Research Article - Journal of Fisheries Research (2023) Volume 7, Issue 4
Evaluation of the toxicity of copper oxide and nanoparticles using the biochemical, hematological, and histological biomarkers in Nile tilapia, (Oreochromis niloticus)
Mohamed Yahya*
Department of Fisheries, National Institute of Oceanography and Fisheries Egypt
- Corresponding Author:
- Mohamed Yahya
Department of Fisheries
National Institute of Oceanography and Fisheries
Egypt
Tel: +20 1202195174
E-mail: myahya120@yahoo.com
Received: 27-June-2023, Manuscript No. aajfr-22-103169; Editor assigned: 01-July-2023, PreQC No. aajfr-22-103169(PQ); Reviewed: 19-July-2023, QC No. aajfr-22-103169; Revised: 25-July-2023, Manuscript No. aajfr-23-103169(R); Published: 04-Aug-2023, DOI:10.35841/aajfr-7.3.156
Citation: Yahya M. Evaluation of the toxicity of copper oxide and nanoparticles using the biochemical, haematological, and histological biomarkers in Nile tilapia, (Oreochromis niloticus). J Fish Res. 2023;7(4):156
Abstract
This study aimed to evaluate the toxicity of synthesized copper nanoparticles (CuO-NPs) on biochemical and histological changes in Nile tilapia. Silver nano colloids were characterized using visible and ultraviolet light spectroscopy and high-resolution transmission electron microscopy (HRTEM). The results showed that the exposure of Oreochromisniloticus to CuO-NPs (0.525 mg/L) at pH 7, 15 °C and 25 °C showed a significant decrease in the values of RBCs, hemoglobin, hydrogen, MCV, MCH and MCHC but a significant increase in WBCs The histological changes and apparent damage to the gills were more than that of the control group and the other treatments exposed to the same concentration of CuO-NPs at higher pH (9) and salinity (34 ppm) which revealed normal values and more or less gill structure. Hence it is recommended to reduce the toxicity of CuO-NPs in Oreochromisniloticus fish farms by increasing the water pH up to 9 or increasing the water salinity.
Keywords
CuO-NPs toxicity, Oreochromis niloticus, Histopathological, Blood parameters.
Introduction
The widespread use of MONMs in the aquatic environment may cause serious concern and severe impacts on vulnerable aquatic organisms, including fish and bivalve molluscs. In this interest, reviewed investigations of the Nano toxicity of CuONPs in different fish species including their bioavailability, bioaccumulation, mechanisms of action and health effects on exposed fish. Previous studies have shown that the toxic effects of CuONPs in fish are generally affected by particle size and method of application agglomeration, solubility, and concentration of nanoparticles in exposure medium. Reports showed that exposure of fish to CuONPs induced oxidative stress damage and malformation in zebrafish (Danio rerio) embryos and haematological biochemistry changes in Caspian trout (Salmo trutta caspius) Oxidative stress and increased copper accumulation in liver and muscle tissues of African catfish (Clarias gariepinus). Furthermore, CuONPs induced serious histopathological changes in organs of naked rainbow trout (Oncorhynchus mykiss), common carp (Cyprinus carpio) Goby (Poecilia reticulata) [1]. Silver nanoparticles (AgNPs) are novel silver agents that are synthesized at the nanoscale and typically have diameters from 1 to 100nm. The physical and chemical properties, including size, shape, surface area, charge and solubility, make AgNP both interesting and challenging for industrial markets worldwide. To date, AgNP has been increasingly used in global markets for its antibacterial properties. Silver nanoparticles (CuO-NPs) possess many unique properties, which have inspired their widespread application in antimicrobial materials, biomarkers, optoelectronics, and catalysis systems. Silver nanoparticles have very large surface area-to-mass ratios and have a high surface-to-surface ratio of their constituent atoms, which gives them unique biological activity or toxicity. It is important to study the toxic effect of nanomaterials on fish and aquatic organisms because most of the pollutants released into the environment are consumed by aquatic species. The results of this study provide new insights and add information to better elucidate the toxicity mechanisms of CuONPs in Nile tilapia by evaluating histopathological parameters (gills, liver and kidneys) and serum biochemistry [2].
Materials and methods
To study the identification of Clupisoma garua and Clupisoma montana in Indrapuri dam.
