- Biomedical Research (2015) Volume 26, Issue 1
Evaluation of the efficacy of Nawalution woundcare® solution used against various microorganisms.
Keramettin Yanik1*, Kemal Bilgin2, Adil Karadag1, Nevzat Ünal3, Hakan Odabaşı1, Hamza Kadı4, Şaban Esen5, Murat Günaydın61Ondokuz Mayis University, Faculty of Medicine, Department of Medical Microbiology, Samsun, Turkey
2Ondokuz Mayis University, Vocational School of Health Services, Department of Medical Services and Techniques, Samsun, Turkey
3Adana Numune Education and Research Hospital, Laboratory of Microbiology, Adana, Turkey
4Veterinary Control Institute, Department of Virology, Samsun, Turkey
5Ondokuz Mayis University, Faculty of Medicine, Department of Infectious Diseases and Clinical Microbiology, Samsun, Turkey
6İstanbul University, Cerrahpaşa Faculty of Medicine,Department of Medical Microbiology, İstanbul, Turkey
- *Corresponding Author:
- Keramettin Yanik
Ondokuz Mayis University
Faculty of Medicine
Department of Medical Microbiology
Samsun, Turkey
Accepted date: November 02 2014
Abstract
The aim of the present study was to evaluate the activity of the NAWAlution WoundCare® solution on various bacteria and to determine its suitability for use in critical care units. The effect of the antiseptic solution, NAWAlution® WoundCare(NAWA Heilmittel GmbH, Germany) was evaluated with and without the addition of albumin, using the broth microdilution method. A total of eight gram negative, four gram positive type bacteria that may be isolated in an intensive care unit and spore-forming bacteria Bacillus spp., as well as C.albicans were studied. E.coli, K.pneumonia, E.faecium, S.aureus and ATCC strains were used in the study. In vitro cytotoxic activity of the NAWAlution WoundCare® antiseptic solution was determined by 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide test. NAWAlution WoundCare® was found to be effective against K.pneumoniae at a concentration of 1/32; against A.baumanii, P.aeruginosa, Chryseobacterium spp, S.maltophilia and Shewanella at a concentration of 1/64; and against E. coli at a concentration of 1/256. However, no activity was observed against B.cepacia. The substance was effective against MRSA at a concentration of 1/64; against voncomycin-resistant E.faecium, vancomycinsensitive E.faecium and Bacillus spp. at a concentration of 1/128; against methicillin-sensitive S.aureus and methicillin-resistant coagulase negative staphylococci at a concentration of 1/256; and against C.albicans at a concentration of 1/256. However, NAWAlution WoundCare solution at a concentration of between 1/10 and 1/2560 had no cytotoxic effect on the Vero cells. In conclusion NAWAlution WoundCare® antiseptic solution was found to be effective against many microorganisms that could cause wound infection, and is also appropriate for direct topical application.
Keywords
NAWAlution WoundCare®, various microorganisms, efficiency.
