techniques have been rarely used to study cerebral hemodynamics in experimental animals. Due to the nonlinear optical effects of two-photon absorption in biological tissue, the two photon microscopy technique allowed researchers to observe single vessels in the cerebral cortex of living brain [9]. Two photon imaging has a deeper penetration over several hundred micrometers of depth, higher resolution, more concentrated space focus, less tissue damage compared to laser confocal microscope and other conventional single photon microscopes.
During pregnancy, approximately 2-8% of women have some form of hypertensive disorder such as preeclampsia [10]. Preeclampsia accounts for 16%-38% of all maternal deaths [11], Clinical imaging of preeclampsia demonstrated brain edema and cerebrovascular hemorrhage [12-15], the cerebral circulation has been suggested to play a central role in neurological complications of preeclampsia, and thus understanding how pregnancy and preeclampsia affect the cerebrovasculature is of interest [15,16]. Therefore, preeclampsia is thought to be a hypertensive encephalopathy. Investigating how cerebral hemodynamics change during pregnancy including microvascular structure, may provide important mechanisms underlying preeclampsia. Understanding how normal pregnancy affects the cerebral vascular environment is important for neurological conditions such as preeclampisa. Therefore, the present study aimed to investigate dynamic changes in the blood flow and vessel diameter from prepregnancy to late pregnancy by in vivo twophoton imaging.
Materials and Methods
Animals
A total of 12 female adult c57 mice (weight 20-22 g, 10-12 weeks) were obtained from experimental animal center of southern medical university (Guangzhou, Guangdong, China), the mice were acclimatized to the laboratory conditions for 1 week prior to the start of the experiments. Each mouse used in all the pregnancy groups was separately mated overnight. Day 0 of pregnancy was defined as the day when a sperm plug was found in a vaginal smear [17]. Animals were grouped as following: the non-pregnant group (n=3), early-pregnant group (n=3, day=3), middle-pregnant group (n=3, day=10), late-- pregnant group (n=3, day=17). Experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Guangzhou Medical University (Permit Number: 2012-50) in accordance with the NIH Guidelines (NIH Publications No. 8023, revised 1978) for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used as well as their suffering.
Thinned-skull window preparation for in vivo imaging
To make a cranial window for imaging, mice were anaesthetized with 1.5% tribromoethanol (20 ml/kg, i.p.), the most part of brain skin was removed to expose the skull. The position of window was 2.0 mm posterior to the bregma and 2.0 mm lateral from the middle line. At the beginning, a micro drill (Ideal, Cat# SN3150) was used to thin a 1-2 mm diameter circular skull region, after removing the majority of the spongy bone, remaining concentric cavities within the bone can usually be seen under the dissecting microscope, indicating that drilling is approaching the internal compact bone layer. At this stage, skull thickness should still be more than 50 μm. Then a 10# surgical blade was used to slowly and carefully thin the skull until the blood vessels on the cerebral cortex were clearly visible under a light microscope (a ~20 μm preparation). During the whole thinning process, 0.9% physiological saline was added to the skull surface from time to time to reduce heat. After the thinned skull became thoroughly dry, a small drop of thin glue (TED PELLA, INC. Cat#1003) was applied and a small piece of cover glass (about 1-1.5 × 1-1.5 mm size) was glued onto the thinned skull. The remaining area of the skull was covered with a layer of the thin glue. The mice were allowed to recover for least 1 day before imaging.
Two-photon imaging
The mice were anaesthetized with 1.5% tribromoethanol (20 ml/kg, i.p.) and received an intraperitoneal injection of 0.1 ml Rhodamin B sothiocyanate-dextran solution (70 KDa, 3 mg/ml in distill water) at least 15 min before imaging to label the blood plasma. The mice were placed on a head holder (Narishigie, Cat#SG-4N) and the head position was adjusted so that the cortical surface was in a horizontal position to the microscope objective. The mice were placed onto of a healing pad to maintain the body temperature at 37°C, and imaging was made with a two photon microscopy. We used 870 nm excitation wavelength (Maitai DeepSee, 10 RP52-2, Newport, USA). A 20X objective lens was used to image individual blood vessels and take line scan images along with the central axis of a vessel at 3X optical zoom. Each session of line scan consisted 5000 lines. The line scan parameters were automatically recorded in the ZEN software.
