Research Article - Biomedical Research (2017) Volume 28, Issue 21
Evaluation of the diagnostic value of microRNA143b for osteoporosis
Ning Wang and Shijun Zhang*
Department of Orthopedic Surgery, QiLu Hospital of Shandong University, Jinan, Shandong, China
- *Corresponding Author:
- Shijun Zhang
Department of Orthopedic Surgery
QiLu Hospital of Shandong University, China
Accepted on September 23, 2017
Abstract
Objective: Osteoporosis has become a threat to patients’ health. The search for reliable biomarkers for the diagnosis of osteoporosis is of important clinical significance. microRNAs are involved in the regulation of various physiological and pathological processes. The current study aimed to investigate the potential diagnostic value of microRNA143b for early osteoporosis.
Patients and Methods: This study included 156 osteoporosis patients (69 mild osteoporosis and 87 severe osteoporosis) who were treated. Sixty-nine healthy volunteers were used as controls. Patients were given alendronate hydroxyethyl (5 mg/d) for 2 w. Normal Bone Mineral Density (BMD) is considered effective treatment. The microRNA143b level in blood cells was determined by quantitative Real-Time PCR (qRT-PCR) before and after the treatment. The association between microRNA143b level and osteoporosis was analysed by Pearson’s correlation test.
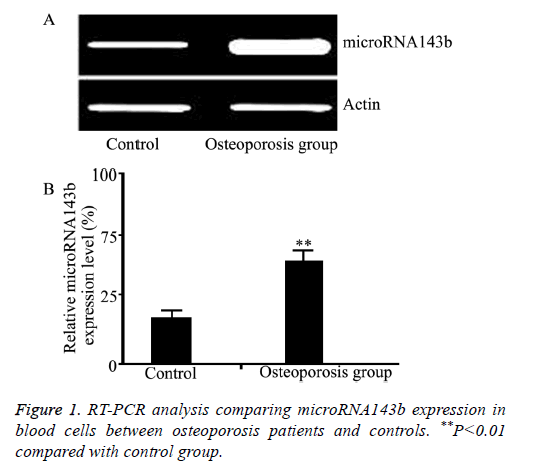
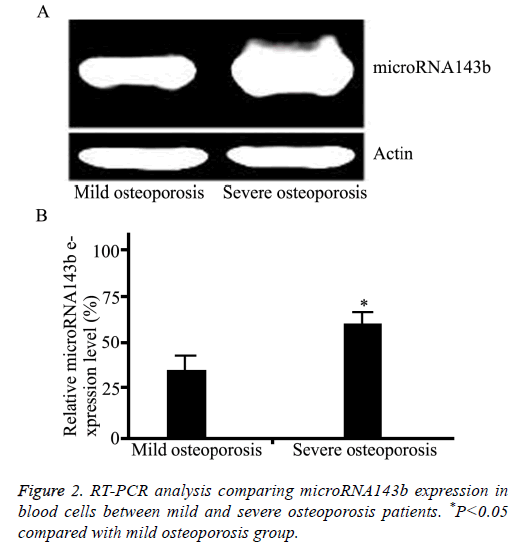
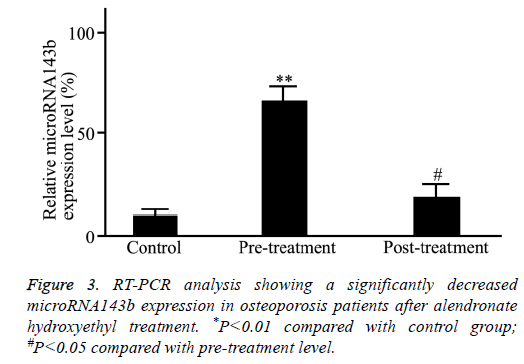
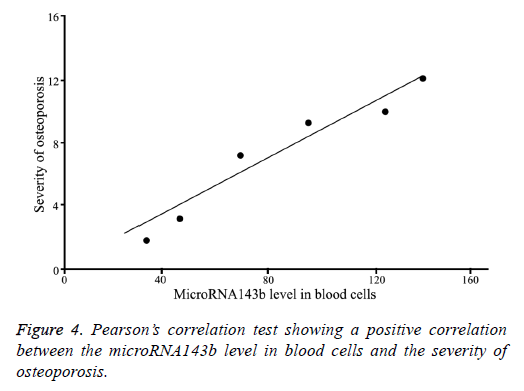
Results: The microRNA143b level in osteoporosis patients was significantly higher compared with healthy controls (P=0.0017). The post-treatment microRNA143b expression in osteoporosis patients was significantly reduced to a level close to that in control group (P=0.45). Moreover, microRNA143b level in severe osteoporosis was significantly higher compared with mild osteoporosis (P=0.0072). The microRNA143b level in blood cells was positively correlated with the severity of osteoporosis (r=0.91, P=0.011).
