Research Article - Biomedical Research (2017) Volume 28, Issue 16
Evaluation of the cement/dentin thickness of the mesiobuccal root of maxillary first molars by optical microscopy in a Chilean sample
Daniela Matus1*, Mario Cantin2, Pablo Navarro3 and Gabriel M. Fonseca4
1Magister Program in Dentistry, Department of Integral Adult Dentistry, Faculty of Dentistry, University of La Frontera, Temuco, Chile
2Magister Program in Dentistry, Faculty of Dentistry, CIMA Research Group, University of La Frontera, Temuco, Chile
3Magister Program in Dentistry, Faculty of Dentistry, CICO Research Centre in Dental Sciences, University of La Frontera, Temuco, Chile
4Magister Program in Dentistry, Faculty of Dentistry, Center for Observation and Management of Health Risk, University of La Frontera, Temuco, Chile
- *Corresponding Author:
- Daniela Matus
Magister Program in Dentistry, Department of Integral Adult Dentistry
Faculty of Dentistry, Universidad de La Frontera, Chile
Accepted date: June 27, 2017
Abstract
Vertical root fractures in maxillary first molars affect the mesiobuccal root in most cases. Among intrinsic factors directly related to increasing susceptibility to vertical root fractures, the thickness of the cement/dentin walls is a factor over which the clinician has a direct influence during the chemomechanical preparation. In a cross-sectional in vitro study, the mesiobuccal roots of fifty extracted human maxillary first molars were sectioned horizontally at 1, 3 and 5 mm from the apex. Cement/ dentin thickness was measured in the resulting 300 surfaces by using optical microscopy to an accuracy of X20 magnification. The obtained data were summarized and values were assessed statistically by ANOVA and post-hoc Tukey's range test. Buccal and lingual walls had the greatest thicknesses, whereas mesial and distal were variable and thinnest at the 3 mm level. The buccal wall had statistically significant differences in all surfaces (p<0.05). The 3 mm apical can be considered as a "danger zone" for instrumentation due to the variability of the thinner walls of the canal. The great variability of the buccal wall and the thinness of the proximal walls may explain the frequent buccolingual direction of vertical mesiobuccal root fractures.
Keywords
Maxillary first molar, Mesiobuccal root, Thickness, Cement/dentin walls, Vertical root fracture, Endodontics
Introduction
The treatment of root canals is a dental procedure, which consists of thoroughly shape, clean and subsequently fills all pulp spaces with an inert filling material [1]. First maxillary molars, particularly their Mesiobuccal (MB) roots, are usually treated endodontically with a low success rate [2]. Difficulty in preparing this root is due not only to its complex internal morphology (with double-canalled configurations in 80% of the cases, accessory canals, loops and isthmuses mainly located in the apical third) [3], but also to other intrinsic root and canal factors which might increase susceptibility to Vertical Radicular Fractures (VRF) [4].
Pradeep et al. [5] reported that after premolars, the first molar is the maxillary tooth that most frequently suffers VRFs. VRF is defined as a longitudinally oriented fracture of the root, extending from the root canal to the periodontium [6,7], showing a combination of several clinical findings: pain of various kinds, swelling, mobility, pockets (located adjacent to the site of the fracture) and fistulous tract [6]. VRF is considered an important clinical problem since it is been reported as a cause of loss of teeth treated endodontically in a 13.4% of cases [8]. Once produced, the only therapeutic alternatives are radical: tooth extraction or resection of the fractured root [6].
Seo et al. [4] reported that the dentin thickness, radius of canal curvature, and external root morphology are factors directly related to increased susceptibility to VRF. These factors must be considered during the various stages of endodontic treatment. The latter, especially since the thickness of the cement/dentin walls is a clinician-dependent factor directly determined by appropriate Chemomechanical Preparation (CMP) [5]. Marchi et al. conclude that the remaining thickness of these walls after CMP is the most important iatrogenic factor correlated to incoming fracture resistance [9]. Incorrect instrumentation during CMP with consequent excessive intraradicular dentin removal or even perforation, can lead to crack initiation or weakening of the cement/dentin walls. The resulting high-stress concentration areas can cause a VRF when force is applied during excessive lateral condensation, restorative procedures or even from occlusal stresses during mastication [6-11]. Despite the importance of knowledge about thickness of the cement/dentin walls, most studies on the MB root of maxillary first molars have been focused on its complex morphology [12] with minimal information regarding wall thickness [13].
