Research Article - Biomedical Research (2019) Volume 30, Issue 1
Evaluation of the pharmaceutical quality of modified-release tablets containing 60 mg gliclazide, applied in the therapy of diabetes mellitus type 2.
Nadezhda Marchokova1*, Valentina Petkova1 and Milen Dimitrov2
1Department of Social Pharmacy, Medical University of Sofia, Sofia, Bulgaria
2Department of Pharmaceutical Technology and Biopharmacy, Medical University of Sofia, Sofia, Bulgaria
- *Corresponding Author:
- Nadezhda Marchokova
Department of Social Pharmacy
Medical University of Sofia
Bulgaria
Accepted date: February 12, 2019
DOI: 10.35841/biomedicalresearch.30-19-073
Visit for more related articles at Biomedical ResearchAbstract
Doctors and patients often think that the increasing number of adverse reactions and variable therapeutic effect are due to individual variability and quality of generic medicines. Wide-spread opinion among patients is that the original medicinal product is characterized by superior quality and effectiveness compared to generic products and also that there is difference in the quality among generic products. The aim of the present study is to evaluate the pharmaceutical quality of three generic products with modified-release containing the anti-diabetic active substance gliclazide and compare them with the original drug product Diaprel MR 60 mg modified-release tablets. The pharmaceutical quality of the chosen modified-release products containing 60 mg gliclazide was evaluated with respect to mass and uniformity of mass, resistance to crushing, friability and in vitro release behavior in three dissolution media. Also calculated was the factor of similarity. The results evidenced no significant difference in relation to pharmaceutical quality between the original and the generic products. All tested products complied with the requirements of Ph.Eur. in terms of mass and uniformity of mass and friability. The results obtained for resistance to crushing were similar. The dissolution behavior of the original and the generic products in the three dissolution media was also similar. A factor of similarity above 50 was achieved for all generic products in the three dissolution media. The study gives rise to confidence among patients when the original drug product is substituted with a generic drug product thus enhancing their compliance.
Keywords
Gliclazide, Modified-release tablets, Pharmaceutical quality
Introduction
Diabetes mellitus is a metabolic disease, characterized by chronic hyperglycemia, disturbance of carbohydrate, fat and protein metabolism, which is due to defect in insulin secretion, insulin action or both of them [1]. In 2017 the number of people suffering from diabetes mellitus aged between 20 and 79 was 425 million and in 2045 the number is expected to be 629 million people. In Europe the number of people suffering from diabetes mellitus in the same age group was 58 million and their number is expected to increase to 67 million individuals [2].
Sulfonylureas are used for treatment of diabetes mellitus type 2. A lot of similarities exist among sulfonylurea products, yet there are differences in their efficacy and frequency of adverse reactions as well [3].
Gliclazide was included in the Model List of Essential Medicines 2013 by the World Health Organization owing to its safety profile in elderly patients. It causes hypoglycemia to the least extent because of quick binding and reversal to the β-cell receptor [4]. Diamicron MR allows 24 hour control of blood glucose level with a once-daily dosage. Diamicron MR 60 mg is the first scored modified-release diabetic tablet. The tablet could thus be halved for better patient compliance and dose flexibility [5].
Generic medicinal product is a medicinal product which has the same qualitative and quantitative composition in active substances and the same pharmaceutical form as the reference medicinal product, and whose bioequivalence with the reference medicinal product has been demonstrated by appropriate bioavailability studies [6].
It is a common practice doctors and patients to accuse the individual variability as well as possible problems associated with the quality of generic medicines as a potential reason for increasing number of adverse drug reactions or a variable and/or unsafe therapeutic effect. Variability in the therapeutic response, although familiar to clinicians, is still under-discussed and even not yet well understood by patients [7].
Wide-spread opinion among patients is that the original medicinal product is characterized by superior quality and effectiveness compared to generic products and also that there is difference in the quality among generic products. Difference could exist between the definition of equivalence and clinical equivalence for non-narrow therapeutic index drugs. Clinical equivalence could be proven only in everyday medical practice [8].