Methodology
Experimental fish
Healthy Nile tilapia, Oreochromis niloticus fish were obtained from unpolluted fish farm at Menofeya governorate, Egypt. The initial body length was 18±2 cm and the weight was 100±3g. Fish were acclimated to laboratory conditions for 2 weeks before start experiment in fiberglass tanks containing dechlorinated tap water. Fish were randomly distributed at a rate of 8 fish per aquarium and water quality parameters were analyzed according to the methods of American Public Health Association (APHA 2005). The water quality was water temperature (25.1-25.3°C), pH 7.2-7.4, EC 635-640(μS/cm), dissolved oxygen 6.6–6.8 mg/l, BOD 2.4-2.7mg/l, COD 4.8– 5.0 mg/l,, Ammonia 0.120-0.170 mg/l, nitrite 9.1-9.4(μg/l) and nitrate 33.6-33.9 (μg/l) respectively [3]. The photoperiod was 12 h light: 12 h dark. CuOBPs were purchased from El- Nasr pharmaceutical chemicals Co. Egypt, while, CuONPs were obtained from sigma-Aldrich, St. Louis, MO, USA, with average size?50nm and 99%purity level. The different suspension concentrations (1/4 and 1/8 of 96h LC50) of both CuO (BPs & NPs) were freshly prepared by weighing CuOBPs or CuONPs powder in dechlorinated water (pH = 7.4), then exposed to ultrasonication (100W, 40 kHz) for 60 min to increase their dispersal.
Experimental diets
Fish were fed on artificial diet in small pellet form containing 30% protein, 13% fish meal, 15% soya been, 18% cotton seed, 22% wheat brain, 1% cotton seed oil and 1% vitamin. The diet was given twice daily at 8:00 (am) and 6:00 (pm). Fish were starved for 48h prior to experimentation to avoid metabolic differences [4]. Water were renewed with routine clearing of the aquaria, leaving no faecal matter or unconsumed food as well as any fish showing any unusual performances were excluded.
Toxicity bioassay
Experiments were conducted to determine the half lethal concentration after 96 h (96 h LC50) of copper oxide bulk particles (CuOBPs) and copper oxide nanoparticles (CuONPs) in O. niloticus according to Behrens-Karber’s method. After acclimatization period (2 weeks), fish groups were distributed at a rate of 8 fish per aquarium, 8 fish were used per concentration per replicate. A total of three replicates were carried out for each concentration and the control group. The different suspension concentrations of CuOBPs and CuONPs were prepared and dispersed using bath sonicator for 1h immediately prior to use without addition of any stabilizing agents. A control group was setup with dechlorinated tap water without addition of particles. The feeding stopped 24 h before the start of the experiment and during the exposure period to prevent the absorption of particles in food and the production of faeces [5-7].
Experimental design
All fish of Nile tilapia, O. niloticus with approximately the same body weight were divided into two experiments (both bulk and nano ZnO forms) as following: Fish studied groups were exposed to different sub-lethal concentrations (1/4, and 1/8LC50) of CuOBPs and CuONPs for exposure periods of 45 days.
T1: Control group, fish reared in dechlorinated tap water.
T2: Fish exposed to (1/4 LC50) of CuOBPs.
T3: Fish exposed to (1/8 LC50) of CuOBPs.
T4: Fish exposed to (1/4 LC50) of CuONPs.
T5: Fish exposed to (1/8 LC50) of CuONPs
Blood sampling and examination
Blood samples were withdrawn from the arteria caudali of control group fish and other treatments at the end of the experimental period [8]. Sodium citrate is the most satisfactory anticoagulant since heparin and oxalate were found to be unsatisfactory in completely preventing coagulation (Falkner & Houston, 1966). Blood samples were examined immediately for the following: Red and White blood cells count: Total number of erythrocytes (RBCs) and leukocytes (WBCs) were counted using improved Neubauer Haemocytometer. Haemoglobin content (Hb) was estimated using cyanmethemoglobin method described by Van Kampen and Zijlstra [9]. Haematocrit value: Packed cell volume (PCV) was carried out in small haematocrit tubes using haematocrit centrifuge at 3000 rpm for 15 minutes. Blood indices Mean corpuscular volume (MCV), Mean corpuscular haemoglobin (MCH) and Mean corpuscular haemoglobin concentration (MCHC) were calculated according to Gupta method.
Biochemical analyses
Glucose was measured using Boehringer Mannheim kits according to Trinder [10]. Aspartate amino transferase (AST) and alanine amino transferase (ALT) activities were determined calorimetrically using transaminase kits according to the method described by Reitman’s and Frankel. Creatinine was measured using colorimetric method described by Henry. Uric acid was measured using enzymatic reaction according to Barham and Trinder.
Histological preparations
Fish from control and different treatments were rapidly dissected at the end of the experiment (45 days) from the ventral line beginning with the anal papilla till the gill operculum. The second gill arch from the right branchial chamber of each fish was removed [11]. These specimens were fixed directly in neutral buffered formalin and Bouin’s fixatives then dehydrated, cleared and embedded in paraplast plus (m.p.56- 58°C). The gills were oriented with their middle hemibranchs placed downwards. Paraffin sections 4-6μm thick were then cut with a rotary microtome and stained with Haematoxylin and eosin (HE) according to Harris for the study of the general morphology of the gill [12-15].
