- Biomedical Research (2014) Volume 25, Issue 3
Evaluation of triceps brachii muscle strength during grip force exercise through surface electromyography.
Md. Asraf Ali1, Kenneth Sundaraj1, R. Badlishah Ahmad1, Nizam Uddin Ahamed1, Md. Anamul Islam1 and Sebastian Sundaraj MD21AI-Rehab Research Group, Universiti Malaysia Perlis (UniMAP), Kampus Pauh Putra, Perlis, Malaysia
2Medical Officer, Malaysian Ministry of Health, Malaysia
- *Corresponding Author:
- Md. Asraf Ali
AI-Rehab Research Group
Universiti Malaysia Perlis (UniMAP)
Kampus Pauh Putra, 02600 Arau, Perlis, Malaysia
Accepted date: March 05 2014
Abstract
The objective of the present study was to investigate the strength of the three heads of triceps brachii muscle during handgrip force exercise with full elbow extension through surface electromyography. The myoelectric signals were recorded from the lateral, long and medial heads of triceps brachii muscle of 11 healthy men during maximal isometric contractions for 10-s of concurrent handgrip force and elbow extension. The subjects were asked to perform their contraction task five times with 3-min interval between the two successive contractions. Among the three heads of triceps brachii muscle, the higher normalize root mean square amplitude were found at the first 5-s duration (FD) compare to the last 5-s duration (LD). The normalized root mean square amplitude changes were obtained between FD and LD in lateral head (7.995%), long heads (5.435%), and medial head (1.144%). The electromyographic activities between the period of FD and LD were not statistically significant within the head for lateral (p = 0.178), long (p = 0.491), and medial (p = 0.448). These findings supported that the triceps brachii muscle strength is decreased by the increasing of contraction duration of the muscle fiber, where the muscle strength of the medial head might be more stable and followed the long and lateral head.
Keywords
Surface electromyography, triceps brachii muscle, isometric contraction, grip force, muscle strength
Introduction
Muscle activity is one of the major factors associated with human body movements during sports, exercise, and survival in different daily life tasks. In general, surface electromyography (sEMG) is used to measure the activity of superficial muscles and is an essential tool in biomechanical and biomedical assessments [1]. In addition, myoelectric (EMG) signals are generated in human skeletal muscle during contraction of the muscle fibre, which is always random [2,3]. These EMG signals provide evidence of the anatomy and functional activity of a muscle [4,5].
The functional activities of muscles are generally analysed to increasing its ability in the performance of various human activities. However, the grip force exercise is a type of activity in which the subject grasps a handgrip dynamometer with the maximum force possible for an instant. During the grip force exercise, the EMG signals are generated from the following group muscles of the upper limb: arm [6], forearm [7], and hand [8]. The triceps brachii (TB) is one of the main arm muscles. Only a few studies have assessed the activity of arm muscles, particularly the TB, using an electromyography sensor during the grip force exercise. For example, the authors [6] measured the TB activity during a hand grip exercise that was performed in the seated position with the elbow supported at a 90° angle. These researchers analysed the EMG activity on the TB based on three temperature conditions (thermoneutral air, cold air, and cold air with a cold drink); however, they only measured one anatomical location of the TB without mentioning the exact anatomical location on the muscle. More recently, another study [9] measured only the lateral head of the TB during the hand grip exercise, which was performed with an elbow angle of 120°.
From the biomedical and biomechanical perspective, understanding the function of the TB is essential because the electromyography signal is generated as a result of electrode placement and voluntary contraction. The TB has three proximal attachments: the long head, which originates from the infraglenoid tubercle, the lateral head, which originates from the humerus superior to the radial groove and the lateral intermuscular septum, and the medial head, which extends from the humerus inferior to the radial groove and the medial intermuscular septum [10]. However, the medial head is mostly covered by the lateral and long heads and is only visible closer to the elbow joint. Furthermore, the author [11] demonstrated that the TB is the long muscle on the posterior humerus, consists of a three-headed and fusiform arrangement, and operates as a third-class lever because the force is applied between the joint axis and the load. However, the various sEMG findings associated with the TB and the processing methods used to analyse the TB have revealed a large number of muscular activities, for example muscle fatigue [12-15], force [16,17], and motor unit action potential [18,19]. These activities of the TB are generally investigated because of their importance physiological exercise [20-22], rehabilitation [23-25], sports science [26-28], and signal processing for prosthesis control applications [29-31].