The purpose of this in vitro study was to evaluate cement/ dentin thickness of the MB root of maxillary first molars sectioned horizontally at 1, 3 and 5 mm from the apex, by using optical microscopy in a Chilean population.
Methodology
This study was approved by the University of La Frontera (UFRO) Scientific Ethics Committee (Protocol no. 002/2015). A descriptive, observational, cross-sectional in vitro study was performed on the MB root of fifty mature extracted permanent first molars from individuals from Chile's Araucania Region, from both sexes older than 18 years. Teeth were collected by random sampling taking into account that MB root had no signs of radectomy or resorption, carious lesions, defects, calcifications of the canalicular system, fusion with the distobuccal root, dilacerations greater than 30º, or signs of previous restorations, endodontic or orthodontic intervention.
Once extracted, molars were cleaned in 5% NaOCl solution for 24 h, debrided of periodontal tissue and calculus, washed under running water, blotted dry and stored in saline solution. Teeth were cleaned using ultrasonic scaler P5-Newtron® (Satelec®, Acteon®, France) and tips Start-X® (Denstply®, Maillefer®, Switzerland). All MB roots were sectioned at the furcation level; a previously calibrated investigator made specific measurements to establish cut marks at 1, 3 and 5 mm from the apex (A-C respectively) by using a Vernier Calliper Standard Model, 0-180 mm (Mitutoyo®, Kawasaki, Japan). Every MB root was placed at the coronal extreme in the front notch of a bead-crimping plier (BeadSmart™, Eurotool®, Grandview, MO, USA) and clamped gently. Following the perpendicular plane determined by the jaws of the tool, the roots were then horizontally sectioned by diamond disk (0.1 mm thickness) mounted on hand piece under refrigeration with water and air. The ovoid shape of the notches prevented the rotation of the roots during the cuts. The sections were polished on grinding stone slabs of various grades using pumice and water paste, to avoid artefacts and stored in Eppendorf tubes with 10% buffered formalin to prevent dehydration and bacterial contamination.
The sections were stained with methylene blue to be observed with trinocular optical microscope CX31 (Olympus®, Hamburg, Germany) to an accuracy of 20X magnification. Each section was analysed on apical and coronal surfaces determining a sample of 300 surfaces. Photographs were taken with Moticam® 480 digital camera (Wetzlar, Germany) integrated into the microscope, and the images were analysed with software Image J® 1.49 for Mac OS X (NIH imagen.nih.gov/ij, USA) by using a 10 mm printed grid. For each section, the presence of canals was identified as MB1, MB2 and MB3 (one, two or three canals respectively), and thicknesses were determined by the distance between the external limit of buccal, lingual, distal and mesial root surfaces, and the centre of the canals.
All the root thicknesses were summarized, and values were assessed statistically by ANOVA and post-hoc Tukey's range test. A value of p<0.05 was accepted as statistically significant and SPSS for Windows v20.0 (Chicago, IL, USA) was used for statistical analyses.
Results
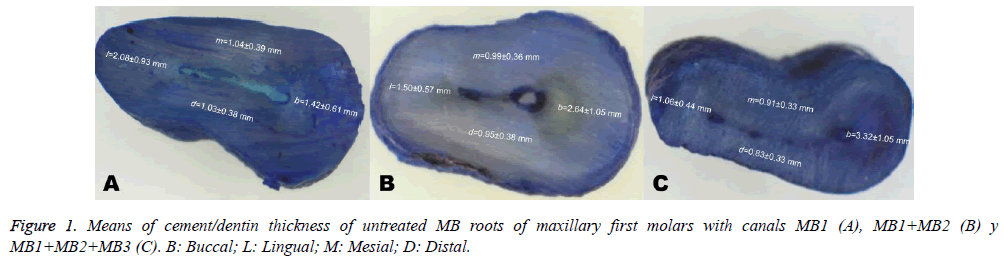
Of fifty maxillary first molars included in this study, 60% (30 teeth) had two canals, 28% (14 teeth) had one canal, and 12% (6 teeth) had three canals in the MB root. Three hundred surfaces were obtained and analysed from 150 sections of all50 MB roots: 52% (n=156) had a MB1 configuration (one canal), 40.7% (n=122) had a MB2 configuration (two canals), 5% (n=15) had a MB3 configuration (three canals), and 2.3% (n=7) had no a visible canal (foramen did not coincide with the root apex). The means of all thicknesses according to the number of canals observed per section are shown in Figure 1.