Statistical Analysis
The results were statistically analyzed using analysis of variance (F-test) followed by Duncan's multiple range test to determine differences in means using Statistical Analysis Systems, Version 6.2.
Results
Hematological and biochemical aspects
Hematological parameters of blood samples are presented in (Table 1&2). It is obvious that Oreochromis niloticus exposed to control group, CuOBPs, and CuONPs after 45 days of the exposure period. The results showed the mean concentrations of (RBC, Hb, HT, MCV, MCH, MCHC and WBC). The statistical analysis of mean values of Hb, MCV, MCH, and MCHC in the blood of Nile tilapia decreased in the serum of treated fish by T2 while it was RBC, and HT decreased in T4 [16-21]. On the contrast, HT MCV and MCH, in the blood of fish increased in the serum of treated fish by T1. Also increase RBC, Hb and MCHC increased in T5 while WBC the highest value T4 and lowest valueT5.
| RBCs X106/mm3) |
Hb (g/100ml) |
Ht (%) | MCV (μm3/cell) |
MCH (pg/cell) |
MCHC (g/100ml) |
WBCs (X103/mm3) |
|
| T1 | 1.2 ± 0.054 | 6.18 ± .053 | 21.2 ± .083 | 177 ± 0.99 | 51.5± 0.69 | 29.7± 0.31 | 18.85± 0.34 |
| T2 | 0.88 ± 0.186 | 2.41 ± 0.41 | 9.18 ± 0.42 | 104.2 ± .58 | 27.4 ± 1.145 | 26.3± 0.88 | 39.2 ± 9.18 |
| T3 | 1.29 ± 0.034 | 4.6 ± 0.12 | 15.38±0.16 | 119.2 ± 0.78 | 35.7 ± 0.28 | 29.9 ± 0.79 | 16.9 ± 0.77 |
| T4 | 0.68 ± 0.023 | 2.7 ± 0.11 | 9.2 ± 0.26 | 135.66 ± 5.58 | 39.7± 0.46 | 29.3± 1.69 | 57.56 ± 0.997 |
| T5 | 1.44 ± 0.18 | 4.7 ± 0.78 | 15.9 ± 2.7 | 115.6 ± 6.99 | 36.5 ± 1.09 | 30.2 ± 1.23 | 18.5± 0.9 |
Table 1: Changes in serum Hematological parameters (mean ± SD) in Nile Oreochromis niloticus exposed to CuOBPs and CuONPs for a long exposure period (45 days).
| Glucose (mg/100 ml) | AST (u/l) |
ALT (u/l) |
Creatinine (mg/100 ml) | Uric acid (mg/100 ml) | Glucose (mg/100 ml) | |
|---|---|---|---|---|---|---|
| T1 | 59.0 ± 0.33 | 10.24± 0.005 | 6.3± 0.005 | 0.29 ± 0.014 | 2.45 ±0.076 | 59.0 ± 0.33 |
| T2 | 84.1 ± 2.24 | 17.5 ± 1.3 | 14.0 ± 0.285 | 0.81± 0.12 | 4.18 ± 0.079 | 84.1± 2.24 |
| T3 | 58.0 ± 0.26 | 9.46± 0.404 | 6.65 ± 0.21 | 0.39 ± 0.004 | 3.25 ± 0.29 | 58.0 ± 0.26 |
| T4 | 98.16 ±1.16 | 18.22 ± 1.33 | 11.16 ± 1.71 | 0.97 ± 0.04 | 4.28 ± 0.63 | 98.16 ± 1.16 |
| T5 | 55.33 ± 0.05 | 8.19± 0.235 | 7.7 ± 0.33 | 0.35± 0.005 | 2.39 ± 0.048 | 55.33 ± 0.05 |
Table 2: Biochemical biomarkers Changes in (mean ± SD) Nile Oreochromis niloticusexposed to to CuOBPs and CuONPs for a long exposure period (45 days).
The mean concentrations of cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glucose, uric acid and creatinine, in blood serum of Nile tilapia. Differences were observed in the mean values of all the detected biochemical variables among the treatments. The mean concentrations of the biochemical Parameters increased in the serum of treated fish by T4 compared to those sampled from other treatment except alanine aminotransferase (ALT), it was increased inT2 whereas mean concentrations of biochemical variables in serum decreased for treatmentT1 and T5.
