Research Article - Biomedical Research (2017) Volume 28, Issue 4
Evaluation of the prognostic role of androgen receptor positivity in breast cancer
Eda Erdis1*, Tulay Koc2, Birsen Yucel1 and Sahande Elagoz21Department of Radiation Oncology, Medical School of Cumhuriyet University, Sivas, Turkey
2Department of Pathology, Medical School of Cumhuriyet University, Sivas, Turkey
- *Corresponding Author:
- Eda Erdis
Department of Radiation Oncology
Medical School of Cumhuriyet University, Turkey
Accepted date: August 08, 2016
Abstract
Aim: Estrogen Receptors (ER) and Progesterone Receptors (PR) are known to play a role in breast cancer as both predictive and prognostic markers. However, the influence of Androgen Receptors (AR), a member of the steroid receptor family, remains controversial. In this study, we aimed to determine the relationship between Androgen Receptors (AR) expression and the prognostic factors of breast cancer.
Materials and Methods: Pathologic specimens of 100 breast cancer patients were stained with an Androgen Receptors (AR) immunohistochemical stain, and the nuclear staining was positive. The intensity and distribution scores of the stainings were summed, and then the total score was calculated. A total score from 0-1 was accepted as negative, while an overall score of 2 and above was positive. The obtained data were evaluated with Chi-square and descriptive statistical methods. A survival analysis was carried out using a Kaplan-Meier test.
Findings: The median age of patients was 53 years (range: 17-92 years). Androgen Receptor (AR) positivity was present in 66 (66%) of participants. There were statistically significant relationships between Androgen Receptor (AR) positivity and tumour type (p=0.012), grade (p<0.001), Estrogen Receptor (ER) positivity (p<0.001), Progesterone Receptor positivity (p=0.002), triple negativity of breast cancer (p=0.001), the Ki-67 Index (p=0.007), and the recurrence rate (p=0.043). There were no statistically significant associations between Androgen Receptor positivity and menopausal status, stage, tumour diameter, lymph node involvement, multicentricity, lymphovascular invasion, human epidermal growth factor positivity, or distant metastasis (p>0.05).
Conclusion: Androgen receptor may be a good prognostic factor because of its association with lowgrade breast cancer), estrogen receptor positivity, progesterone receptor positivity, and low Ki-67 Index values. However, this finding will need to be confirmed by large cohort studies.
Keywords
Breast cancer, Androgen receptor, Prognosis
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer deaths in females in economically developing countries worldwide [1]. Therefore, new strategies are being developed for breast cancer prevention and treatment [1]. A patient’s hormone receptor status is known to affect a breast cancer diagnosis as well as its histological typing and treatment response evaluation [2].
The identification and definition of the estrogen and progesterone receptor status in breast cancer is a standard histological evaluation procedure [3,4]. The prognostic significance of Estrogen Receptor (ER) and Progesterone Receptor (PR) expression in breast cancer and their roles in therapy are well recognized, but the significance of AR expression is less well characterized [5].
In a previous study, Androgen Receptor (AR) expression was present in 80% of primary breast cancer patients and 60% of metastatic breast cancer patients [6]. Androgen receptor expression varies across breast cancer subtypes and may be correlated with a patient’s Estrogen receptor status. The rates of androgen receptor expression were reportedly 84-95% for estrogen receptor-positive tumours [7,8]. Currently, one of the main subjects in hormone research is androgen receptor positivity, particularly in Triple-Negative Breast Cancer (TNBC). In an evaluation of 19 published studies, the rates of androgen receptor positivity in Triple-Negative Breast Cancer (TNBC) cases ranged from 0-53% in all Triple-Negative Breast Cancer (TNBC) patients; this difference may be largely due to the methodological differences used to analyse androgen receptors [9].
In this study, we aimed to research the relationship between androgen receptor expression and the prognostic factors of breast cancer.
Materials and Methods
We retrospectively investigated 100 patients who had been diagnosed with breast cancer, were followed up at the Department of Radiation Oncology at Cumhuriyet University College of Medicine, and whose specimens had been accessed at the Department of Pathology at Cumhuriyet University College of Medicine between 2008 and 2013. The data of patients were retrospectively analysed and evaluated.