Conclusion: MicroRNA143b expression is closely associated with the severity of osteoporosis, and might be a specific biomarker for the disease.
Keywords
Osteoporosis, microRNA143b, microRNA143b, Diagnosis.
Introduction
Osteoporosis is a disease of decreased bone strength which increases the risk of a broken bone. It is a common disease, especially in the elderly and children [1]. Osteoporosis often leads to fractures and pain, and thereby seriously affects the quality of patients’ life [2,3]. Bone Mineral Density (BMD) is the current method for the diagnosis of osteoporosis. In 2015, WHO suggested diagnostic criteria based on the grades of BMD or Bone Mineral Content (BMC) of the axial or peripheral skeleton [4,5]. However, the method is timeconsuming, and is not sensitive in the diagnosis of early osteoporosis. Therefore, the development on an effective and reliable diagnostic method for osteoporosis is of great clinical significance [6,7].
Biomarkers can be used for accurate and sensitive evaluation of some early, low-level damages, and thus has provided valuable diagnostic values for clinicians [8]. Currently, biomarkers for osteoporosis have been frequently reported including several serum proteins such as sphingosine, periostin, fibroblast growth factor, etc. [2,6,8]. Nevertheless, the measurement of these biomarkers requires expensive detection kit and instrument, and thus is not suitable for less developed areas [9]. Some studies have suggested calcitonin as a marker for osteoporosis [10]. However, the method is not suitable for all patients. For instance, calcitonin level is quite low in some patients. Therefore, a reliable and sensitive biomarker is needed for effective diagnosis of osteoporosis.
MicroRNA are a class of small non-coding RNA molecules (about 22-nucleotide lone) that are involved in a wide range of physiological and pathological processes such as inflammation and even cancer thought the regulation of cell proliferation, growth and apoptosis. Recent studies have suggested microRNA-18a as a molecular marker for prostate cancer [11], microRNA 25, microRNA 145 and microRNA 210 as markers for lung cancer [12], and microRNA-200c-141 as a marker for human solid malignancies [13]. To date, there are quite few studies on the potential of microRNAs as biomarkers for osteoporosis [14,15]. While microRNA143b has been known to be involved in inflammatory response [12], the occurrence of osteoporosis is also closely associated with inflammation [13], suggesting that microRNA143b might be a biomarkers for osteoporosis. This study investigated the microRNA143b level in osteoporosis patients, and the correlation between and microRNA143b expression and the severity of osteoporosis in order to evaluate its diagnostic value for the disease.
Materials and Methods
Subjects
A total of 156 osteoporosis patients who were treated in QiLu Hospital of Shandong University between January 2012 to January 2017 were selected for this study based on the following criteria: no age limit, no other medical history, primary osteoporosis, no history of chemotherapy or other treatment history causing osteoporosis. Osteoporosis was defined as previously described based on BMD [16,17]. The BMD of the axial or peripheral skeleton was measured using a DSC-600EVX ultrasound bone density meter (Hitachi Biotech, Japan). Patients with 600 g/cm2 ≤ BMD<1000 g/cm2 were defined as mild osteoporosis and those with BMD<600 g/cm2 were considered severe osteoporosis. Patients were given alendronate hydroxyethyl (5 mg/d) for 2 w. Normal Bone Mineral Density (BMD) is considered effective treatment. Sixty-nine healthy volunteers were used as controls.
The research was approved by the Ethic Committee of QiLu Hospital of Shandong University. All participants were required to sign the informed consent form.
Samples collection
Blood samples were collected from all participants before and after the treatment. Blood cells were isolated by centrifugation at 500 rpm for 6 min, and stored at -20°C.