A number of studies have reported that the results from sEMG signals vary and are influenced by the placement of the electrode(s) on the TB during different contractions. For example, the authors [32] noted the effect of the anatomical location “over the muscle belly in line with the muscle fibres on the TB” during the voluntary contraction generated during an arm swing movement. Other study [33] examined another anatomical location used for electrode placement, namely “the muscle belly in line with the muscle fibre direction and between the innervations zone and the terminal tendon”, during a maximal voluntary isometric contraction.
Moreover, the authors [34] placed the electrode “over the muscle belly along the long axis” during recumbent stepping with active, passive, and resting arm efforts. In addition, surface electromyography for the non-invasive assessment of muscles (SENIAM) is part of a larger project that analyses the effects of sEMG sensors and sensor placement locations in the human skeleton and body muscles, including the anatomical location of the TB, and recommends that the electrodes be placed on the lateral and long heads [35].
However, the various anatomical locations on the TB that have been previously used for electrode placement and identified the three most effective anatomical locations, namely the three heads of the TB. According to cadaveric studies, the three heads of the TB do not work as a single unit throughout an extension movement [36]. Thus, the purpose of the present study was to examine the muscle strength of the heads of TB during grip force exercise performed with a maximum elbow extension concurrently. We choose our present investigation because static contractions at very low force levels have been shown to cause spectral compression in the EMG signals from the TB [37].
Materials and Methods
Subjects
Eleven healthy male university students between the ages of 22 and 34 years participated in this study. The mean and standard deviations of the characteristics of the participants were the following: age = 24.3 ± 4.1 years; height = 171.1 ± 5.6 cm; weight = 71.1 ± 6.2 kg. Participants with a history of shoulder, elbow, or wrist injury were excluded from the study. All of the participants read and signed an informed consent form before their participation in this study, and the protocol used in this study was approved by the institution’s Research Ethics Board.
Experimental protocol
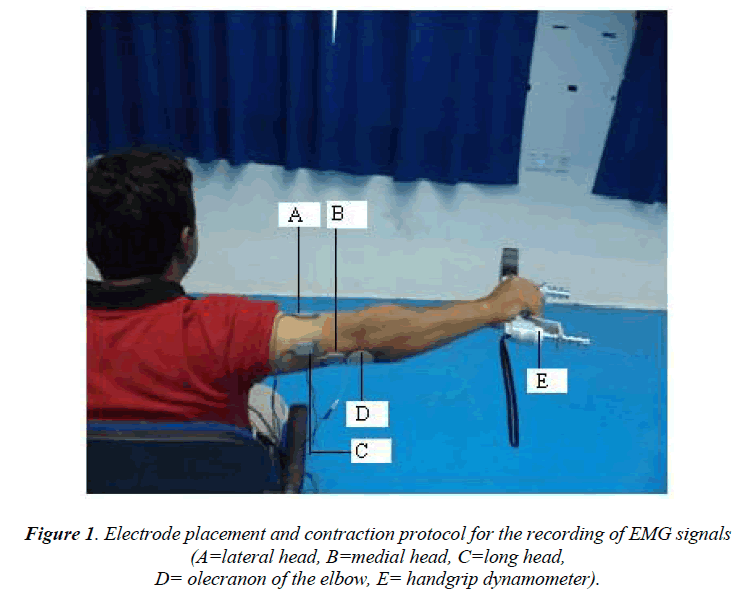
To optimise the participants, university students particularly cricketers, were recruited for the EMG signal recordings due to their athletic abilities and low subcutaneous fat. The EMG signals on the lateral, long, and medial heads of the TB of the dominant arm of each of the participants were recorded. During the EMG signal recording, the subjects were asked to fully extend their elbow and concurrently apply a maximum grip force using a handgrip dynamometer. Additionally, the subjects were asked to maintain their arm at the way of the shoulder abduction with scapula fixed during their task performance. We chose this arm position because it is the neutral position of the upper limb with full extension of the forearm between abduction and adduction. To reduce the influence of any other parts of the body to the TB contractions, subjects were asked to maintain their exercise without trunk movement. All of the measurements were performed as the subjects sat erect in a rigid chair furnished with an approximately vertical backrest.