Introduction
In the human body, the skin is the first line of defence against pathogenic bacteria [1]. Skin and soft tissue infections are very common among humans, specially in hospital settings after various interventional procedures, due to the entry of pathogenic microorganisms as a result of pathological processes or disruption of skin integrity [2]. Wound infections can manifest as minor complications, but may also have life threatening consequences if counter measures are not taken timely [3]. Wound infections can cause delay in the healing of wounds, particularly after surgical operations. Despite the advances in modern surgery, wound infections are still of significant concern, in hospitalized patients specially those in critical care or intensive care units. These infections also occur as nosocomial surgical site infections thereby significantly reducing the success rate of surgery [3,4]. The rate of nosocomial infections occurring in these patients is higher than the rates observed in patients hospitalized in other units. For this reason, infections occurring in patients housed in these units constitute a higher risk of carriage and disease transmission [5]. Infections occurring in hospitalized patients are often severe, prolonging the treatment period, causing additional costs, as well as increasing morbidity and mortality [3,6]. Antisepsis and disinfection with reliable chemical substances according to the instructions is a key factor in the prevention of such infections, halting the spread of pathogenic microorganisms and eliminate foci of colonization [1,5,7]. A topical application of such chemicals prevents the development of resistance and the diffusion into the wound site, and also toxic side effects commonly encountered with systemic antibacterial agents. These topical agents are widely preferred in prophylaxis and treatment [1]. The prophylactic use of these agents, particularly in wound infections, has an important place in infection control [1,7]. For such critical units as intensive care wards, there is a limited number of antimicrobial substances that may be used against these agents and applied directly and topically to the wound surfaces [1,8]. Most chemical substances that are currently used for the care of wounds do not meet the requirements of an ideal antiseptic due to the fact that they are not appropriate for topical application. In addition, the protein-rich structure of wounds decreases the efficiency of these substances and contributes to bacterial growth [1,9,10]. These substances must be made appropriate for use inside and outside the hospital setting, must be effective against agents that cause wound infections, be easily applicable and not cause irritation to the wound [11]. The local use of antiseptics circumvents the side effects associated with systemic antimicrobials [1]. The increasing resistance to antimicrobial agents has resulted in an increased need for an ideal antiseptic that can be used topically in wound infections [12].
Our aim was to investigate the effects of NAWAlution WoundCare® against various bacteria and C. albicans in the presence or absence of albumin, and evaluate its utility in critical care units.
Materials and Methods
Recovery of Bacteria
K. pneumoniae, A. baumanii, P. aeruginosa, Chryseobacterium spp, S. maltophilia and Shewanell, E.coli, S. aureus (MRSA), methicillin-sensitive S. aureus (MSSA), methicillin-resistant coagulase negative staphylococci (MRCNS), vancomycin-resistant E. faecium (VRE), vancomycin-sensitive E. faecium (VSE) and spore-forming bacillius B.cepaiai were isolated in the microbiology laboratory as the cause of nosocomial infection using a fully automatic system (Biomeriux, France). In addition, one strain of C. albicans was used as rare cause of wound infection, as well as Gram negative E.coli ATCC and K.pneumonia ATCC strains and gram positive E.faecalis ATCC and S.aureus ATCC strains.
Preparation of Broth and Microdilution Plates
Mueller-Hinton agar (MHA) was measured and diluted as per manufacturer’s instructions. The broth was sterilized in an autoclave and left at +4oC overnight before use. The broth was supplemented with 0.1 ml calcium chloride for cation adjustment. U plates with 96 wells were used for microdilution, and 100 μl of MHA was added to each well. The first column was left empty for growth control, and the antiseptic solution was prepared, with a final concentration being 2double the desired concentration. A 100 μl NAWAlution WoundCare® (Cocamidopropyl betaine Polyaminopropyl Biguanide Disodium EDTA, NAWA Heilmittel GmbH, Germany) antiseptic solution was added to each well in the second column to reach a final concentration of between 1/4 and 1/2048. The final volume of the antiseptic solution and the bacterial suspension was 200 microliter, and serial dilution was carried out across the plate until column 11 and column 12 was set as the sterile controls [13].
Due to the presence of protein structures that decrease the efficiency of antiseptic solutions, dilutions of the same strains were also studied in the presence of 20% albumin (Human albumin 20%, Eczacıbaşı-Baxter) at the final concentration in order to simulate a real life situation [9,14].
Preparation of the Inoculum
The microorganisms were incubated in blood sheep agar at 37oC for 24 hours, and each microorganism was studied twice. The plates were evaluated after incubation at 37oC for 18–20 hours.