Quantitative real-time PCR
Total RNA in blood cells was extracted using the Trizol reagent. RNA concentration was determined using a spectrometer under a wavelength of 260 nm. Total RNA was reverse transcribed into cDNA using a reverse transcription kit (Tiangen Biotech., Beijing) following the manufactures instructions. The level of microRNA143b was measured by qRT-PCR using cDNA as template and the following primers: microRNA143b forward: 5'- CTACAATGAGCTGCGTGTGG-3', reverse: 5'- AAGGAAGGCTGGAAGAGTGC-3'; actin forward: 5'- CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG-3' and reverse: 5'- CTCAACTGGTGTCGTGGAGTCGGCAATTC-3'. The reaction mixture was prepared: 0.5 μl of cDNA, 1 μl of forward primer, 1 μl of reverse primer, 2 μl of dNTP mixture, 1 μl of reaction buffer, 0.25 μl of Taq enzymes, and 0.5 μl of cDNA template. The reaction condition was as follows: 95°C, 9 min, followed by 26 cycles of 95°C 45 s, 57°C 36 s, and 72°C 30 s, and 72°C10 min. The experiment was repeated three times. The PCR products were analysed by agarose gel electrophoresis. The intensity of bands was detected by a BDA BOX1 System (Boyue Instruments, China). The gray value of bands was analysed by Image J 1.48 u (National Institutes of Health). The relative expression level of microRNA143b was calculated as its grey value to that of actin.
Statistical analysis
Numeric data was subjected to normality tests and expressed as mean ± standard deviation. Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Differences among groups were compared by ANOVA and difference between groups were analysed by t-tests. Association between microRNA143b level and the severity of osteoporosis was analysed using Pearson's correlation test. P<0.05 was considered significantly different.
Results
Basic information of subjects
A total of 156 osteoporosis patients were selected in this study including 69 cases of mild osteoporosis and 87 severe osteoporosis. The mean age of osteoporosis group was 55. 3 ± 18.4 y (range: 18-88 y), which was close to that of control group (58.3 ± 17.4 y, range: 25-83 y, P>0.05). As shown in Table 1, there was no significant difference in the male/female ratio or mean age among the mild osteoporosis, severe osteoporosis and control groups (P>0.05). Before the treatment, the BMD in both mild and severe osteoporosis groups was significantly lower compared with controls (P=0.003). The BMD in severe osteoporosis was significantly lower than that in mild osteoporosis (P=0.023). The posttreatment BMD in mild and severe osteoporosis groups was similar to that in control group (P>0.05).
| Variable | Mild osteoporosis | Severe osteoporosis | Healthy control |
|---|---|---|---|
| Male/female | 39/39 | 39/39 | 78/784 |
| Age (y) | 56.1 ± 8.1 | 52.1 ± 4.2 | 57.1 ± 18.2 |
| Pre-treatment BMD | 698 g/cm2 ± 3.6& | 579 g/cm2 ± 3.6&* | 1228 g/cm2 ± 2.6 |
| Post-treatment BMD | 1398 g/cm2 ± 1.9 | 1299 g/cm2 ± 1.9 | 1310 g/cm2 ± 5.5 |
Note: &P<0.01 when compared with control group; *P<0.05 when compared with mild osteoporosis group.
Table 1. Basic information of subjects in mild osteoporosis, severe osteoporosis and control groups (x ± s).
Comparison of microRNA143b expression before treatment
The microRNA143b level in blood cells in the three groups was compared by qRT-PCR. As shown in Figure 1, the pretreatment microRNA143b level in osteoporosis patients was significantly higher than that in controls (P=0.005). Moreover, the pre-treatment microRNA143b level in severe osteoporosis was significantly higher compared with mild osteoporosis (P=0.012, Figure 2).
Comparison of pre- and post-treatment microRNA143b expression
As shown in Figure 3, the treatment of alendronate hydroxyethyl significantly reduced the microRNA143b level in osteoporosis patients (P=0.0072).
Correlation between microRNA143b level and the severity of osteoporosis
As shown in Figure 4, Pearson’s correlation test revealed a positive correlation between the microRNA143b level in blood cells and the severity of osteoporosis (r=0.91, P=0.011).