Analysis of blood flow and vessel diameter
A program in MatLab written by Dr Xiao Ming Jin and his student was used for calculating the velocity of Red Blood Cells (RBC) in cerebral blood vessels according to the line scan parameters. The vessel diameter was measured by NIH Image J.
Statistical analysis
A one-way Analysis of Variance (ANOVA) was used to compare the average values between the different groups, which was followed by an appropriate Least Significant Difference (LSD) post hoc test (homogeneity test of variance is neat) or else Dunnett's test (homogeneity test of variance is not neat). Values were presented as Mean ± SE and statistical significance was denoted as P<0.05.
Results
The morphologies, blood flow signals and blood flow velocity of Rhodamin stained microvessels
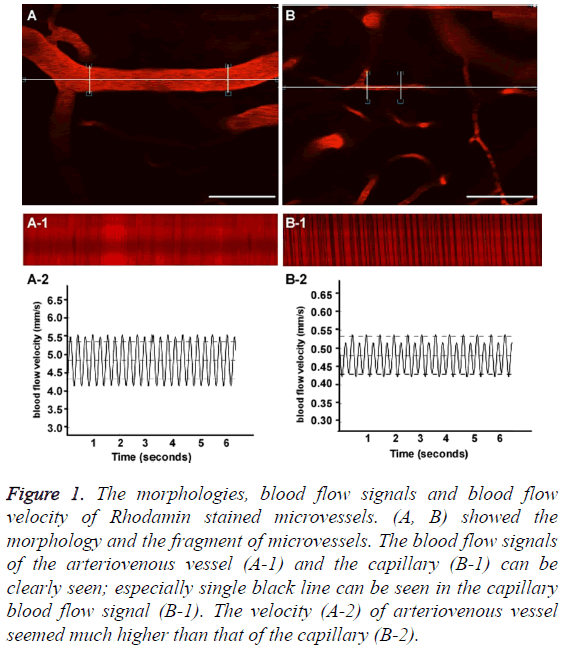
Figures 1A and 1B showed the morphology and the fragment of an arteriovenous vessel and a capillary. The blood flow signals of the arteriovenous vessel (Figure 1A-1) and the capillary (Figure 1B-1) can be clearly seen; especially single black line can be seen in the capillary blood flow signal (Figure 1B-1), as we all know the capillary allows only one blood cell to go across, single black line indicates one blood cell. The velocity (Figure 1A-2) of arteriovenous vessel seemed much higher than that of the capillary (Figure 1B-2). Scale bar=50 μm.
Figure 1: The morphologies, blood flow signals and blood flow velocity of Rhodamin stained microvessels. (A, B) showed the morphology and the fragment of microvessels. The blood flow signals of the arteriovenous vessel (A-1) and the capillary (B-1) can be clearly seen; especially single black line can be seen in the capillary blood flow signal (B-1). The velocity (A-2) of arteriovenous vessel seemed much higher than that of the capillary (B-2).