Immunohistochemical staining was used to evaluate the cells’ androgen receptor expression status. Nuclear staining was accepted as a positive result. The intensity score and distribution score of these stainings were summed, and the total score was calculated. A total score from 0-1 was negative, while a total score of 2 and above was positive. The estrogen receptor, progesterone receptor, Human Epidermal Growth Factor 2 (HER2), and Ki-67 status of the cells were reexamined.
We performed all analyses using the Statistical Package for the Social Sciences (SPSS) software, version 15.0 (SPSS Inc., Chicago, IL, USA). The Chi-square test and descriptive statistical methods were used to evaluate the work data. Kaplan-Meier test was used for the survival analysis. We considered the differences to be statistically significant at a p-value< 0.05 (two-sided test), and the results were evaluated at the 95% Confidence Interval (CI).
Results
One hundred breast cancer patients’ data were analysed, and the median age of these patients was 53 years (range: 17-92 years). Forty-three patients (43%) had been diagnosed in the premenopausal period, and 57 (57%) were diagnosed during the postmenopausal period.
The tumour types diagnosed in this study’s participants were Invasive Ductal Carcinoma (IDC) in 61 patients (61%) and all other types of carcinoma in 39 patients (39%). These other types of carcinomas included mixed carcinoma (9%), metaplastic carcinoma (7%), medullary carcinoma (2%), medullary differentiated carcinoma (9%), apocrine breast carcinoma (5%), invasive papillary carcinoma (2%), invasive micro papillary carcinoma (2%), invasive cribriform carcinoma (2%), and tubular carcinoma (1%). Out of the 100 total patients, 19 (19%) were graded as having stage I breast cancer, 43 (43%) were stage II, 29 (29%) were stage III, and 9 (9%) were stage IV. The lymph nodes were negative in 47 patients (47%) and positive in 53 patients (53%).
Fourteen patients (14%) were rated as grade 1, 58 (58%) were grade II, and 26 (26%) were grade III. The tumour diameter was 5 cm or larger in 21 patients (21%) and was smaller than 5 cm in 79 patients (79%). Lymphovascular invasion was noted in 47 patients (47%), while multicentricity was identified in 22 (22%).
Estrogen receptor positivity was found in 60 patients (60%); 54 participants (54%) demonstrated progesterone receptor positivity, while androgen receptor positivity was identified in 66 (66%). HER2 positivity was present in 41 patients (41%). A triple negative hormone profile was found in 21 (21%) patients. The Ki-67 proliferation index score was low (≤ 15) in 36 patients (36%) and high (>15) in 64 patients (64%).
There was no statistically significant relationship between androgen receptor and menopausal state (p=0.211), disease stage (p=0.367), tumour diameter (<5c m vs. ≥ 5 cm; p=0.420), lymph node status (p=0.502), multicentricity (p=0.156), lymphovascular invasion (p=0.580), HER status (p=0.147), or distant metastasis (p=0.478).
However, a statistically significant relationship was identified between androgen receptor and tumour type (p=0.012), grade (p<0.001), estrogen receptor (p<0.001), progesterone receptor (p=0.002), triple negative hormone profile (p=0.001), Ki-67 proliferation index (p=0.007), and rate of recurrence (p=0.043). The androgen receptor-related parameters are shown in Table 1. A comparison of the parameters that were in accordance with an estrogen receptor or triple negative hormone profile is demonstrated in Tables 2 and 3.