When these sections were related to the thickness of the cement/dentin walls, the buccal wall was variable in every observed section followed by the lingual wall. In particular, proximal walls presented statistically significant differences at 3 mm. In MB1, lingual walls were the thickest in all cuts; in MB2 were lingual and buccal, and in MB3 were the buccal walls were the thickest in all cuts. In all root types, the thinnest walls were distal at 3 and 5 mm, whereas mesial were the thinnest walls at 1 mm (Table 1). In all root types, the thinnest walls were distal at 3 and 5 mm, whereas mesial were the thinnest walls at 1 mm (Table 1).
| Canal | Section level (mm from apex) | ||||
|---|---|---|---|---|---|
| 0 mm | 1 mm | 3 mm | 5 mm | ||
| MB1 | b | 0.67 ± 0.35** | 0.95 ± 0.43** | 1.71 ± 0.37** | 2.05 ± 0.30** |
| MB2 | 1.25 ± 0.81** | 1.90 ± 1.15** | 3.11 ± 0.58** | 3.39 ± 0.60** | |
| MB3 | 2.37 ± 2.53** | 4.17 ± 0.15** | 3.65 ± 0.15** | 3.16 ± 0.83** | |
| p-value | 0.000* | 0.000* | 0.000* | 0.000* | |
| MB1 | l | 1.26 ± 0.80 | 1.56 ± 0.82 | 2.36 ± 0.69** | 2.75 ± 0.75** |
| MB2 | 1.42 ± 0.60 | 1.32 ± 0.60 | 1.44 ± 0.45** | 1.91 ± 0.50** | |
| MB3 | 0.48 ± 0.19 | 1.16 ± 0.15 | 0.87 ± 0.1 | 1.32 ± 0.24 | |
| p-value | 0.238 | 0.455 | 0.000* | 0.000* | |
| MB1 | m | 0.69 ± 0.41 | 0.81 ± 0.38 | 1.17 ± 0.27 | 1.28 ± 0.26 |
| MB2 | 0.69 ± 0.41 | 0.81 ± 0.38 | 1.17 ± 0.27 | 1.28 ± 0.26 | |
| MB3 | 0.92 ± 0.38 | 0.7 ± 0.15 | 0.84 ± 0.15 | 1.03 ± 0.24 | |
| p-value | 0.298 | 0.883 | 0.019* | 0.331 | |
| MB1 | d | 0.71 ± 0.41 | 0.88 ± 0.36 | 1.17 ± 0.30 | 1.26 ± 0.27 |
| MB2 | 0.79 ± 0.41 | 0.81 ± 0.40 | 0.97 ± 0.31 | 1.17 ± 0.32 | |
| MB3 | 0.5 ± 0.11 | 0.94 ± 0.1 | 0.65 ± 0.1 | 0.99 ± 0.43 | |
| p-value | 0.562 | 0.778 | 0.013* | 0.096 | |
| *p-value according to ANOVA test (p<0.05). **Significant differences according to Tukey's range test (p<0.05). B: Buccal; L: Lingual; M: Mesial; D: Distal. | |||||
Table 1: Evaluation of cement/dentin thickness around untreated MB root canals (mean and standard deviation, in mm).
Discussion
While there exists previous investigation on root morphology of the first maxillary molars of Chilean populations [12,14,15], we have not found any reports that quantitatively evaluate the cement/dentin thickness of their MB roots, or studies that use the methodology that we performed in this population. Although embedding the roots in acrylic blocks is the technique most commonly reported to perform the horizontal sections in vitro [13,16], we preferred to use the crimp bead pliers as this allowed to better evaluate the perpendicularity of these sectioning due to the usual curvature and complexity of the MB root [17,18]. This technique must be very precise and gentle, but it allows a better control of the perpendicularity of the cut. While this could be considered a limitation of the study, we believe that this technical option should be explored more deeply.