Data collection hardware characteristics
Single differential sEMG sensors (DE-02, Delsys Inc., Bangnoli-4, Boston, MA, USA) were used for the EMG recording. Briefly, parallel bar silver electrodes (length = 10 mm, diameter = 1 mm) with a fixed interelectrode distance of 10 mm were placed on the three heads of the TB. The myoelectric activities on the three heads of the TB were collected using customised software (Delsys EMGWorks, Boston, MA, USA). The myoelectric signals were recorded at a sampling rate of 2 kHz during a 10-s period before A-D conversion and stored on a compatible computer. The raw EMG was band-pass filtered (4th order Butterworth) between 10-500 Hz (CMRR > 92 db, input noise < 1.21 V, impedance of 1012Ω in parallel with 5 pF), and the gain was fixed for all of the channels at 1000.
Electrode placement and muscle contraction testing procedure
To record the EMG signals from the three heads of the TB using three channels of single differential sEMG sensors, the electrodes were placed over the muscles parallel to the fibre orientation in the muscle belly of the lateral, long, and medial heads. The electrodes were placed on the muscles using a two-slot adhesive skin interface (Delsys Inc.) to firmly stick the sensors to the skin. Before the electrodes were placed on the three heads of the TB, the appropriate skin areas were shaved, cleaned with alcohol, and abraded with emery paper. The reference electrode (2 cm × 2 cm) was attached to the lateral epicondyle of the humerus (approximately ±1 inch from the olecranon of the elbow) of the arm used for the EMG signal recording. The placements of the electrodes are shown in Figure 1.
Once the electrodes were placed, the EMG values were obtained as the subjects performed the maximum grip force exercise with a full extension of the forearm using a handgrip dynamometer. The participants performed 10-s isometric contractions against the manual resistance provided by a researcher. The participants were asked to perform the muscle contraction test at least five times with maximal effort. The participants were allowed a 3-min rest period between the tests to minimise the potential effects of fatigue.
EMG analysis
The EMG amplitudes (in V) of the three heads of the TB were recorded from each of the subjects as they performed the repeated tasks according to the instructions of the researchers. The myoelectric data from each contraction task performed by the participants was processed in an identical manner. The myoelectric signals were first filtered using the EMG analysis software (Delsys EMG-Works, Boston, MA, USA), and the EMG signals were then processed using the root mean square algorithm with a time constant of 5-s.
Statistical analysis
To obtain biologically meaningful data for the analysis of EMG activities in FD and LD, descriptive statistics were used. The mean, peak amplitude and root mean square (RMS) values (in μV) of FD and LD were calculated for each of the individual heads of the TB from each of the individual subject. The significance of the changes in the activity between the heads of the TB were tested by paired t-tests (two-tailed) at the p < 0.05 significance level. All of the statistical calculations were executed using the SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA) statistical package.
Results
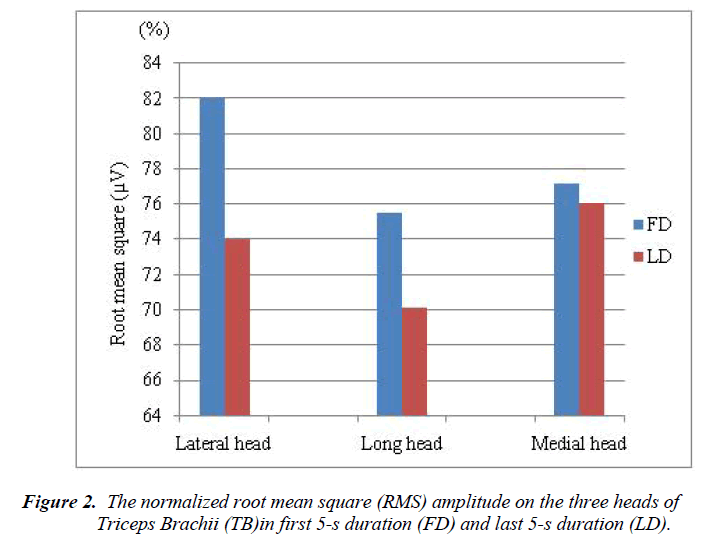
The sEMG signals on the lateral, long, and medial heads of TB of eleven individual subjects were recorded for the analysis of muscle strength. All subjects are asked 5 times to perform their contraction task of the TB. RMS amplitudes in FD and LD for the three heads of TB were calculated based on the peak amplitude of each contraction of the each subject. The normalized RMS amplitudes on the lateral, long, and medial heads of TB in FD and LD are shown in Figure 2.