| Patient number: N:100 | AR (-) N: 34 (34%) | AR (+) N: 66 (66%) | p value | 95% Confidence interval of the difference (Lower-upper) | |
|---|---|---|---|---|---|
| Tumour type | |||||
| IDC | 61 | 15 (44) | 46 (70) | 0.012 | 0.056-0.456 |
| Others | 39 | 19 (56) | 20 (30) | ||
| Grade | |||||
| Grade I | 14 | 3 (9) | 11 (17) | <0.001 | 0.236-0.766 |
| Grade II | 58 | 12 (35) | 46 (70) | ||
| Grade III | 26 | 19 (56) | 9 (13) | ||
| ER | |||||
| Negative | 40 | 22 (65) | 18 (27) | <0.001 | 0.568-0.181 |
| Positive | 60 | 12 (35) | 48 (73) | ||
| PR | |||||
| Negative | 46 | 23 (68) | 23 (35) | 0.002 | 0.528-0.128 |
| Positive | 54 | 11 (32) | 43 (65) | ||
| HER | |||||
| Negative | 59 | 23 (68) | 36 (55) | 0.147 | 0.078-0.216 |
| Positive | 41 | 11 (32) | 30 (45) | ||
| Triple (-) | |||||
| Absent | 79 | 20 (59) | 59 (89) | 0.001 | -0.005-0.007 |
| Present | 21 | 14 (41) | 7 (11) | ||
| Ki67 | |||||
| Low (<15) | 36 | 6 (18) | 30 (46) | 0.007 | 0.221-0.908 |
| Medium (15-30) | 27 | 9 (26) | 18 (27) | ||
| High (>30) | 37 | 19 (56) | 18 (27) | ||
| Recurrence | |||||
| Absent | 93 | 29 (85) | 64 (97) | 0.043 | 0.012-0.074 |
| Present | 7 | 5 (15 | 2 (3) | ||
| Distant metastasis | |||||
| Absent | 84 | 28 (82) | 56 (85) | 0.478 | 0.038-0.576 |
| Present | 16 | 6 (18) | 10 (15) | ||
| AR: Androgen Receptor; IDC: Invasive Ductal Carcinoma; ER: Estrogen Receptor; PR: Progesterone Receptors; HER: Human Epidermal Growth Factor. | |||||
Table 1: Comparison of androgen receptor subgroups for several parameters.
| ER (-) N: 34 (34%) | ER (+) N: 66 (66%) | p value | 95% Confidence interval of the difference (Lower-upper) | |
|---|---|---|---|---|
| Tumour type | ||||
| IDC | 17 (42) | 44 (73) | 0.002 | 0.113-0.503 |
| Others | 23(58) | 16 (27) | ||
| Grade | ||||
| Grade I | 2 (5) | 12 (20) | <0.001 | 0.455-0.912 |
| Grade II | 14 (35) | 44 (73) | ||
| Grade III | 24 (60) | 4 (7) | ||
| PR | ||||
| Negative | 22 (65) | 18 (27) | <0.001 | 0.568-0.181 |
| Positive | 12 (35) | 48 (73) | ||
| PR | ||||
| Negative | 32 (80) | 14 (23) | <0.001 | 0.736-0.397 |
| Positive | 8 (20) | 46 (77) | ||
| HER | ||||
| Negative | 24 (60) | 35 (58) | 0.518 | 0.382-0.6654 |
| Positive | 16 (40) | 25 (42) | ||
| Triple (-) | ||||
| Absent | 20 (59) | 59 (89) | 0.001 | 0.005-0.007 |
| Present | 14 (41) | 7 (11) | ||
| Ki 67 | ||||
| Low (<15) | 5 (12) | 31 (52) | <0.001 | 0.462-1.088 |
| Medium (15-30) | 11 (28) | 16 (26) | ||
| High (>30) | 24 (60) | 13 (22) | ||
| ER: Estrogen Receptor; PR: Progesterone Receptors; IDC: Invasive Ductal Carcinoma; HER: Human Epidermal Growth Factor. | ||||
Table 2: A comparison of the estrogen receptor subgroups across several parameters.
| Absent triple (-) | Present triple (-) | p value | 95% Confidence interval of the difference (Lower-upper) | |
|---|---|---|---|---|
| Tumour type | ||||
| IDC | 15 (44) | 46 (70) | 0.012 | -0.009-0.033 |
| Others | 19 (56) | 20 (30) | ||
| Grade | ||||
| Grade I | 14 (18) | - | <0.001 | -0.005-0.007 |
| Grade II | 50 (63) | 8 (38) | ||
| Grade III | 15 (19) | 13(62) | ||
| Ki 67 | ||||
| Low (<15) | 33 (42) | 3 (14) | 0.001 | -0.005-0.007 |
| Medium (15-30) | 24 (30) | 3 (14) | ||
| High (>30) | 22 (28) | 15 (71) | ||
| LVI | ||||
| Absent | 38 (48) | 15 (71) | 0.047 | 0.006-0.088 |
| Present | 41 (52) | 6 (29) | ||
| IDC: Invasive Ductal Carcinoma; LVI: Lymphovascular Invasion. | ||||
Table 3: Comparison of triple negative subgroups for several parameters.