Cytotoxic activity
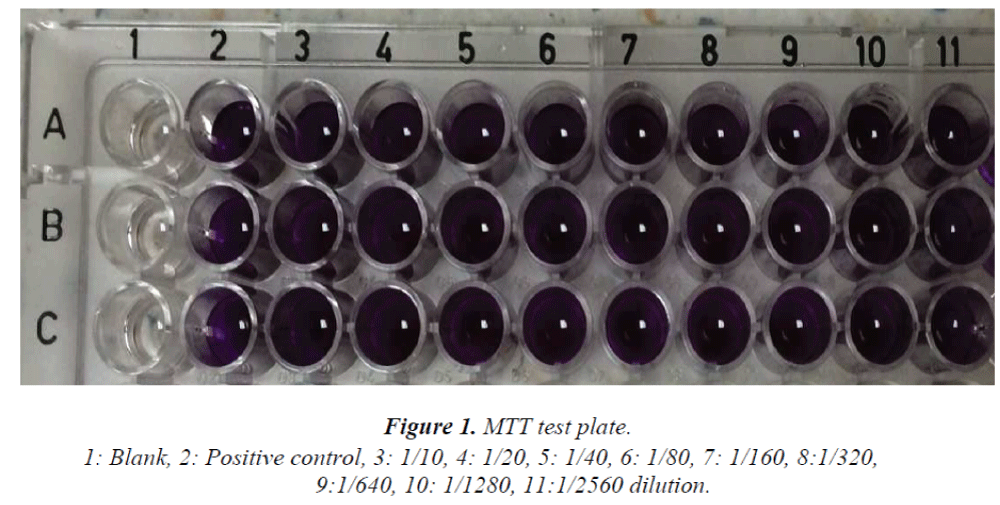
In vitro cytotoxic activity of the NAWAlution Wound- Care® antiseptic solution was determined with 3-(4.5- dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide (MTT) test [15] using African green monkey kidney (Vero) cells. The cells were inoculated on 96-well cell culture plates at a concentration of 2x104 cell/well, and incubated overnight at 37oC. After incubation, the antiseptic solution was added to each well at a concentration of between 1/10 and 1/2560. After overnight incubation at 37oC, the supernatant fluid was aspirated and MTT was added to each well. The number of cells was then evaluated by measurement of formazan crystals. The cell culture plate produced a reading of 490 nm using ELISA.
Results
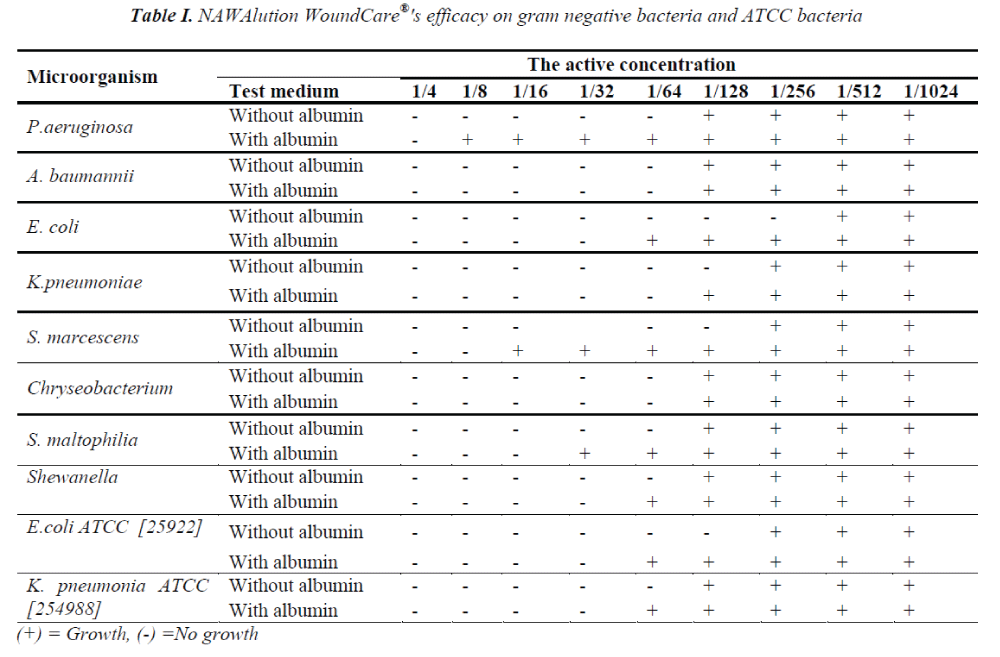
NAWAlution WoundCare® was found to be effective against gram negative K .pneumoniae at a concentration of 1/32; against A. baumanii, P. aeruginosa, Chryseobacterium spp, S. maltophilia and Shewanella at a concentration of 1/64; and against E. coli at a concentration of 1/256. However, no activity was observed against B. cepacia. The data is presented in Table I.