A study conducted in Medellin-Columbia proves the clinical equivalence between the original product Diamicron and the generic Gliclazide [9].
Several studies have been conducted to evaluate the pharmaceutical quality of generic anti-diabetic products versus the original product. A study by Sakr et al. conducted in the Kingdom of Saudi Arabia, evaluates the quality and pharmacoeconomics of glibenclamide and metformin generic drugs versus their original products. The study established that the generic products are quantitatively and qualitatively equivalent to the original products, but are essentially cheaper when compared with them [10].
Unlike the results obtained by Sakr et al. contradictory results for the quality of generic products are reported in the studies conducted by El-Sabawi et al., Elhamili et al. and Shazly and Mahrous. The study by El-Sabawi et al. evaluates the pharmaceutical quality of glibenclamide products available in Jordanian pharmacies. All products provide satisfactory results in regard to identification and related substances. Assay of the active substance shows compliance with the requirements of assay in USP (90-110%), but the generics differ in terms of dissolution profile as compared against the original product [11]. Elhamili et al. examine the pharmaceutical quality of three generic products containing glibenclamide that are available in the Libyan market. The results obtained by them show again considerable differences in the dissolution profile, while the results for the other tested items (friability, disintegration, identification, assay) are similar and comply with the requirements of the British Pharmacopoeia [12]. A study carried out by Shazly and Mahrous also investigates the physicochemical properties and in vitro dissolution of glibenclamide tablets. A test for release of the active substance from the original and the generic products was carried out in a phosphate buffer with pH 7.4 and pH 7.8 and in a borate buffer with pH 9.5. The dissolution profiles of the generic and original products differ [13].
The objective of the present study is to evaluate the pharmaceutical quality of three generic products with modified-release of the active substance gliclazide and to compare them with the original product Diaprel MR 60 mg modified-release tablets.
Materials and Methods
Materials
Gliclazide, hydrochloric acid 37% (Merck, Germany), Sodium acetate (Merck, Germany), acetic acid (Merck, Germany), potassium phosphate (Merck Germany), sodium base (Merck Germany), purified water were used in the preparation of dissolution media. The original product Diaprel MR 60 mg modified-release tablets, batch № 601062, (Les Laboratoires Servier) and the three generic products (Normodiab MR 60 mg modified-release tablets, batch № 248216, (Actavis Group PTC ehf.), Gliclazide Zentiva 60 mg modified-release tablets, batch № 5151700, (Zentiva), Madras MR 60 mg modified-release tablets, batch № 5151952, (Stada Arzneimittel AG)), each of them containing 60 mg of gliclazide, were purchased from pharmacies in Sofia, Bulgaria. All products were within their shelf life at the time of the conducted study. The products were denoted with the first letter of their trade name, respectively D, N, G and M.
Methods
Mass and uniformity of mass-a test was conducted in accordance with the requirements of Ph.Eur 9.0, chapter 2.9.5. “Uniformity of mass of single-dose preparations”. Defined were an individual mass of 20 tablets and an average mass and deviations there from were calculated for all products.
Resistance to crushing-a test was conducted in accordance with the requirements of Ph.Eur 9.0, chapter 2.9.8. “Resistance to crushing of tablets”. Use was made of an apparatus Erweka type TBH 30, Germany. The force needed to disrupt tablets by crushing was measured.