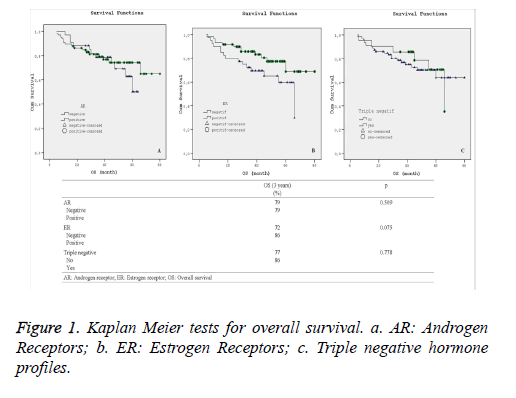
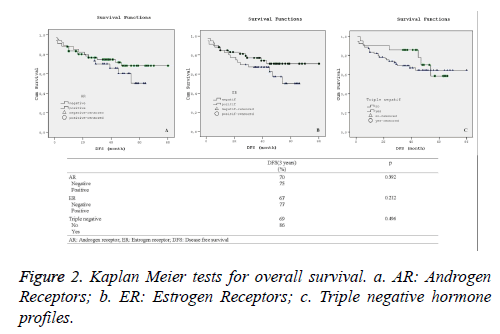
Statistical comparison of overall and disease-free survival rates along with their corresponding varying hormone profiles are presented in Figures 1 and 2. Additionally, Kaplan-Meier curves for the patients’ overall survival and disease free survival in relation with their hormone profiles are shown separately in Figures 1a-1c and 2a-2c.
Although there was no substantial difference in the overall and disease free survival 3-year rate in terms of other hormone profiles, the 3-year Kaplan-Meier survival curves for overall survival as shown in Figure 2b demonstrated visual improvement (p=0.075) survival across in estrogen receptor positive patients.
Discussion
Some cancer deaths may potentially be avoided, particularly when breast cancer is controlled [10]. The early findings from a community-based intervention that was conducted to raise the general awareness of the signs and symptoms of three common cancers (breast, colorectal, and lung) and promote an earlier presentation and diagnosis are presented. The prevention, early diagnosis, and treatment of breast cancer are particularly effective [10,11].
The hormone receptor status of the tumour is an important marker in breast cancer. Estrogen receptors and progesterone receptors are the most commonly studied hormone receptors in breast cancer patients. It has been reported that 60-65% of tumours have positive estrogen and progesterone receptors [12]. These hormonal receptors are important, especially to determine the best medical treatment and assess the treatment response. Tumours that are histologically estrogen receptor (+) are usually well differentiated and have a better prognosis than other types [12,13]. According to these studies, estrogen receptors and progesterone receptors are important parameters that play a role in breast cancer development and prognosis [14]. Although studies regarding this issue are on-going, it is generally accepted that estrogen receptors and progesterone receptors have a basic role in breast cancer prognosis. However, there is not yet a consensus regarding the influence of androgen receptor in breast cancer, and conflicting results have been reported. Although the role of androgen receptor in breast cancer has been neglected, one study noted that the issue of the relationship between androgen receptor and other factors that affect tumour biology and development must be evaluated using a multidisciplinary approach [15]. Androgen receptor positivity is usually found in progesterone receptor (-) breast cancer patients; therefore, it is thought that the androgen receptor signalling pathway has an important and critical role in breast cancer carcinogenesis [16]. Previous studies have reported that androgen receptor positivity was higher in estrogen receptor (+) breast cancer patients than it was in estrogen receptor (-) breast cancer patients [17,18]. The androgen receptor positivity was also previously identified in 9-56% of breast cancer patients who were estrogen receptor-and progesterone-negative [19-22]. In our study, estrogen receptor positivity was found in 73% of patients who were androgen receptor-positive, while progesterone receptor positivity was identified in 65% of androgen receptor-positive patients. As a distinction, the association between progesterone receptor and androgen receptor was higher in our study; there were also statistically significant relationships between androgen receptor and estrogen receptor positivity and also androgen receptor and progesterone receptor positivity.