Changes in blood flow velocity from non-pregnancy to late-pregnancy
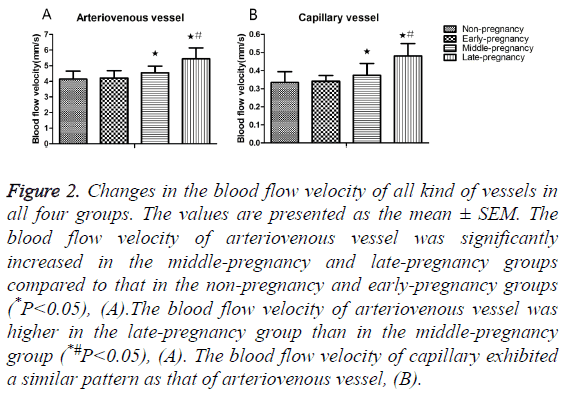
300 arteriovenous vessels or capillaries in each group were selected at random for the measurement of velocity. Quantitative analysis demonstrated that the blood velocity of arteriovenous vessels significantly increased in the middlepregnancy group (4.54 ± 0.44 mm/s) compared to the values in the non-pregnancy (4.14 ± 0.51 mm/s) and early-pregnancy (4.21 ± 0.47 mm/s) groups (P<0.05, Table 1); the blood velocity of arteriovenous vessels in the late-pregnancy group (5.44 ± 0.70 mm/s) was significantly higher than in the nonpregnancy, early-pregnancy and middle-pregnancy groups (P<0.05), Figure 2A. The velocity of capillaries in the latepregnancy group (0.48 ± 0.07 mm/s) also significantly increased compared to the values in the non-pregnancy (0.33 ± 0.06 mm/s), early-pregnancy (0.34 ± 0.03 mm/s) and middlepregnancy (0.37 ± 0.07 mm/s) groups (P<0.05), Figure 2B.
| Group (n) | Number (n) | Non-pregnancy (mm/s) | Early-pregnancy (mm/s) | Middle-pregnancy (mm/s) | Late-pregnancy (mm/s) |
|---|---|---|---|---|---|
| Arteriovenous vessel | 1200 | 4.14 ± 0.33 | 4.21 ± 0.41 | 4.54 ± 0.29 | 5.44 ± 0.58# |
| Capillary vessel | 1200 | 0.33 ± 0.12 | 0.34 ± 0.09 | 0.37 ± 0.07 | 0.48 ± 0.10# |
#p<0.05, compared with the Non-pregnancy group; Early-pregnancy group and Middle-pregnancy group
Table 1: Blood flow velocity during pregnancy.
Figure 2: Changes in the blood flow velocity of all kind of vessels in all four groups. The values are presented as the mean ± SEM. The blood flow velocity of arteriovenous vessel was significantly increased in the middle-pregnancy and late-pregnancy groups compared to that in the non-pregnancy and early-pregnancy groups (*P<0.05), (A).The blood flow velocity of arteriovenous vessel was higher in the late-pregnancy group than in the middle-pregnancy group (*#P<0.05), (A). The blood flow velocity of capillary exhibited a similar pattern as that of arteriovenous vessel, (B).
Changes in morphology of vessels from nonpregnancy to late-pregnancy
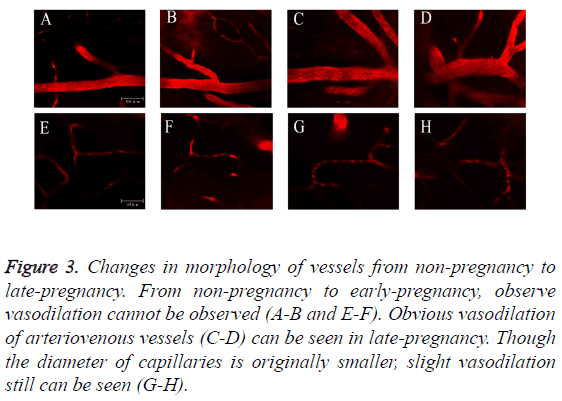
From non-pregnancy to early-pregnancy, we didn’t observe vasodilation (Figures 3A, 3B, 3E and 3F). Obvious vasodilation of arteriovenous vessels (Figures 3C and 3D) can be seen in late-pregnancy. Though the diameter of capillaries is originally smaller, slight vasodilation still can be seen (Figures 3G and 3H). Figures 3A-3D: Scale bar=50 μm. Figures 3E-3H: Scale bar=20 μm.
Figure 3: Changes in morphology of vessels from non-pregnancy to late-pregnancy. From non-pregnancy to early-pregnancy, observe vasodilation cannot be observed (A-B and E-F). Obvious vasodilation of arteriovenous vessels (C-D) can be seen in late-pregnancy. Though the diameter of capillaries is originally smaller, slight vasodilation still can be seen (G-H).