Friability-a test was conducted in accordance with the requirements of Ph.Eur 9.0, chapter 2.9.7. “Friability of uncoated tablets”. An Erweka type TA 100, Germany, apparatus was used. 20 tablets were accurately weighed up and then placed in a drum. They were rotated for 4 min at 25 rpm. The tablets were then dedusted and accurately weighed up again. The loss in their weight was calculated after the following formula:
%=((initial weight-obtained weight)/initial weight) × 100
In vitro drug release studies-a test was carried out using the apparatus RC-8D Dissolution tester, Minhua Pharmaceutical Machinery Co., Limited, China. Use was made of an apparatus 2 (paddle method). The dissolution media used were 0.1 M HCl with pH 1.2, an acetate buffer with pH 4.5 and a phosphate buffer with pH 7.4. The test was carried out at a rotation speed of 75 ± 2 rpm, in a 900 ml volume and set temperature of 37 ± 0.5°C. Tablets of each product were investigated in the dissolution apparatus. 10 ml samples were withdrawn every hour and the quantity withdrawn was replaced with 10 ml dissolution medium. Each sample was filtered through a membrane filter and the amount of released drug was determined using a spectrophotometric method. A RayLeigh-UV-9200, Beijing Beifen-Ruili Analytical Instrument Co., Ltd, China spectrophotometer was used. The amount of released drug was determined at 226 ± 2 nm wavelength. The percent of released gliclazide was calculated by utilizing a standard calibration curve obtained in advance.
Determining the similarity factor (f2): the similarity factor (f2) was defined, as follows:
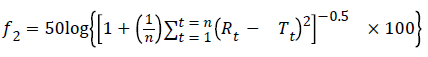
where n is the number of time points, Rt is the average percentage of original drug dissolved at time t after initiation of the study, Tt is the average percentage of generic drug dissolved at time t after initiation of the study. The dissolution profiles are similar when the values for f2 are between 50 and 100 [6].
Results
Investigation of physico-chemical characteristics
Physico-chemical characteristics, including mass and uniformity of mass, resistance to crushing and friability, were analysed for all drug products and the obtained results are presented in Table 1.
| Test items | D | N | G | M | |
|---|---|---|---|---|---|
| Mass and uniformity of mass | Average mass, g | 0.3259 | 0.3395 | 0.3214 | 0.3219 |
| Deviation from the average mass, % | +1.46/-0.9 | +1.61/-1.78 | +1.49/-1.9 | +1.69/-1.98 | |
| RSD, % | 0.81 | 1.11 | 1.31 | 1.16 | |
| Resistance to crushing | Average, N | 82.8 | 139.3 | 99.2 | 99.7 |
| Minimum, N | 77 | 119 | 87 | 90 | |
| Maximum, N | 88 | 149 | 109 | 108 | |
| RSD, % | 4.37 | 6.56 | 8.1 | 5.35 | |
| Friability, % | 0.15 | 0.03 | 0.02 | 0.26 | |
Table 1: Investigation of physico-chemical characteristics.
The uniformity of mass of all tested products was compliant with the requirement of Ph. Eur., chapter 2.9.5. as none of the tablets deviated from the average mass by more than ± 5%. The deviation was very low: ± 2%, which was also confirmed by the results obtained for relative standard deviation (RSD)-below 1.5%. The average mass of the original drug product (D) and the generics G and M was almost the same. It was only the generic product N that had a slightly higher average mass-0.340 g. The average resistance to crushing for the tested products was in the range of 80-140 N. The highest resistance to crushing was obtained for generic product N, which could be associated with the higher value of mass of one tablet. For products G and M the average resistance to crushing was almost the same. The smallest deviation for the tested item was observed in the original product-RSD 4.37%. Friability for all tested products was below 0.3%, which complied with the requirement of Ph.Eur., chapter 2.9.7-not to exceed 1%.
In vitro drug release studies
In vitro drug release of gliclazide was investigated in three dissolution media-0.1 M HCl with pH 1.2, an acetate buffer with pH 4.5 and a phosphate buffer with pH 7.4.
In a phosphate buffer with pH 7.4 the percentage of drug released after 2 hours was between 17 and 21%, after 4 hours between 41-44% and after 9 hours-more than 80%. In an acetate buffer with pH 4.5 the release of gliclazide was similar to that in a phosphate buffer with pH 7.4 after 2 and 4 hours, 15-21% and 37-47% respectively, but after that the release was slower and after 9 hours the percentage of dissolved drug was between 63-75%. At 0.1 M HCl with pH 1.2 the release of active substance was quicker. This tendency was observed right from the beginning of the process-after 2 hours (20-35%), after 4 hours (51-62%) and after 7 hours-more than 80% of the active substance was released from all tested products.