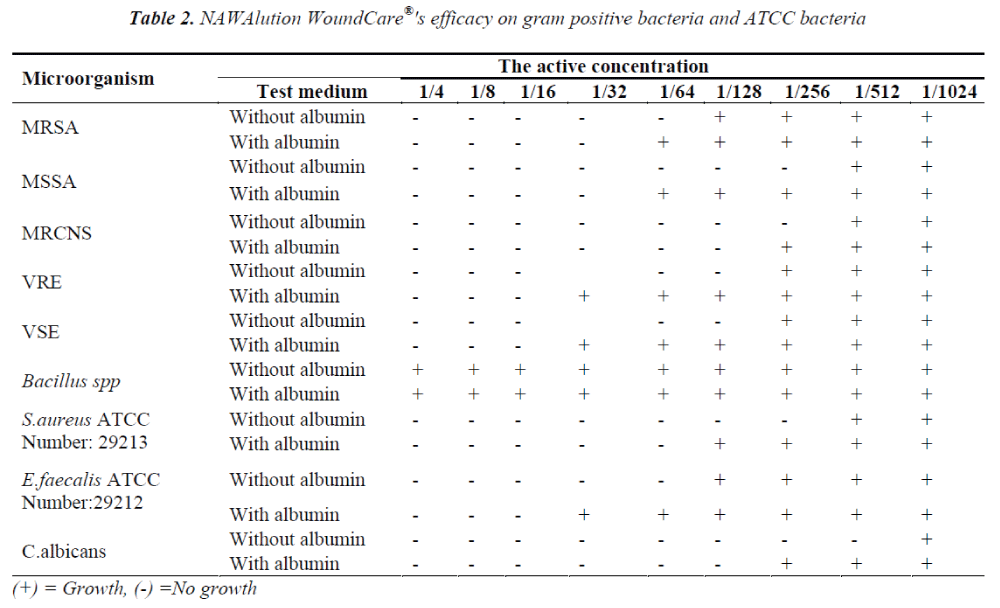
The solution was found to be effective also against gram positive bacteria MRSA at a concentration of 1/64; against VRE, VSE and Bacillus spp. at a concentration of 1/128; and against MSSA and MRCNS at a concentration of 1/256. It was also found to be effective against C.albicans at a concentration of 1/256. The data is presented in Table II.
After analysis of the two gram positive and two gram negative ATCC strains used in the study, the solution was found to be effective against K. pneumonia ATCC (254988) at a concentration of 1/128; against E. coli ATCC (25922) at 1/128; against E. faecalis ATCC (29212) at a concentration of 1/64; and against S. aureus (ATCC 29213) at a concentration of 1/256. The results are presented in Tables I and II.
The presence of albumin significantly decreased the efficacy of NAWAlution WoundCare® solution against gram negative P. aeruginosa, Chryseobacterium spp and S. maltophilia, gram positive MSSA and Enterococci. The presence of albumin had no effect on the efficacy of NAWAlution WoundCare® solution against gram negative A. baumanii and Chryseobacterium spp. Furthermore, the solution also proved to be ineffective against Bacillus spp. The results are presented in Tables I and II.