Changes in vessel diameter from non-pregnancy to late-pregnancy
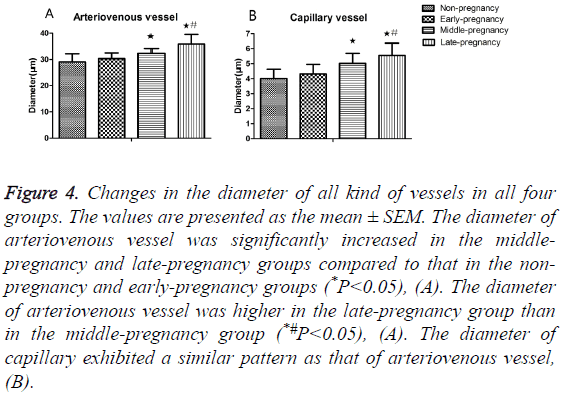
300 arteriovenous vessels and 300 capillaries in each group were selected at random for the measurement of diameter. The Image J was used to measure the diameter by using the parameters which were corresponding with the morphological pictures. Statistical analysis revealed that the diameter of arteriovenous vessel significantly increased in the middlepregnancy (32.36 ± 1.82 μm) and late-pregnancy (35.88 ± 3.64 μm) groups than in the non-pregnancy (29.05 ± 3.13 μm), early-pregnancy (30.29 ± 2.18 μm) groups (P<0.05, Table 2); the diameter of arteriovenous vessel in the late-pregnancy group also became bigger compared to that in the middlepregnancy group (P<0.05), Figure 4A. The diameter of capillary also increased significantly middle-pregnancy (5.02 ± 0.67 μm) and late-pregnancy (5.55 ± 0.82 μm) groups than in the non-pregnancy (4.01 ± 0.62 μm), early-pregnancy (4.31 ± 0.64 μm) groups (P<0.05), Figure 4B.
| Group (n) | Number (n) | Non-pregnancy (um) | Early-pregnancy (um) | Middle-pregnancy (um) | Late-pregnancy (um ) |
|---|---|---|---|---|---|
| Arteriovenous vessel | 1200 | 29.05 ± 2.96 | 30.29 ± 3.41 | 32.35 ± 2.17 | 35.88 ± 4.58# |
| Capillary vessel | 1200 | 4.01 ± 1.41 | 4.31 ± 1.62 | 5.02 ± 1.68 | 5.55 ± 1.95# |
#p<0.05, compared with the Non-pregnancy group; Early-pregnancy group and Middle-pregnancy group.
Table 2: Vessel diameter during pregnancy (um).
Figure 4: Changes in the diameter of all kind of vessels in all four groups. The values are presented as the mean ± SEM. The diameter of arteriovenous vessel was significantly increased in the middlepregnancy and late-pregnancy groups compared to that in the nonpregnancy and early-pregnancy groups (*P<0.05), (A). The diameter of arteriovenous vessel was higher in the late-pregnancy group than in the middle-pregnancy group (*#P<0.05), (A). The diameter of capillary exhibited a similar pattern as that of arteriovenous vessel, (B).
Discussion
This study showed that the blood flow velocity of arteriovenous vessels and capillaries in late-pregnancy and middle-pregnancy increased by 8%-30% in comparison with that in non-pregnancy and early-pregnancy; the vessel diameter in late-pregnancy and middle-pregnancy increased by 7%-24% compared to that in non-pregnancy and early-pregnancy. The changing tendency of blood flow velocity is consistent with that of vessel diameters. We concluded that maternal cerebral blood flow is gradually increasing during normal pregnancy; the morphological changes of vessels might be related to the blood flow changes.
During pregnancy, there is a large increase in cardiac output and plasma volume, the uterine blood flow in late-gestation increased 10-fold over the non-pregnancy; but the brain is enclosed in a rigid skull, dramatic increase in cerebral blood flow could result in detrimentally increased intracranial pressure that can cause serious neurological symptoms, brain edema, cerebral hemorrhage and even death. So the magnitude by which cerebral blood flow change needs to be assessed accurately.