Histological Investigations
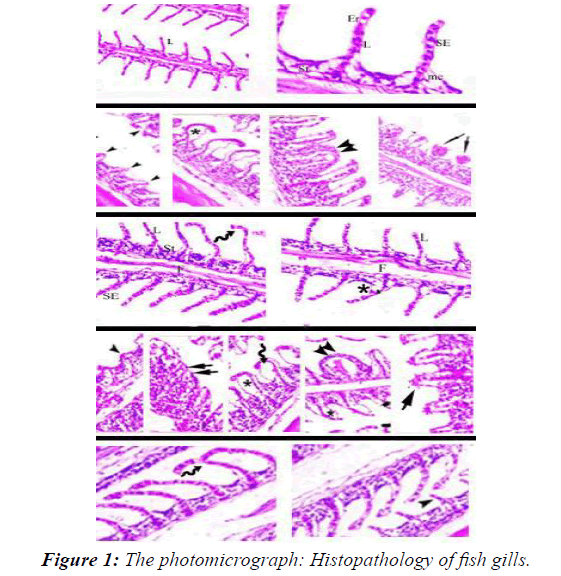
Histopathology of fish gills
The histopathological study of the gills showed that the typical structural organization of the lamellae in the control group without pathological changes showed the normal histological structure of the gills, in addition to the primary and secondary gill lamellae (T1). A series of histopathological changes occurred in the gills of fish exposed after 45 days of exposure to different sub lethal concentrations of CuOBPs and CuONPs observed in all experimental groups. Primary and secondary lamellae counts decreased. External epithelial hyperplasia, dilated blood vessels (telangiectasia), and epithelial necrosis led to secondary platelet fusion [22]. In the second and third treatments (T1 and T2) epithelial hyperplasia showed widening and curvature of the secondary lamellae with incomplete fusion due to edema and slight congestion, which led to increased expansion of the secondary lamellae and blood spots on the surface of the gills. In the fourth and fifth treatments (T4 and T5) showed increased fusion and incomplete curvature of the pr to aneurysms and imary and secondary lamellae with hyperemia. In addition bleeding telangiectasias compared to the control group [23].
The photomicrograph shows the effect on the gills of Nile tilapia after exposure for 45 days T1control group. T2 and T3Fish exposed to 1/4 and 1/8 LC50 CuOBPs, T4 and T5Fish exposed to 1/4 and 1/8 LC50of CuONPs (Figure 1). Fish in the control group (T1)) showed normal histological structure of the gill, primary and secondary gill lamellae [24]. (T2) and (T3) Dilation and curvature of the secondary lamellae with incomplete fusion due to edema and slight engorgement showed increased dilatation of the secondary lamellae and blood spots on the surface of the gills (T4) and (T5) Increased fusion and incomplete curvature of the primary and secondary lamellae with hyperemia In addition to aneurysms and bleeding telangiectasias.
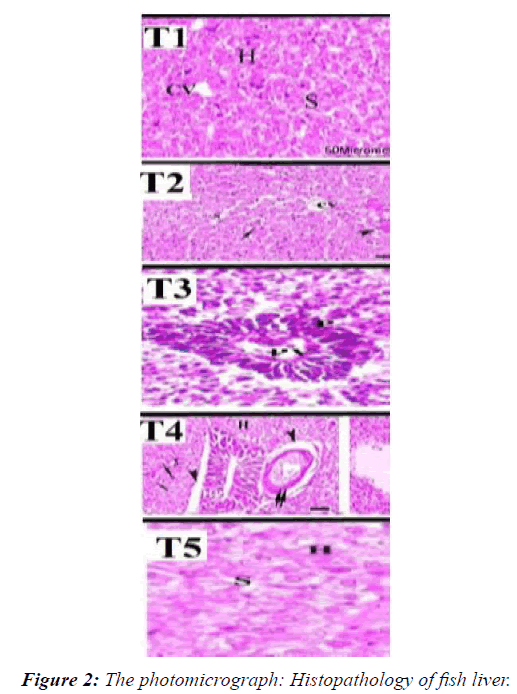
Histopathology of fish liver
Fish in the control group (T1) showing normal hepatic cells, intact hepatic lobules that border Cytoplasmic vacuolation and sinusoid with normal hepatic cord. The histopathological alterations of the liver tissues in fish groups exposed to sublethal levels of CuOBPs and CuONPs showed dose-dependent alterations ranged from Shows vacuolation and blood congestion of Cytoplasmic vacuolation, diffuse vacuolated hepatocytes and moderate necrosis, alterations ranged from slight vascular congestion; diffuse fatty vacuolization in hepatocytes moderate congestion, diffuse fatty vacuolized hepatocytes and moderate necrosis, and severe necrosis, fatty degeneration of hepatocytes and severe congestion of blood sinusoids [25-29]. In the second (T2) Shows vacuolation and blood congestion of Cytoplasmic vacuolation, diffuse vacuolated hepatocytes and moderate necrosis. In the third (T3) alterations vascular congestion and diffuse fatty vacuolization in hepatocytes In the fourth and fifth treatments (T4 and T5) Portal vein, Necrosis congestion, diffuse fatty visualized hepatocytes, severe necrosis, and fatty degeneration of hepatocytes and severe congestion of blood sinusoids [30].