HER2 stimulates cell proliferation by activating the intracellular signalling pathway. Yu et al. found that HER positivity was evident in 65.9% of androgen receptor-positive breast cancer patients [16]. Another study evaluated 599 androgen receptor-positive breast cancer patients, and HER positivity was found in 12% [23]; in the present study, HER positivity was identified in 45% of androgen receptor (+) breast cancer patients.
Ki-67 is a nuclear antigen that is a marker for cell proliferation; it is commonly thought that active tumour cells express high rates of Ki-67 [24,25]. In an Italian study (N=953), the Ki-67 Index was ≥ 10% in 272 androgen receptor (+) breast cancer patients (51%) and was present at a rate of <10% in 257 androgen receptor (+) breast cancer patients (49%) [23]. In our study, the Ki-67 Index was <15% in 46% of androgen receptor (+) breast cancer patients, between 15-30% in 27% of androgen receptor (+) breast cancer patients, and >30% in 27% of androgen receptor (+) breast cancer patients.
Triple-negative breast cancer has recently been recognized as an important subgroup of breast cancer that has a high risk and often requires aggressive clinical behaviour [13,16,26]. The role of androgen receptors in triple negative breast cancer is not clear; in some studies, it has been reported that androgen receptor positivity has a positive influence on survival, while other studies have suggested that the androgen receptor positivity negatively affects survival. In their study of 282 breast cancer cases [27], Rakha et al. found that a lack of androgen receptor expression was more likely to be present in patients with cancer of a higher histological grade and was also associated with a higher recurrence rate and the presence of distant metastasis. Luo et al. reported that the androgen receptor expression was correlated with a high five-year disease-free survival in a study of 137 triple negative breast cancer cases [28]. In our study, a triple negative hormone profile was found in 11% of androgen receptor (+) breast cancer patients. However, we did not conduct a survival analysis due to our low number of patients.
Androgen receptors have also been examined in prostate cancer patients and have been determined to play important roles in this type of cancer. Emphasis was placed on the effect of androgen receptors on epithelial cell proliferation, which led to the investigation of androgen receptor’s role in breast cancer [29,30]. The relationship between androgen receptors and other receptors was studied, and the predictive ability of androgen receptors for producing a therapeutic response was evaluated [30]. It was found that androgen receptor expression correlates well with estrogen receptor expression; therefore, the androgen receptor expression status may more accurately identify patients with breast cancer who are most likely to respond to hormonal treatment. These results indicate that the effect of androgen receptors in breast cancer patients must be evaluated both alone and along with other receptors [30]. It was reported that androgen receptor (+) breast cancer patients have higher survival rates and better therapeutic responses than androgen receptor (-) breast cancer patients [31]. New studies have suggested that androgen receptors may modify the clinical outcomes in early breast cancer stages [32]. There were positive associations for a high androgen receptor positivity, low recurrence rates, and low death rates [27]. In our study, the recurrence rates were 3% in androgen receptor (-) patients and 15% in androgen receptor (+) patients. These results were statistically significant. Low recurrence rates were also found in androgen receptor (+) breast cancer patients.
Consequently androgen receptor positivity is related to better clinical outcomes (association with low grade, low Ki-67 Index, low local recurrence rates, low triple negativity). Its correlation with both estrogen receptor and progesterone receptor positivity suggests that the predictive and prognostic value of this receptor may be enhanced by evaluating the estrogen receptor and progesterone receptors status of the patient. Large multidisciplinary studies will be needed to reveal this correlation and the effect of androgen receptor on clinical outcome.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90.
- Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, Gelber RD, Curigliano G, Torrisi R, Luini A, Intra M, Galimberti V, Renne G, Nole F, Peruzzotti G, Goldhirsch A. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res 2004; 10: 6622-6628.
- Allemani C, Sant M, Berrino F, Aareleid T, Chaplain G. Prognostic value of morphology and hormone receptor status in breast cancer - a population-based study. Br J Cancer 2004; 91: 1263-1268.
- Molino A, Micciolo R, Turazza M, Bonetti F, Piubello Q, Corgnati A, Sperotto L, Recaldin E, Spagnolli P, Manfrin E. Prognostic significance of estrogen receptors in 405 primary breast cancers: a comparison of immunohistochemical and biochemical methods. Breast Cancer Res Treat 1997; 45: 241-249.
- Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 2003; 120: 725-731.
- Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW, Lee KS. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol Off J Eur Soc Med Oncol Esmo 2011; 22: 1755-1762.
- Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumours and in estrogen receptor-negative tumours with apocrine differentiation. Mod Pathol Off J U S Can Acad Pathol Inc 2010; 23: 205-212.
- Qi JP, Yang YL, Zhu H, Wang J, Jia Y, Liu N, Song YJ, Zan LK, Zhang X, Zhou M, Gu YH, Liu T, Hicks DG, Tang P. Expression of the androgen receptor and its correlation with molecular subtypes in 980 Chinese breast cancer patients. Breast Canc Basic Clin Res 2012; 6: 1-8.
- McNamara KM, Yoda T, Takagi K, Miki Y, Suzuki T. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol 2013; 133: 66-76.
- Richards MA. The National Awareness and Early Diagnosis Initiative in England: assembling the evidence. Br J Cancer 2009; 101 Suppl 2: S1-4.
- Lyon D, Knowles J, Slater B, Kennedy R. Improving the early presentation of cancer symptoms in disadvantaged communities: putting local people in control. Br J Cancer 2009; 101: 49-54.
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF. Prognostic markers in triple-negative breast cancer. Cancer 2007; 109: 25-32.
- Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol 2005; 18: 26-35.
- Pervaiz F, Rehmani S, Majid S, Anwar H. Evaluation of hormone receptor status (ER/PR/HER2-neu) in breast cancer in Pakistan. J Pak Med Assoc 2015; 65: 747-752.
- Moe RE, Anderson BO. Androgens and androgen receptors: a clinically neglected sector in breast cancer biology. J Surg Oncol 2007; 95: 437-439.
- Yu Q, Niu Y, Liu N, Zhang JZ, Liu TJ. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol 2011; 22: 1288-1294.
- Schippinger W, Regitnig P, Dandachi N, Wernecke KD, Bauernhofer T. Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch 2006; 449: 24-30.
- Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD. Androgen receptor inhibits estrogen receptor alfa activity and is prognostic role in breast cancer. Cancer Res 2009; 69: 6131-6140.
- Rakha EA, El-Sayed ME, Green AR, Paish EC, Lee AH, Ellis IO. Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathol 2007; 50: 434-438.
- Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer 2003; 98: 703-711.
- Micello D, Marando A, Sahnane N, Riva C, Capella C. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch 2010; 457: 467-476.
- Park S, Koo J, Park HS, Kim JH, Choi SY. Expression of androgen receptors in primary breast cancer. Ann Oncol 2010; 21: 488-492.
- Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G, Sapino A. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010: 124: 607-617.
- Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB-1 and 3) detect proliferating cells in microwave-processed formalin- fixed parafin sections. J Pathol 1992; 168: 357-363.
- Comin CE, Messerini L, Novelli L, Boddi V, Dini S. Ki-67 antigen expression predicts survival and correlates with histologic subtype in the WHO classification of thymic epithelial tumors. Int J Surg Pathol 2004; 12: 395-400
- Fulford LG, Easton DF, Reis-Filho JS, Sofronis A, Gillett CE, Lakhani SR, Hanby A. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathol 2006; 49: 22-34.
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF. Prognostic markers in triple-negative breast cancer. Cancer 2007; 109: 25-32.
- Luo X, Shi YX, Li ZM, Jiang WQ. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin J Cancer 2010; 29: 585-590.
- Birrell SN, Hall RE, Tilley WD. Role of the androgen receptor in human breast cancer. J Mammary Gland Biol Neoplasia 1998; 3: 95-103.
- Mishra AK, Agrawal U, Negi S, Bansal A, Mohil R. Expression of androgen receptor in breast cancer & its correlation with other steroid receptors & growth factors. Indian J Med Res 2012; 135: 843-852.
- Maggiolini M, Donze O, Jeannin E, Ando S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res 1999; 59: 4864-4869.
- Wang Y, Yang HRE. Androgen Receptor Expression and Outcomes in Early Breast Cancer: A systematic review and meta-analysis. J Natl Cancer Inst 2015; 107.