Among the three heads of triceps brachii muscle, the higher normalized RMS amplitudes were found at FD compare to LD. The normalize RMS amplitude changes were found between FD and LD in lateral head (7.995%), long heads (5.435%), and medial head (1.144%). Within the head of TB, the electromyographic activities between the duration of FD and LD were not statistically significant for lateral (p = 0.178), long (p = 0.491), and medial (p = 0.448).
Discussion
In our present study, we evaluated changes the EMGderived signal amplitude on the three heads of TB during handgrip force exercise. Changes in RMS amplitude parameter by the isometric contractions indicated that the muscle strength was decreased by the increasing of contraction duration of the muscle fiber, where the muscle strength of the medial head was more stable in longer duration of isometric contraction, and followed the long and lateral heads of TB.
According to previous studies [38-40], the generation of handgrip force requires the combined activities of extrinsic forearm muscles and intrinsic hand muscles. Later, Meigal et al. [6] examined the EMG activity from proximal, middle and distal arm muscles during handgrip force exercise, and resulted increased the EMG activity in all the arm muscles. The arm muscle consists of a group of muscles, where TB is the largest muscle and generally acknowledged as an extensor of the forearm across the elbow joint [41]. Thus, the protocol of the present study is reliable to expect the EMG signals from the three heads of TB. Because there are some precise roles of TB muscle those are as follows; it is an antagonist muscle and is posterior to the arm skeleton. Also, the long head of the TB muscle is isolated from the surrounding tissue at its insertion.
In general, the RMS amplitude or median power frequency used to compare the muscle activity of between muscles, between subjects, or between different locations within the muscle. According to the aim of the present study, we analysed the RMS amplitude to compare the activity of different locations within a muscle. The RMS amplitude were obtained from the EMG signals through contractions of the lateral, long and middle heads of the TB of each subject. The normalized RMS values were overall decreased in LF compare to FD for all the three heads of TB (Figure 2). Previous study [9] compared the EMG activity on the lateral head of TB by 4-s duration and found that the EMG activity was decreased in second 4-s duration than first 4-s duration which is supported our present study. But the authors of this study examined only the lateral head of TB, whereas our study examined all the three heads of TB because the three heads of the TB do not work as a single unit throughout an extension movement [36].
In the present study, the greater changes of normalized RMS values between FD and LD were obtained at lateral head and followed the long and medial heads of TB (Figure 2). The RMS decreased associated with the increment duration of muscle fiber contraction was observed at the three heads of TB. Even though the normalized RMS values were decreased in the medial head, it could be consider as stable because the normalized RMS values almost same for the both FD and LD (Figure 2). However, it is not so easy to explain muscle action during isometric contraction through hand grip force exercise. According to previous studies [6-8], the generation of hand grip force requires the combined activities of hand muscle, forearm muscle and arm muscle. The decreases in muscle activity during contraction through hand grip force exercise led to the decrements in hand grip force [9]. This hand grip force decreases due to decrement of the muscle strength. It is therefore suggested that the decreases in lateral and long, and medial heads of TB activity during the present isometric contraction tests led to the decrements of muscle strength. Additionally, it could be concluded that the muscle strength is high in longer duration for medial head compared to other two heads of TB. On the other hand, the greater EMG activity change (decrease) was observed in the lateral head compared to other heads of TB. It might be happened due to reduce the muscle strength at early stage in the lateral head compare to the other heads of TB.
Conclusion
This study evaluated the muscle strength of the three heads of TB during handgrip force exercise with the full extension of the forearm, in which was being expected maximum elbow extension occurs and reliable amount of isometric contraction of TB. Based on the study findings, it is concluded that the triceps brachii muscle strength is decreased by the increasing of contraction duration of the muscle fiber; but the muscle strength of the medial head is more stable, and muscle strength of the lateral head is less stable and followed the long heads. These outcomes are assumed to need more research for better understanding the mechanisms behind these compensatory strategies.