The photomicrograph shows the effect on the liver of Nile tilapia after exposure for 45 days T1control group. T2 and T3Fish exposed to 1/4 and 1/8 LC50 CuOBPs, T4 and T5Fish exposed to 1/4 and 1/8 LC50of CuONPs (Figure 2). (T1) in the control group showing normal hepatic cells, intact hepatic lobules that border Cytoplasmic vacuolation and sinusoid with normal hepatic cord (T2) Shows vacuolation and blood congestion of Cytoplasmic vacuolation, diffuse vacuolated hepatocytes and moderate necrosis [31]. (T3) showed that is alterations ranged from slight vascular congestion; diffuse fatty vacuolization in hepatocytes (T4 and T5) Portal vein, Necrosis congestion, diffuse fatty visualized hepatocytes, severe necrosis, and fatty degeneration of hepatocytes and severe congestion of blood sinusoids.
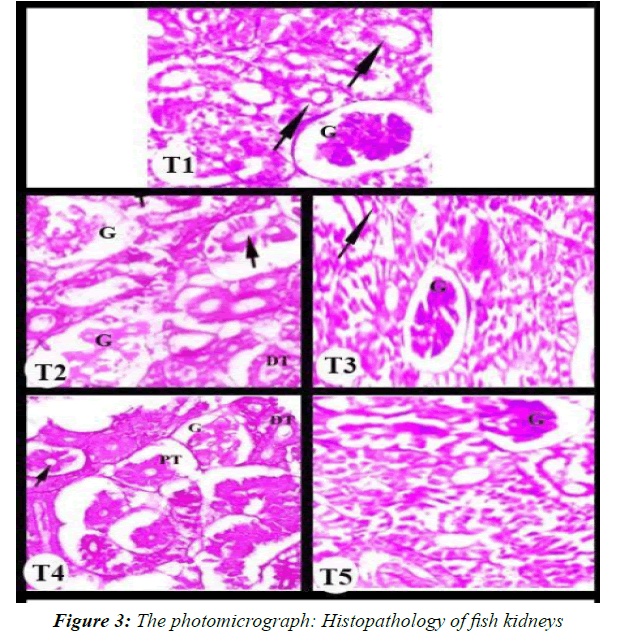
Histopathology of fish kidneys
Fish in the control group (T1) showed normal histological structure of kidney tissue, normal renal corpuscle, glomerulus and renal tubules [32]. The histopathological alterations of the kidney tissues in fish groups exposed to sub-lethal levels of CuOBPs and CuONPs showed dose-dependent alterations ranged from Shows vacuolation and blood congestion of Cytoplasmic vacuolation, diffuse vacuolated hepatocytes and moderate necrosis. Alterations vascular congestion and diffuse fatty vacuolization in hepatocytes in the fourth and fifth treatments Portal veins, Necrosis congestion, diffuse fatty visualized hepatocytes, severe necrosis, and fatty degeneration of hepatocytes and severe congestion of blood sinusoids. (T2) and (T3) granuloma central necrosis in the kidney tissue, Glomerular expansion and with increase hemorrhage in interstitial tissue cells. (T4) and (T5) several histopathological lesions, large area necrosis, degenerated and deterioration of glomerulus, detachment of edema forming tubular epithelial cells and Constricted disappearance [33].
The photomicrograph shows the effect on the kidney of Nile tilapia after exposure for 45 days. T1 control group. T2 and T3Fish exposed to 1/4 and 1/8 LC50 CuOBPs, T4 and T5Fish exposed to 1/4 and 1/8 LC50of CuONPs (Figure 3). Fish in the control group (T1)) showed normal histological structure of kidney tissue, normal renal corpuscle, glomerulus and renal tubules. (T2) and (T3) granuloma central necrosis in the kidney tissue Glomerular expansion and with Increase Hemorrhage in interstitial tissue cells (T4) and (T5) several histopathological lesions, large area necrosis, degenerated and deterioration of glomerulus, detachment of edema forming tubular epithelial cells and Constricted disappearance [34].