In this study, antiseptic solutions at a concentration of between 1/10 and 1/2560 had no cytotoxic effect on the Vero cells. Figure I.
Discussion
Wound and surgical site infections continue to pose a serious problem in hospitals, particularly in intensive care units even in the modern age of medicine [4,5]. The limited number of antimicrobial agents that can be applied directly to the wound, and the inability to apply chemicals directly onto the wound surface that would normally be used only in wound care, raise difficulties in the management of wound infections [9,10].
Several studies have been conducted evaluating the efficacy of antimicrobial agents used in wound care; however, the present study is unique in its evaluation of the efficacy of NAWAlution WoundCare® solution when applied directly to the wound surface against various microorganisms that are common in wound infections, and will hopefully guide future studies.
The study has shown that NAWAlution WoundCare® antiseptic solution had bactericidal activity against 15 clinical and four different standard strains that were isolated from patients in the intensive care unit. The study also evaluated the effectiveness of the solution in the presence of human albumin in order to simulate the wound environment with the same organisms. Using the same method, the solution was found to be effective.
In 2009, Dettenkoffer et al. from Germany compared the efficacy of octenidine with chlorhexidine in catheter site infections over a period of 3 years, and although there was a decrease in skin colonization, no significant decrease was reported in the frequency of infections. It was further reported that octenidine exerted less toxicity when compared to chlorhexidine [16]. Sedlock et al. compared the effectiveness of octenidine and chlorhexidine against S. auerus, E. coli, Serratia, pseudomonas and K. pneumonia, and reported a lower efficacy against E. coli [17]. In 2008, Sopata et al performed a wound dressing with octenidine-moistened gauzes three times a day in 21 patients with advanced stage liver disease. Consequently, E. coli and Pseudomonas infections persisted in one patient, while octenidine was shown to reduce necrosis, exudation, erythema and edema [18]. In a similar study by Tietz et al. from the United States, a decrease in catheter-based skin colonization was reported along with KNS reproduction. Tredgian made an analysis of octenidine wound gel, and reported faster wound healing when compared to an octenidine solution [19]. In the study by Uygur et al. comparing the topical antibacterial effect of octenidine, polyhexanide and povidone iodine in mice with full thickness skin burns, and contaminated with pseudomonas, octenidine was found to have a higher efficacy and decreased the frequency of wound dressings [20]. Tazegül et al. performed a study using the agar diffusion method, reporting a higher efficacy of octenisept on enterococcus fecalis when compared to chlorhexidine solution [21].
The in vitro cytotoxic activity of NAWAlution Wound- Care® solution was determined using an MTT test on Vero cells. A 50 percent or higher inhibition in the MTT test when compared to a positive control indicates cytotoxic activity. In one study, antiseptic solutions at concentrations of between 1/10 and 1/2560 had no cytotoxic effect on the Vero cells. The substance used in our study was considered nontoxic due to the lack of a 50 percent or higher inhibition at any dilution [15]. We suggest that studies using different cell types would be useful.
There have been various studies evaluating the antibacterial action of such disinfectants as alcohol and its derivatives, chlorhexidine and povidone iodine. However, these substances are not commonly used in wound care due to differences in the spectrum of actions and side effects but rather used in the disinfection of intact skin [19]. The antibacterial spectrum of action for various disinfectants is not at the desired level, and there are ongoing studies to identify an ideal disinfectant in wound care due to the side effects of the available products. In this regard, NAWAlution WoundCare® solution was found to be efficient against many microorganisms that might cause wound infection, and is also appropriate for direct topical application. Accordingly, it can be suggested as an appropriate antimicrobial agent for wound care, although further studies should be under-taken to identify any side effects and other applications for this substance.
Conflict of interest/ Funding
There is no conflict of interests and no funding source to declare.