The results of our study lead us to suggest that the thickness of the cement/dentin walls of the MB root of the permanent maxillary first molar is an important factor to consider in all stages of endodontic treatment, especially during CMP, since it may increase the vulnerability of the root to suffer VFR. It is recommended to work during this stage with abundant irrigation and to use instruments gently, in order to obtain smooth walls and avoid weakening of the proximal walls. The findings in this study are similar to those given by Sathorn et al. [11], who reports that the thickness of the proximal walls in the MB root of the maxillary first molars tends to be half that of the buccal and lingual walls. This is an important factor to be considered during the CMP of the apical third, specifically at 3 mm, where we found greater thickness variability in the proximal walls. We agree with Dagerness and Bowles in that this zone could be considered as a "danger zone" [18]. It should also be taken into account that over instrumentation at this level may lead to a perforation or excessive wall weakening during orthograde preparation of the canal, when using ultrasonic instruments in retrograde preparations since their use requires a minimum thickness of 1 mm to avoid microfractures [13].
Although the buccal and lingual walls have the greatest thickness [16], it has been reported that cases of VF occurs more frequently in the buccolingual direction [6,7,11]. This is a frequent finding in ovoid-shaped roots, such as the MB root of the maxillary first molar, more prone to fractures [11]. Degerness and Bowles [18] reported that a decrease in the thickness of the proximal wall increases the concentration of stress to the buccal and lingual walls, and therefore, predisposition to fractures. These authors suggest that in response to excessive forces, the thinner (proximal) walls are forced to expand faster than the thicker (buccal and lingual) walls, and this asymmetric expansion would create additional stress on the internal surface, therefore increasing the possibility of VRF in a buccolingual direction. In addition, they indicated that another factor that directly influences the susceptibility to fractures is the presence of irregularities in the canal, which may lead to a higher concentration of stress [18]. Our data coincides with these appreciations, since the buccal wall presented variability in its thickness in all the sections observed, being able to favour this phenomenon.
Another clear limitation of this study is the small sample size; however, we consider that our findings are relevant since they allow the clinician to recognize the level of complexity of the endodontic treatment to be performed. It has been documented that the root canal system of the MB root of the maxillary first molar presents a challenging morphology [19-23], and the major goal of our research is to confirm, by using a novel and simple technique in vitro, this demanding morphological pattern. This complexity is not limited only to possible anatomical variations, but also to the intrinsic characteristics of that specific root and canal, which make this it more prone to VFR [6]. This information is important to be considered during all stages of endodontic treatment, especially during the CMP, since the clinician can directly influence the thickness of the cement/dentin canal walls. We coincide with Akhlaghi et al. in that "real root thicknesses are always less than what appears in the pre-operative radiographs" [16]. Instrumentation procedures should be as conservative as possible, achieving cleaning and conformation with abundant irrigation and gentle preparation to minimize crazing [24]. Canal conformation should allow smooth walls by eliminating irregularities, thus reducing the stress concentration and uniformly distributing forces, avoiding excessive wear on the dentinal walls or applying excessive forces during the obturation stage [6,17,25], especially in the thinner areas of the canal: the proximal walls.
Conclusion
Considering the limitations of this in vitro study, the observations made with this unreported technique of sectioning and the subsequent evaluation by using optical microscopy, give a better understanding of the maxillary MB canal root system. Although the sample studied is not representative of the entire Chilean population, these quantitative findings may improve endodontic therapies. Based on these results, the cement/dentin thickness of mesiobuccal roots of the maxillary first molars is a factor that must be carefully considered during all stages of endodontic treatment, and special attention should be paid at the 3 mm level due to the variability of the thinner walls of the canal.
References
- European Society of Endodontology. Quality guidelines for endodontic treatment: consensus report of the European Society of Endodontology. Int Endod J 2006; 39: 921-930.
- Coutinho Filho T, La Cerda RS, Gurgel Filho ED, de Deus GA, Magalhães KM. The influence of the surgical operating microscope in locating the mesiolingual canal orifice: a laboratory analysis. Braz Oral Res 2006; 20: 59-63.
- Somma F, Leoni D, Plotino G, Grande NM, Plasschaert A. Root canal morphology of the mesiobuccal root of maxillary first molars: a micro-computed tomographic analysis. Int Endod J 2009; 42: 165-174.
- Seo DG, Yi YA, Shin SJ, Park JW. Analysis of factors associated with cracked teeth. J Endod 2012; 38: 288-292.