Discussion
Early diagnosis and treatment of osteoporosis is extremely important to prevent bone damages. Measurement of BMD has been the gold standard for diagnosis of osteoporosis [5]. However, the method is time-consuming and might be insensitive in cases of early osteoporosis [5]. Therefore, the development of a rapid and reliable diagnostic method is of great importance.
It has been well known that microRNAs are involved in the regulation of a variety of genes and are thus closely associated with several diseases. Studies have detected stable microRNAinduced silencing complex in human plasma [16-19]. Moreover, while osteoporosis is a chronic inflammationinduced diseases, microRNA143b is closely related to the inflammation of the body [20], indicating that microRNA143b might be a potential biomarker for osteoporosis. In this study, we investigated the prognostic value of microRNA143b for osteoporosis. It was found that the microRNA143b level in osteoporosis patients was significantly higher compared with healthy controls. More importantly, the microRNA143b level in blood cells was positively correlated with the severity of osteoporosis. To our best knowledge, our study is the first on the potential of microRNA143b as a biomarker for osteoporosis.
MicroRNA143b has been known to be closely associated with inflammation of human body, and therefore might be involved in the occurrence and development of osteoporosis through inflammatory responses [20,21]. Furthermore, microRNA143b is one of the most important regulators in the nervous system, and plays an important role in the growth, development, and differentiation of neurons, and the regeneration of injured nerves [22]. Numerous studies have been performed on the application of microRNA143b in the treatment of nervous diseases such as peripheral nerve injury, peripheral neuropathy, optic nerve damage and Alzheimer's disease [22]. In addition, microRNA143b also maintains the balance among, immune and endocrine system. Decreased estrogen levels due to immune senescence can induce the changes in microRNA143b expression [18]. These findings suggest that microRNA143b might be involved in the development and progression of osteoporosis through the regulation on immune and endocrine systems. We therefore speculate that increased microRNA143b level induces immune disorders in the body, leading to apoptosis of bone cells, necrosis of bone tissues, and ultimately the occurrence of osteoporosis. Nevertheless, further mechanism studies are needed to validate our speculation.
The current study has several limitations such as small sample size. Researches on a larger number of cases shall be performed to further evaluate the diagnostic value of microRNA143b in osteoporosis. Additionally, whether the microRNA143b level in osteoporosis patients is affected by chemotherapy needs to be investigated, since a large portion of osteoporosis cases are induced by chemotherapy. Furthermore, the potential of microRNA143b as the molecular target for osteoporosis gene therapy shall be explored in animal models in the future.
Conclusion
In summary, microRNA143b expression is positively correlated with the severity of osteoporosis, and might be a specific biomarker for the disease.
References
- Ji X, Chen X, Yu X. MicroRNAs in osteoclastogenesis and function: potential therapeutic targets for osteoporosis. Int J Mol Sci 2016; 17: 349.
- Li G, Bu J, Zhu Y, Xiao X, Liang Z, Zhang R. Curcumin improves bone microa OArchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int J Clin Exp Pathol 2015; 8: 15684-15695.
- Zhao N, Han D, Liu Y, Li Y, Zeng L, Wang Y, Feng H. DLX3 negatively regulates osteoclastic differentiation through microRNA-124. Exp Cell Res 2016; 341: 166-176.
- Ma Y, Shan Z, Ma J, Wang Q, Chu J, Xu P, Qin A, Fan S. Validation of downregulated microRNAs during osteoclast formation and osteoporosis progression. Mol Med Rep 2016; 13: 2273-2280.
- Lucato P, Trevisan C, Stubbs B, Zanforlini BM, Solmi M, Luchini C, Girotti G, Pizzato S, Manzato E, Sergi G, Giannini S, Fusaro M, Veronese N. Nephrolithiasis, bone mineral density, osteoporosis, and fractures: a systematic review and comparative meta-analysis. Osteoporos Int 2016; 133: 341-351.
- De-Ugarte L, Yoskovitz G, Balcells S, Guerri-Fernandez R, Martinez-Diaz S, Mellibovsky L, Urreizti R, Nogues X, Grinberg D, García-Giralt N, Díez-Perez A. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genomics 2015; 8: 75.