Using dual-beam angle-independent digital Doppler ultrasound, Ori et al. found that a maximum increase of 20% in the maternal blood flow of the Internal Carotid Artery (ICA) during the course of pregnancy compared to the nonpregnant baseline, such increase is associated with moderate increase in ICA diameter [18]. Another study showed 8.1% increase in cerebral hemispheric blood flow during the first trimester by using Single-Photon Emission Computerized Tomography (SPECT) before and 6 weeks after pregnancy termination [19]. Our results are in accordance with these reports.
The adaptation of cerebral circulation during pregnancy is related to the vasodilatory effects of Vascular Endothelial Growth Factor (VEGF), estrogens and other circulating factors [18,20]. Rusterholz and Aagaard-Tillery et al. proposed that hormones production including growth factors, cytokines increases significantly throughout pregnancy, reaching a peak in late gestation [2,21]. VEGF is one of the most important growth factors for successful pregnancy, it has been recognized as having a role in angiogenesis, vascular growth, endothelial cell survival, and vasodilation through complex interaction of VEGF with its receptors [22,23]. In the uterine circulation and cerebral veins, VEGF expression is higher than the nonpregnant condition [24,25]. Estrogen (ER) may also promote vasodilation during pregnancy; ER expression in endothelial and vascular smooth muscle layers of the aorta and mesenteric artery increased during pregnancy [26]. The related mechanisms for ER mediated vasodilation may be through inhibition of Ca2+ entry into vascular smooth muscle and increasing NO formation by increasing Ca2+ activated endothelial NOS expression or by increasing substrate availability for NO formation [27,28].
Our previous studies has proposed that normal pregnancy lowered seizure threshold [29,30], one possible mechanism was that mild systemic inflammation might cause neuroinflammation which could increase neuronal excitability during pregnancy [1,29-32]. Another possible mechanism may be related to cerebral blood flow autoregulation breakthrough. When the extent by which blood flow changed was beyond the scope of brain autoregulation during pregnancy, brain blood barrier would be disrupted and then caused cerebral edema formation. Clinical reports have shown that 93% of eclamptic women had cerebral vasogenic edema in diffusion-weighted MRI studies [33]. The present study demonstrated that in latepregnancy the blood velocity increased by 8%-30% compared with that in non-pregnancy. Such changed magnitude of blood flow during pregnancy can served as a reference to assess whether the brain blood barrier is intact and the possibility of eclampsia-like seizures.
Different from clinical imaging techniques, the application of two photon imaging in this study helped us to obtain highresolution images of microvasculature of the cortex in vivo, the diameter and blood flow velocity of each microvessel can be exactly measured. The present findings also provided some basis in investigating how microvascular environment change in preeclampsia and eclampsia which is a leading cause of maternal morbidity and mortality worldwide [34]. However, the CBF changes in preeclampsia and eclampsia is not clear. Since molecular transport across the blood-brain barrier mainly occurs in the microvasculature (especially the capillaries), all the vessels play an important role in the metabolism, energetics, and functionality of the cortex [35]. Further studies needed to be performed to investigate changes in the diameter and blood flow velocity of each microvessel under pathological conditions such as preeclampsia and eclampsia.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by the National Natural Sciences Foundation of China (9681170594), the Guangzhou Science and Technology Project (2016A020218002).
References
- Cipolla MJ, Pusic AD, Grinberg YY, Chapman AC, Poynter ME, Kraig RP. Pregnant serum induces neuroinflammation and seizure activity via TNFalpha. Exp Neurol 2012; 234: 398-404.
- Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Sem Fetal Neonat Med 2006; 11: 279-295.
- Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989; 256: 1060-1065.
- Pritchard JA. changes in the blood volume during pregnancy and delivery. Anesthesiology 1965; 26: 393-399.
- Ryznar V, Erban J. Measurement of regional uteroplacental blood flow. Acta Universitatis Palackianae Olomucensis Facultatis Medicae 1988; 119: 353-359.
- Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxidants Redox Signal 2011; 14: 1889-1903.
- Chiu FY. Validation and absolute quantification of MR perfusion compared with CT perfusion in patients with unilateral cerebral arterial stenosis. Eur J Radiol 2012; 81: 4087-4093.
- Kontos HA. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke J Cerebr Circ 1989; 20: 1-3.