- Pradeep Kumar AR, Shemesh H, Jothilatha S, Vijayabharathi R, Jayalakshmi S, Kishen A. Diagnosis of vertical root fractures in restored endodontically treated teeth: A time-dependent retrospective cohort study. J Endod 2016; 42: 1175-1180.
- Tamse A. Vertical root fractures in endodontically treated teeth: diagnostic signs and clinical management. Endodontic Topics 2006; 13: 84-94.
- Hassan B, Metska ME, Ozok AR, van der Stelt P, Wesselink PR. Detection of vertical root fractures in endodontically treated teeth by a cone beam computed tomography scan. J Endod 2009; 35: 719-722.
- Toure B, Faye B, Kane AW, Lo CM, Niang B. Analysis of reasons for extraction of endodontically treated teeth: a prospective study. J Endod 2011; 37: 1512-1515.
- Marchi GM, Mitsui FH, Cavalcanti AN. Effect of remaining dentine structure and thermal-mechanical aging on the fracture resistance of bovine roots with different post and core systems. Int Endod J 2008; 41: 969-976.
- Kang SH, Kim BS, Kim Y. Cracked teeth: distribution, characteristics, and survival after root canal treatment. J Endod 2016; 42: 557-562.
- Sathorn C, Palamara JE, Palamara D, Messer HH. Effect of root canal size and external root surface morphology on fracture susceptibility and pattern: a finite element analysis. J Endod 2005; 31: 288-292.
- Betancourt P, Fuentes R, Aracena Rojas S, Cantín M, Navarro Cáceres P. Prevalence of a second canal in the mesiobuccal root of maxillary first molars by cone-beam computed tomography. Av Odontoestomatol 2013; 29: 31-36.
- Degerness R, Bowles W. Anatomic determination of the mesiobuccal root resection level in maxillary molars. J Endod 2008; 34: 1182-1186.
- Betancourt P, Aracena Rojas S, Navarro Caceres P, Fuentes R. Anatomical configuration of canalicular system in mesiobuccal root of maxillary first molar. Av Odontoestomatol 2015; 31: 11-18.
- Betancourt P, Navarro P, Munoz G, Fuentes R. Prevalence and location of the secondary mesiobuccal canal in 1,100 maxillary molars using cone beam computed tomography. BMC Med Imaging 2016; 16: 66.
- Mohammadzadeh Akhlaghi N, Ravandoust Y, Najafi M, Dadresanfar B. An in vitro study of mesiobuccal root thickness of maxillary first molars. Iran Endod J 2012; 7: 31-35.
- Oliveira MA, Venâncio JF, Raposo LH, Barbosa Júnior N, Biffi JC. Morphometric evaluation and planning of anticurvature filing in roots of maxillary and mandibular molars. Braz Oral Res 2015; 29: 1-9.
- Degerness RA, Bowles WR. Dimension, anatomy and morphology of the mesiobuccal root canal system in maxillary molars. J Endod 2010; 36: 985-989.
- Jung IY, Seo MA, Fouad AF, Spangberg LS, Lee SJ. Apical anatomy in mesial and mesiobuccal roots of permanent first molars. J Endod 2005; 31: 364-368.
- Smadi L, Khraisat A. Detection of a second mesiobuccal canal in the mesiobuccal roots of maxillary first molar teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 77-81.
- Baratto Filho F, Zaitter S, Haragushiku GA, de Campos EA, Abuabara A, Correr GM. Analysis of the internal anatomy of maxillary first molars by using different methods. J Endod 2009; 35: 337-342.
- Matus D, Cantín M. Evaluation of isthmus frequency, location, and types in mesiobuccal roots of first maxillary molars. An ex vivo study. Int J Morphol 2016; 34: 804-810.
- Betancourt P, Cantin M, Fuentes R. In vitro and in vivo frecuency of MB2 canal in maxillary first molars. A systematic review. Av Odontoestomatol 2014; 30: 11-22.
- Lertchirakarn V, Palamara JE, Messer HH. Patterns of vertical root fracture: factors affecting stress distribution in the root canal. J Endod 2003; 29: 523-528.
- Chai H, Tamse A. The effect of isthmus on vertical root fracture in endodontically treated teeth. J Endod 2015; 41: 1515-1519.