Dissection
Biochemical indices
Copper is important for biological functions. Copper can be harmful to fish. These effects include behavioral changes, disruption of blood and biochemical parameters, imbalances in antioxidant defense, as well as pathological lesions in tissues, and the accumulation of copper in liver tissues is related to its concentration in the environment and time of exposure [35-39]. Investigating the effects of nanomaterials on the aquatic ecosystem, which ultimately receives overflow and wastewater from domestic and industrial sources, has become an important topic. These concerns have prompted a number of studies examining the release of these materials from functional Nano products. Nano CuO is one of the most interesting innovations in medical care, as it reduces microbial infections within hospitals. In order to evaluate the possibility of using nanoparticle-induced modifications in the tested organism as biomarkers of nanoparticle water contamination, the present investigation showed a dose-dependent increase in the activities of biochemical and haematological indicators in groups exposed to CuOBPs and CuONPs compared to a control group after a long-term exposure 45 days [40]. In line with our findings, Abdel-Khalek et al. described a marked elevation of uric acid and creatinine values and of ALP, ALT, and AST activities in Nile tilapia exposed to 15 or 7.5 mg/L CuONPs after long-term 30 days water exposure. In the same sense, the values of urea, creatinine, ALP, ALT, and AST were significantly increased in Nile tilapia exposed for 14 days to different sub-lethal levels of CuONPs [42]. Moreover, Kaviani et al., showed a similar increase in ALP and AST activities in Caspian trout exposed to sublethal levels of CuONPs for 28 days. Similarly, Abdel-Daim et al. reported a similar increase in blood urea, creatinine, and ALP, AST, and et al., ALT values in Nile tilapia exposed to sub-lethal doses of zinc oxide NPs for 30 days. Increased serum urea and creatinine levels indicate impaired renal function, which may be closely related to renal tubular dysfunction and insufficient glomerular infiltration. Moreover, the release of ALT, ALP, and AST enzymes into the bloodstream and the subsequent increase in their serum levels are biomarkers of liver damage and hepatitis following exposure to aqueous pollutants and toxicants. Blood parameters can be affected by a wide range of internal and external factors. The hematopoietic the response is nonspecific toward chemical stressors; however, it could indicate that the fish are under environmental stress. Changes in the red blood cell profile are likely an adaptive response to impaired gas exchange in gills exposed to Cu and increased energetic demand in fish. In the blood of fish subjected to stress, an increase in erythrocyte counts, hemoglobin concentrations, and hematocrit levels was frequently observed. In our study, increased erythrocyte counts, white blood cell counts, hematocrit values, MCV, MCH, and MCHC values could be due to erythropoiesis due to chronic metal exposure [41].
Histopathological Alterations of gills
Pathologic alteration of the gills may lead to impairment of several functions, including respiration, osmoregulation, acid-base balance, and metabolite secretion. Thus, the histopathology of gills appears to be a good biomarker for assessing the effects of environmental stress on fish. In this study, histopathological changes in gills of fish exposed to sub-lethal levels of CuOBPs and CuONPs for 45 days showed varying degrees of necrosis, epithelial fusion, rupture, hyperplasia, and edema of the primary and secondary gill lamellae corresponding to different exposure dose (Figure 1). Abdelkhalek et al. reported similar results in Nile tilapia exposed to sub-lethal doses of CuONPs. Al-Bairuty et al. showed that exposure to CuONPs resulted in edema, hypertrophy, lamellar fusion, tortuous tips, aneurysms, and necrotic changes in secondary gill plates of rainbow trout. Gupta, et al., showed that edema of the gill epithelium, hypertrophy at the base of the secondary gill lamellae, zigzag tips, lamellar fusion, transverse aneurysm, and swelling of the secondary lamellae were evident in common carp exposed to CuONPs. Besides, shortening of the primary gill lamellae and fusion of the secondary gill lamellae was evident in Siberian sturgeon (Acipenser baerii) after CuONPs exposure for 21 days.