- Sigler A, Murphy TH. In vivo 2-photon imaging of fine structure in the rodent brain: before, during, and after stroke. Stroke J Cerebr Circ 2010; 41: 117-123.
- van Veen TR, Panerai RB, Haeri S, Griffioen AC, Zeeman GG. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet Gynecol 2013; 122: 1064-1069.
- Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiol 2000; 217: 371-376.
- Dangel AR, Atlas RO, Matsuo K. Headaches in pre-eclampsia: a clinical dilemma in diagnosing intracranial hemorrhage. Eur J Obstet Gynecol Reprod Biol 2009; 146: 232-233.
- Loureiro R. Diffusion imaging may predict reversible brain lesions in eclampsia and severe preeclampsia: initial experience. Am J Obstetr Gynecol 2003; 189: 1350-1355.
- Roberts JM, Pearson G, Cutler J, Lindheimer M, NHLBI Working Group on Research on Hypertension During Pregnancy. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 2003; 41: 437-445.
- Zunker P, Happe S, Georgiadis AL, Louwen F, Georgiadis D, Ringelstein EB, Holzgreve W. Maternal cerebral hemodynamics in pregnancy-related hypertension. A prospective transcranial Doppler study. Ultrasound Obstetr Gynecol 2000; 16: 179-187.
- Engelter ST, Provenzale JM, Petrella JR. Assessment of vasogenic edema in eclampsia using diffusion imaging. Assessment of vasogenic edema in eclampsia using diffusion imaging. Neuroradiol 2000; 42: 818-820.
- McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta 2011; 32: 413-419.
- Nevo O, Soustiel JF, Thaler I. Maternal cerebral blood flow during normal pregnancy: a cross-sectional study. Am J Obstetr Gynecol 2010; 203: 471-476.
- Ikeda T, Ikenoue T, Mori N, Nagamachi S, Jinnouchi S. Effect of early pregnancy on maternal regional cerebral blood flow. Am J Obstet Gynecol 1993; 168: 1303-1308.
- Cipolla MJ. The adaptation of the cerebral circulation to pregnancy: mechanisms and consequences. J Cereb Blood Flow Metab 2013; 33: 465-478.
- Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol 2007; 29: 151-162.
- Breen EC. VEGF in biological control. J Cell Biochem 2007; 102: 1358-1367.
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nat Rev Mol Cell Biol 2006; 7: 359-371.
- Celia G, Osol G. Mechanism of VEGF-induced uterine venous hyperpermeability. J Vasc Res 2005; 42: 47-54.
- Schreurs MP, Houston EM, May V, Cipolla MJ. The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J Off Publ Federation Am Soc Exp Biol 2012; 26: 355-362.
- Mata KM, Li W, Reslan OM, Siddiqui WT, Opsasnick LA, Khalil RA. Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy. Am J Physiol Heart Circ Physiol 2015; 309: 1679-1696.
- Arnal JF. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Nat Acad Sci USA 1996; 93: 4108-4113.
- Kauser K, Rubanyi GM. Potential cellular signaling mechanisms mediating upregulation of endothelial nitric oxide production by estrogen. J Vasc Res 1997; 34: 229-236.
- Huang Q. Decreased seizure threshold in an eclampsia-like model induced in pregnant rats with lipopolysaccharide and pentylenetetrazol treatments. PloS One 2014; 9.
- Liu L. Increased neuronal seizure activity correlates with excessive systemic inflammation in a rat model of severe preeclampsia. Hypertension Res Off J Japan Soc Hypertension 2016; 39: 701-708.
- Riazi K. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Nat Acad Sci USA 2008; 105: 17151-17156.
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstetr Gynecol 1998; 179: 80-86.
- Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol 2004; 190: 714-720.
- Walker JJ. Pre-eclampsia. Lancet 2000; 356: 1260-1265.
- Sunwoo J, Cornelius NR, Doerschuk PC, Schaffer CB. Estimating brain microvascular blood flows from partial two-photon microscopy data by computation with a circuit model. IEEE Eng Med Biol Soc 2011; 2011: 174-177.