- Hackl M, Heilmeier U, Weilner S, Grillari J. Circulating microRNAs as novel biomarkers for bone diseases-complex signatures for multifactorial diseases? Mol Cell Endocrinol 2015; 432: 83-95.
- Li KC, Chang YH, Yeh CL, Hu YC. Healing of osteoporotic bone defects by baculovirus-engineered bone marrow-derived MSCs expressing MicroRNA sponges. Biomaterials 2016; 74: 155-166.
- Liao L, Su X, Yang X, Hu C, Li B, Lv Y, Shuai Y, Jing H, Deng Z, Jin Y. TNF-α inhibits FoxO1 by up-regulating MiR-705 to aggravate oxidative damage in bone marrow-derived mesenchymal stem cells during osteoporosis. Stem Cells 2015; 34: 1054-1067.
- Song Q, Zhong L, Chen C, Tang Z, Liu H, Zhou Y, Tang M, Zhou L, Zuo G, Luo J, Zhang Y, Shi Q, Weng Y. miR-21 synergizes with BMP9 in osteogenic differentiation by activating the BMP9/Smad signaling pathway in murine multilineage cells. Int J Mol Med 2015; 36: 1497-1506.
- Bunyaratavej N, Buranasinsup S. Study of validity of pyridinoline and correlation of pyridinoline and beta crosslap in postmenopausal women. J Med Assoc Thai 2011; 94: 76-78.
- Atbinici H, Sipahioglu S, Aksoy N, Baykara I, Isikan UE. Effects of salmon calcitonin treatment on serum and synovial fluid bone formation and resorption markers in osteoporosis patients. Acta Orthop Traumatol Turc 2015; 49: 160-165.
- Al-Kafaji G, Al-Naieb ZT, Bakhiet M. Increased oncogenic microRNA-18a expression in the peripheral blood of patients with prostate cancer: A potential novel non-invasive biomarker. Oncol Lett 2016; 11: 1201-1206.
- Shi SB, Wang M, Tian J, Li R, Chang CX, Qi JL. MicroRNA 25, microRNA 145, and microRNA 210 as biomarkers for predicting the efficacy of maintenance treatment with pemetrexed in lung adenocarcinoma patients who are negative for epidermal growth factor receptor mutations or anaplastic lymphoma kinase translocations. Transl Res 2016; 170: 1-7.
- Li XY, Li H, Bu J, Xiong L, Guo HB, Liu LH, Xiao T. Prognostic role of microRNA-200c-141 cluster in various human solid malignant neoplasms. Dis Markers 2015; 2015: 935626.
- Lv H, Sun Y, Zhang Y. MiR-133 is involved in estrogen deficiency-induced osteoporosis through modulating osteogenic differentiation of mesenchymal stem cells. Med Sci Monit 2015; 21: 1527-1534.
- Shao B, Liao L, Yu Y, Shuai Y, Su X, Jing H, Yang D, Jin Y. Estrogen preserves Fas ligand levels by inhibiting microRNA-181a in bone marrow-derived mesenchymal stem cells to maintain bone remodeling balance. FASEB J 2015; 29: 3935-3944.
- Srividhya NB, Singh N, Goel N, Gambhir JK, Rathi V, Rajaram S. Comparison of antiresorptive effect of hormone therapy and ibandronate in postmenopausal osteoporotic women by assessing type I collagen C-telopeptide levels. Post Reprod Health 2015; 21: 48-55.
- Chapurlat RD, Confavreux CB. Novel biological markers of bone: from bone metabolism to bone physiology. Rheumatology (Oxford) 2016.
- Li Z, Li X, Wu S, Xue M, Chen W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci 2014; 105: 951-955.
- Fu X, Zeng L, Liu Z, Ke X, Lei L, Li G. MicroRNA-206 regulates the secretion of inflammatory cytokines and MMP9 expression by targeting TIMP3 in Mycobacterium tuberculosis-infected THP-1 human macrophages. Biochem Biophys Res Commun 2016.
- Sheane BJ, Smyth P, Scott K, Aziz R, Buckley M, Lodge E, Kiely N, Kingston M, McGovern E, Healy M, Walsh JB, Sheils O, Cunnane G. An association between microRNA-21 expression and vitamin d deficiency in coronary artery disease. MicroRNA 2015; 4: 57-63.