Histopathological alterations of liver tissues
The liver is the main organ for detoxification of xenobiotics, and histopathological changes in liver and pancreas tissues are often associated with exposure of fish to aquatic pollutants. In this investigation, histopathological changes of liver tissues in groups of fish exposed to sub-lethal levels of CuOBPs and CuONPs showed dose-dependent changes that ranged from the appearance of vacuoles and hyperemia in the cytoplasmic vacuole, to proliferation of vacuolated hepatocytes and moderate necrosis, and the changes ranged from slight vascular hyperemia. Diffuse fatty discharge in hepatocytes moderate congestion, diffuse fatty vacuolating hepatocytes and moderate necrosis, severe necrosis, fatty degeneration of hepatocytes and severe congestion in the sinusoids of blood. Previous reports indicated that fish exposed to CuONPs showed hepatic damage. Al-Bairuty et al. reported the incidence of necrosis, small foci of hepatitis-like injury, enlarged nucleoli, and increased number of melanoma deposits in common carp exposed to CuONPs. Furthermore, Gupta et al., reported that marked increase in the number of demolished nuclei, vacuoles, and necrotic changes in the hepatic and pancreatic tissues of common carp exposed to CuONPs. Ostaszewska et al. also reported lysing hepatocytes from Siberian sturgeon exposed to CuONPs. Similarly, degenerative changes and vacuolization of hepatocytes, fermented nuclei, a damaged central vein, nuclear hyperplasia, and dilated hepatic sinusoids were observed in the hepatic tissue of common carp exposed to CuONPs. Recently, hepatic damage has also been identified in P. lineatus exposed to sub-lethal doses of CuONPs. It should be noted that exposure of fish to toxic substances and aquatic pollutants may lead to disorganization and vacuolization of hepatocytes, alteration of the shape and size of nuclei, and focal necrosis. Furthermore, Al-Bairuty et al. suggested that vacuolation of hepatocytes in fish exposed to CuONPs toxicity may be related to the bioaccumulation of triglycerides in hepatocytes.
Histopathological alterations of kidney
Histopathological changes of kidney tissues in fish groups exposed to sub-lethal levels of CuOBPs and CuONPs showed dose-dependent changes that ranged from the appearance of vacuoles and hyperaemia of the cytoplasmic vacuole, to proliferation of vacuolated hepatocytes and mild necrosis. Vascular congestion changes and proliferation of fatty vacuoles in hepatocytes in the fourth and fifth treatments. Portal veins, hyperemia of necrosis, proliferation of visible fatty hepatocytes, severe necrosis, fatty degeneration of hepatocytes, severe congestion of the sinusoids, epithelial cell damage of the renal tubules, changes in Bowman's voids, and increased foci of melanocytic deposits have been reported in kidneys of rainbow trout exposed to CuONPs. Furthermore, Gupta et al., reported that tubular necrosis, epithelial cell damage of renal tubules, and increased Bowman's distances in common carp exposed to sub-lethal doses of CuONPs.
The results of our study indicate a disturbance in the osmotic regulation mechanisms of fish kidneys. In general, degenerative and necrotic changes in the renal tubular epithelium may be associated with heavy metal and pesticide toxicity in fish. Moreover, the increased Bowman's spaces may be attributed to impaired glomerular filtration resulting from obstruction of the tubule lumen due to damage to the epithelial cells of the renal tubules.
References
- Abdel-Daim MM, Eissa IAM, Abdeen A, et al. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environmental Toxicology and Pharmacology, 2019;69:44-50.
- Abdel-Khalek AA, Kadry MAM, Badran SR, et al. Comparative toxicity of copper oxide bulk and nano particles in Nile Tilapia; Oreochromis niloticus: Biochemical and oxidative stress. The J Basic & App Zoo, 2015;72:43-57.
- Abdel-Latif HMR, Dawood MAO, Menanteau-Ledouble S, et al. Environmental transformation of n-TiO2 in the aquatic systems and their ecotoxicity in bivalve mollusks: A systematic review. Ecotoxicology and Environmental Safety. 2020;200:110776.
- Ajitha B, Reddy YAK, Reddy PS. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater Sci Eng C. 2015;49:373-381.
- Al-Bairuty GA, Shaw BJ, Handy RD, et al. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology. 2013;126:104-115.
- Al-Bairuty GA, Shaw BJ, Handy RD, et al. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aqua Toxicology. 2013;126:104-115.
- Antony JJ, Sivalingam P, Siva D, et al. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophoraapiculata and glucose. Colloids Surf B. 2011;88:134-140.
- Banu A, Vandana R, Ranganath E. Silver nanoparticle production by Rhizopusstolonifer and its antibacterial activity against extended spectrum b-lactamase producing (ESBL) strains of Enterobacteriaceae. Mater Res Bull. 2011;46:1417-1423.
- Canli EG, Dogan A, Canli M. Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environmental Toxicology and Pharmacology. 2018;062:181-187.
- Cazenave J, Wunderlin D, Hued A, et al. Haematological parameters in a Neotropical fish, Corydoras paleatus (Jenyns, 1842) (Pisces, Callichthyidae), captured from pristine and polluted water. Hydrobiologia. 2005;537:25-33.
- Dawood MAO, Abdel-Tawwab M, Abdel-Latif HMR. Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Reviews in Aquaculture 2020;12:2511-2526.
- Duran S, Tuncsoy M, Ay O, et al. Accumulation of copper oxide nanoparticles in gill, liver and muscle tissues of Clarias gariepinus. Toxicology Letters, 2017;280:S186.