Acknowledgement
The authors would like to thank all the researchers of the AI-Rehab Research Group, UniMAP for their cooperation.
References
- Heinonen I, Nesterov SV, Kemppainen J, Fujimoto T, Knuuti J, Kalliokoski KK. Increasing Exercise Intensity Reduces Heterogeneity of Glucose Uptake in Human Skeletal Muscles. PLoS ONE. 2012; 7(12): e52191.
- Komi PV, Viitasalo JHT. Signal Characteristics of EMG at Different Levels of Muscle Tension. Acta Physiologica Scandinavica. 1976; 96(2): 267-276.
- Merletti R, Parker P. Electromyography: physiology, engineering and non-invasive applications. NY, USA: Wiley-IEEE Press; 2004.
- Basmajian JV, De Luca CJ. Muscles alive: their functions revealed by electromyography. NY, USA: Williams & Wilkins; 1985.
- Silva DCdO, Silva Z, Sousa GdC, Silva LFGe, Marques KdV, Soares AB, et al. Electromyographic evaluation of upper limb muscles involved in armwrestling sport simulation during dynamic and static conditions. Journal of Electromyography and Kinesiology. 2009;19(6): e448-e457.
- Meigal AY, Oksa J, Hohtola E, Lupandin YV, Rintamäki H. Influence of cold shivering on fine motor control in the upper limb. Acta Physiologica Scandinavica. 1998; 163(1): 41-47.
- Hoozemans MJM, van Dieën JH. Prediction of handgrip forces using surface EMG of forearm muscles. Journal of Electromyography and Kinesiology. 2005;15(4):358-366.
- Strutton PH, Catley M, Davey NJ. Stability of corticospinal excitability and grip force in intrinsic hand muscles in man over a 24-h period. Physiology & Behavior. 2003; 79(4–5): 679-682.
- Oda S, Kida N. Neuromuscular fatigue during maximal concurrent hand grip and elbow flexion or extension. Journal of Electromyography and Kinesiology. 2001; 11(4): 281-289.
- Hollinshead W. Anatomy for surgeons:The back and limbs. 3rd ed. NY, USA: Harper & Row; 1982.
- Salmons S. Muscle. In: Williams PL BL, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ, editor. Gray's anatomy. NY, USA: Churchii Livingstone; 1995. p. pp. 737-900.
- Stirn I, Jarm T, Kapus V, Strojnik V. Evaluation of muscle fatigue during 100-m front crawl. Eur J Appl Physiol. 2011; 111(1): 101-113.
- Meszaros AJ, Iguchi M, Chang S-H, Shields RK. Repetitive eccentric muscle contractions increase torque unsteadiness in the human triceps brachii. Journal of Electromyography and Kinesiology. 2010; 20(4): 619-626.
- Dearth D, Umbel J, Hoffman R, Russ D, Wilson T, Clark B. Men and women exhibit a similar time to task failure for a sustained, submaximal elbow extensor contraction. Eur J Appl Physiol. 2010; 108(6): 1089-1098.
- Missenard O, Mottet D, Perrey S. The role of cocontraction in the impairment of movement accuracy with fatigue. Exp Brain Res. 2008; 185(1): 151-156.
- Lindinger S, Holmberg H-C, Müller E, Rapp W. Changes in upper body muscle activity with increasing double poling velocities in elite cross-country skiing. Eur J Appl Physiol. 2009; 106(3): 353-363.
- Salomoni S, Graven-Nielsen T. Experimental muscle pain increases normalized variability of multidirectional forces during isometric contractions. Eur J Appl Physiol. 2012; 112(10): 3607-3617.
- Fang Y, Daly JJ, Sun J, Hvorat K, Fredrickson E, Pundik S, et al. Functional corticomuscular connection during reaching is weakened following stroke. Clinical neurophysiology. 2009; 120(5): 994-1002.
- Bottas R, Miettunen K, Komi PV, Linnamo V. Disturbed motor control of rhythmic movement at 2 h and delayed after maximal eccentric actions. Journal of Electromyography and Kinesiology. 2010; 20(4): 608-618.