References
- Lio PA, Kaye ET. Topical Antibacterial. Infect Dis Clin North Am 2009; 23: 945-963
- Bowler PG, Duerden BI, Armstrong DG.Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001; 14: 244-69.
- Henderson DK. Bacteremia due to percutaneous intravasculer devices: In: Principles and Practice of Infectious Diseases. 4th ed Churchil Livingstone., New York 2007; pp 2587-2599.
- Uzunköy A. Surgical site infections: risk factors and methods of prevention. Ulus Travma Derg 2005; 11: 269-281.
- Platt R, Goldman RA, Hopkıns CC. Epidemiology of nosocomial infections. In: İnfectious Diseases. 3rd ed W.B. Sounders Co., Philadelphia 2004; pp 96-106.
- World Health Organization. Prevention of Hospital- Acquired Infections. A Practical Guide. 2nd ed Geneva WHO., Switzerland P. Vanhems 2002.
- Nakipoğlu Y, Gürler B. An Investigation on Antibacterial Efficacy of Various Disinfectants and Antiseptics. Ankem Derg 2004; 18: 220-223.
- Tatnall FM, Leigh IM, Gibson JR. Comparative study of antiseptic toxicity on basal keratinocytes, transformed human keratinocytes and fibroblasts. Skin Pharmacol 1990; 3: 157–63.
- Weber DJ, Barbee SL, Sobsey MD, Rutala WA. The effect of the blood on the antiviral activity of sodium hypochlorite, a phenolic, and a quarternary ammonium compound. Infect Cont Hosp Epidem 1999; 20: 821-827.
- Gröschel DHM. Disinfectant testing in USA. J Hosp Infect 1991; 18: 274-279.
- Russel AD, Hugo WB, Ayliffe GAJ eds. Principles and practice of disinfection preservation and sterilization. London, 2004.
- McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, Action and Resistance. Clin Microbiol Rev 1999; 12: 147-179.
- Reybrouck G. International standardisation of disinfectant testing: is it possible? Hosp Infect J 1991; 18: 280-288.
- prEN 14476. Chemical disinfectants and antiseptics. Virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine. Test method and requirements. Germany 2005.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 16: 55- 63.
- Dettenkofer M, Wilson C, Gratwohl A, Schmoor C, Bertz H, Frei R, Heim D, Luft D, Schulz S, Widmer AF. Skin disinfection with octenidine dihydrochloride for central venous catheter site care: a double-blind, randomized, controlled trial. Clin Microbiol Infect. 2010;16:600-606.
- Sedlock DM, Bailey DM. Microbicidal activity of octenidine hydrochloride, a new alkanediylbis (pyridine) germicidal agent. Antimicrob Agents Chemother 1985; 28:6, 786-790.
- Sopata M, Ciupińska M, Głowacka A, Muszyński Z, Tomaszewska E. Effect of Octenisept antiseptic on bioburden of neoplastic ulcers in patients with advanced cancer. J Wound Care 2008; 17: 24-27.
- Tietz A, Frei R, Dangel M, Bolliger D, Passweg JR, Gratwohl A, Widmer AE. Octenidine hydrochloride for the care of central venous catheter insertion sites in severely immunocompromised patients. Infect Control Hosp Epidemiol 2005; 26: 703-707.
- Uygur F., Özyurt M., Evinç R., Hosbul, T, Çeliköz B, Haznerdaroğlu T. Comparison of octenidine dihydrochloride (Octenisept), polihexanide (Prontosan) and povidon iodine (Betadine) for topical antibacterial effects in Pseudomonas aeruginosa-contaminated, fullskin thickness burn wounds in rats. Cent Eur J Med 2008; 3: 417-421.
- Tazegül S, Koçak MM, Topuz Ö, Özcan S, Çekiç AA, Erten H. Antibacterial Effectiveness of Three Different Mouthrinse Solutions on Streptococcus mutans and Enterococcus faecalis. EÜ Dis Hek Fak Derg 2006; 27: 153-158.