Discussion
It was shown by the results that there were no significant differences in terms of mass and uniformity of mass, resistance to crushing and friability. The dissolution behavior of original and generic products in the three dissolution media was similar. The fact was also confirmed by the calculated factor of similarity (f2). Similarity factors of N as against D in 0.1 M HCl, pH 4.5 and pH 7.4, were 56.4, 55, 68.4 respectively. Similarity factors of G as against D in 0.1 M HCl, pH 4.5 and pH 7.4, were 51.7, 61.6, 84.3 respectively. Similarity factors of M as against D in 0.1 M HCl, pH 4.5 and pH 7.4, were 52.6, 71.3, 65.3 respectively. The requirement for factor of similarity above 50 was achieved for all generic products in the three dissolution media.
Conclusion
All tested products were in compliance with the requirements of Ph.Eur. from the view point of mass and uniformity of mass and friability. The results obtained for resistance to crushing were similar. Dissolution behavior of the generic products was comparable to that of the original product in the three tested dissolution media, which was confirmed by the values obtained for the similarity factor. In general, the conducted study does not show difference in the pharmaceutical quality of generic products compared to the original medicinal product. The study gives rise to confidence among patients when the original drug product is substituted with generic drug product, thus enhancing their compliance.
References
- World Health Organization. Department of Noncommunicable Disease Surveillance. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and Classification of Diabetes mellitus. Geneva: World Health Organization 1999; 66.
- International Diabetes Federation. IDF Diabetes Atlas (8th Edn.). Brussels, International Diabetes Federation 2017; 9.
- Harrower ADB. Comparison of efficacy, secondary failure rate, and complications of sulfonylureas. J Diabetes Compl 1994; 8: 201-203.
- Singh AK, Singh R. Is gliclazide a sulfonylurea with difference? A review in 2016. Exp Rev Clin Pharmacol 2016; 9: 839-851.
- Laroche S. Crowning four decades of evidence-based benefits and advances in diabetes: Diamicron MR 60 mg. Medicographia 2011; 33: 63-71.
- European Medicines Agency. Committee for medicinal products for human use (CHMP) Guideline on the Investigation of Bioequivalence 2010.
- Paveliu MS, Bengea S, Paveliu FS. Generic substitution issues: brand-generic substitution, generic-generic substitution, and generic substitution of narrow therapeutic index (NTI)/critical dose drugs. Maedica (Buchar) 2011; 6: 52-58.
- Reiffel JA. Formulation substitution and other pharmacokinetic variability: underappreciated variables affecting antiarrhythmic efficacy and safety in clinical practice. Am J Cardiol 2000; 85: 46D-52D.
- Amariles P, Muňoz А, Restrepo М, Villegas А. Clinical efficacy of generic gliclazide with respect to the original drug in the treatment of type 2 diabetes in members of the ISS: Medellin-Columbia. Pharm Care Esp 2001; 3: 370-390.
- Sakr FM, Alobaidy KG, Almarri AF, Alkefiri GA, Alhabshee NF. Evaluation of the quality and pharmacoeconomics of some generic drugs versus their reputed counterpart brands in the Saudi market. Saudi J Oral Sci 2016; 3: 97-103.
- El-Sabawi D, Abbasi S, Aljafari S, Hamdan II. Pharmaceutical evaluation of glibenclamide products available in the Jordanian market. Afr J Pharm Pharmacol 2013; 7: 1464-1470.
- Elhamili A, Bergquist J, El-Attug M, Saad S, Saad F, Hemiss G, Almog T. Pharmaceutical evaluation of type II oral antidiabetic agent. Int J Pharma Res Rev 2014; 3: 1-9.
- Shazly GA, Mahrous GM. Assessment of the physicochemical properties and in vitro dissolution of glibenclamide tablets marketed in Saudi Arabia. Dissolution Technologies 2014; 21: 61-66.