- Plattner K, Baumeister J, Lamberts RP, Lambert MI. Dissociation in changes in EMG activation during maximal isometric and submaximal low force dynamic contractions after exercise-induced muscle damage. Journal of Electromyography and Kinesiology. 2011; 21(3): 542-550.
- Krentz J, Farthing J. Neural and morphological changes in response to a 20-day intense eccentric training protocol. Eur J Appl Physiol. 2010; 110(2): 333-340.
- Sakamoto A, Sinclair P. Muscle activations under varying lifting speeds and intensities during bench press. Eur J Appl Physiol. 2012; 112(3): 1015-1025.
- Serrao M, Ranavolo A, Andersen OK, Don R, Draicchio F, Conte C, et al. Reorganization of multimuscle and joint withdrawal reflex during arm movements in post-stroke hemiparetic patients. Clinical Neurophysiology. 2012; 123(3): 527-540.
- Li L, Tong KY, Hu XL, Hung LK, Koo TKK. Incorporating ultrasound-measured musculotendon parameters to subject-specific EMG-driven model to simulate voluntary elbow flexion for persons after stroke. Clinical Biomechanics. 2009; 24(1): 101-109.
- Barker R, Brauer S, Carson R. Training-induced changes in the pattern of triceps to biceps activation during reaching tasks after chronic and severe stroke. Exp Brain Res. 2009; 196(4): 483-496.
- Lohse KR, Sherwood DE, Healy AF. How changing the focus of attention affects performance, kinematics, and electromyography in dart throwing. Human Movement Science. 2010; 29(4): 542-555.
- Neto OP, Magini M. Electromiographic and kinematic characteristics of Kung Fu Yau-Man palm strike. Journal of Electromyography and Kinesiology. 2008; 18(6): 1047-1052.
- Rota S, Hautier C, Creveaux T, Champely S, Guillot A, Rogowski I. Relationship between muscle coordination and forehand drive velocity in tennis. Journal of Electromyography and Kinesiology. 2012; 22(2): 294-300.
- Li G, Li Y, Yu L, Geng Y. Conditioning and Sampling Issues of EMG Signals in Motion Recognition of Multifunctional Myoelectric Prostheses. Ann Biomed Eng. 2011; 39(6): 1779-1787.
- Alkan A, Günay M. Identification of EMG signals using discriminant analysis and SVM classifier. Expert Systems with Applications. 2012; 39(1): 44-47.
- Arslan YZ, Adli MA, Akan A, Baslo MB. Prediction of externally applied forces to human hands using frequency content of surface EMG signals. Computer Methods and Programs in Biomedicine. 2010; 98(1): 36-44.
- Pijnappels M, Kingma I, Wezenberg D, Reurink G, Dieën J. Armed against falls: the contribution of arm movements to balance recovery after tripping. Exp Brain Res. 2010; 201(4): 689-699.
- Holmes MWR, Keir PJ. Posture and hand load alter muscular response to sudden elbow perturbations. Journal of Electromyography and Kinesiology. 2012; 22(2): 191-198.
- Huang HJ, Ferris DP. Upper limb effort does not increase maximal voluntary muscle activation in individuals with incomplete spinal cord injury. Clinical neurophysiology. 2009; 120(9): 1741-1749.
- Seniam. Surface ElectroMyoGraphy for Non-Invasive Assesment of Muscle (SENIAM). http://wwwseniamorg.2010.
- Landin D, Thompson M. The shoulder extension function of the triceps brachii. Journal of Electromyography and Kinesiology. 2011; 21(1):161-165.
- Fallentin N, Sidenius B, JØRgensen K. Blood pressure, heart rate and EMG in low level static contractions. Acta Physiologica Scandinavica. 1985; 125(2): 265-275.
- Landsmeer JMF, Long C. The mechanism of finger control, based on electromyograms and location analysis. Cells Tissues Organs. 1965; 60(3): 330-347.
- Longii C. Intrinsic-extrinsic muscle control of the fingers electromyographic studies. The Journal of Bone & Joint Surgery. 1968; 50(5): 973-984.
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res. 1995; 103(1): 108-122.
- Moore KL, Dalley AF. Clinically oriented anatomy. NY, USA: Lippincott Williams & Wilkins; 1992.