- El Euony OI, Elblehi SS, Abdel-Latif HM, et al. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environmental Science and Pollution Research, 2020;27:23108-23128.
- Fabrega J, Luoma SN, Tyler CR, et al. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ Int 2011;37:517-531.
- Ganesan S, Thirumurthi AN, Raghunath A, et al. Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J Applied Toxicology. 2016;36:554-567.
- Griffitt K, Hyndman ND, Denslow DS, et al. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol Sci. 2009;404-415.
- Gupta YR, Sellegounder D, Kannan M, et al. Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): Biochemical, histological and proteomic approaches. Aquaculture and Fisheries. 2016;1:15-23.
- Hua J, Vijver MG, Ahmad F, et al. Toxicity of different-sized copper nano- and submicron particles and their shed copper ions to zebrafish embryos. Environmental Toxicology and Chemistry, 2014;33: 1774-1782.
- Jezierska B, Witeska A. Metal Toxicity to Fish. 1sted. University of Podlasie, Siedlce, Poland. 2001;318.
- Kanipandian N, Thirumurugan RA. Feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypiumhirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Ind Crop Prod 2014;55:1-10.
- Kaviani E, Naeemi A, Salehzadeh A. Influence of copper oxide nanoparticle on hematology and plasma biochemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution. 2019;5:225-234.
- Kaviani E, Naeemi A, Salehzadeh A. Influence of copper oxide nanoparticle on hematology and plasma biochemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution. 2019;5:225-234.
- Keller AA, Adeleye AS, Conway JR, et al. Comparative environmental fate and toxicity of copper nanomaterials. Nano Impact. 2017;7:28-40.
- Kondera E, Witeska M. Cadmium and copper reduce hematopoietic potential in common carp (Cyprinus carpio L.) head kidney. Fish Physiology and Biochemistry. 2013;39:755-764.
- Lazary A, Irina W, Vatine JJ, et al. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Inter J Infect Dise. 2014;24:23-29.
- Malhotra N, Ger TR, Uapipatanakul B, et al. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials. 2020;10:1126.
- Mansouri B, Rahmani R, Azadi NA, et al. Effect of waterborne copper oxide nanoparticles and copper ions on guppy (Poecilia reticulata): Bioaccumulation and histopathology. J Adv in Environmental Health Res. 2015;3:215-223.
- Moon TW. Glucose intolerance in teleost fish: Face or fiction? Comparative Biochemistry and Physiology B-Biochemistry and Molecular Biology. 2001;129:243-249.
- Nespolo R, Rosenmann F. Intraspecific allometry of haematological parameters in Basilichthys Australis. J Fish Biology. 2002;60:1358-1362.
- Noureen A, Jabeen F, Tabish TA, et al. Assessment of copper nanoparticles (Cu-NPs) and copper (II) oxide (CuO) induced hemato-and hepatotoxicity in Cyprinus carpio. Nanotechnology, 2018;29:144003.
- Ostaszewska T, Chojnacki M, Kamaszewski M, et al. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environmental Sci and Pollution Res. 2016;23:1621-1633.
- Rajkumar KS, Kanipandian N, Thirumurugan R. Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeorohita. Appl Nanosci. 2016;6:19-29.
- Sanchez GR, Castilla CL, Gomez NB, et al. Leaf extract from the endemic plant Peumusboldus as an effective bio product for the green synthesis of silver nanoparticles. Mater Lett. 2016;183:255-260.
- Shaw BJ, Handy RD. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environment International. 2011;37:1083-1097.
- Sun Y, Mayers B, Xia Y. Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process. Nano Lett. 2003;3:675-679.
- Tesser ME, de Paula AA, Risso WE, et al. Sublethal effects of waterborne copper and copper nanoparticles on the freshwater Neotropical teleost Prochilodus lineatus: A comparative approach. Science of the Total Environment. 2020;704:135332.
- Velma V, Tchounwou PB. Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, Carassius auratus. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010;698:43-51.
- Vivek R, Thangam R, Muthuchelian K, et al. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxicity effect on MCF-7 cells. Process Biochem 2012;47(12):2405-2410.
- Wijnhoven SW, Peijnenburg WJ, Herberts CA, et al. Nano-silver-a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3:109-138.
- Witeska M, Kondera A, Lipionoga E, et al. Changes in oxygen consumption rate and red blood pa- rameters in common carp Cyprinus carpio L. after acute copper and cadmium exposures. Fresenius Environmental Bulletin. 2010;19:115-122.
- Xi-Feng Z, Zhi-Guo L, Shen W, et al. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:1534.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref