Research Article - Biomedical Research (2017) Volume 28, Issue 8
Evaluation of the antibacterial effect in vitro of moxifloxacin and linezolid on nontuberculous mycobacterium
Lanfang Fang1, Xiao Chen2*, Zhongkang Ji2 and Kaijin Xu2
1Departments of Infectious Diseases, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, PR China
2State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, PR China
- *Corresponding Author:
- Xiao Chen
State Key Laboratory for Diagnosis and Treatment of
Infectious Diseases
Collaborative Innovation Center for Diagnosis and Treatment
of Infectious Diseases
The First Affiliated Hospital
College of Medicine, Zhejiang University, Hangzhou, Zhejiang, PR China
Accepted on December 30, 2016
Abstract
Objective: To evaluate the antibacterial effect in vitro of moxifloxacin and linezolid on nontuberculous mycobacterium (NTM), and provide scientific evidence for treatment of NTM disease.
Materials and Methods: From January 2012 to June 2014, a total of 98 NTM strains were collected from suspected tuberculosis patients (n=98) in our hospital. The minimal inhibitory concentration (MIC) of the moxifloxacin and linezolid against NTM isolates were detected by microdilution broth method.
Results: The sensitivity of moxifloxacin to Mycobacterium avium complex was 84.5% (49/58), which was significantly higher than that of Mycobacterium abscess (11.5%, 3/26) (χ2=40.505, P=0.000). The sensitivity of linezolid to Mycobacterium avium complex was 65.5% (38/58), which was lower than that 88.5% (23/26) of Mycobacterium abscess. The difference was statistically significant (χ2=4.753, P=0.029). Among Mycobacterium avium complex, the sensitivity of Moxifloxacin to Mycobacterium avium (96.2%, 25/26) was higher than that of Mycobacterium intracellulare (75%, 24/32), the difference was statistically significant (Fisher test, P=0.033); The sensitivity of linezolid to Mycobacterium avium and Mycobacterium intracellulare were 69.2% (18/26) and 62.5% (20/32) respectively. There was no significant difference between the two groups (χ2=0.288, P=0.592).
Conclusion: Different species of NTM differed in the drug susceptibility profiles. It is necessary to identify and perform drug sensitivity test in the diagnosis and treatment of NTM diseases.
Keywords
Nontuberculous mycobacterium, Moxifloxacin, Linezolid, Antibiotics.
Introduction
In recent years, the incidence of nontuberculous mycobacterium (NTM) has increased in many countries [1-3]. The result of China’s epidemiological survey of tuberculosis showed that, the percentage of NTM isolates among mycobacterium isolates increased from 4.3% in 1979 to 11.1% in 2000, while in 2010, the ratio increased to 22.9% [4,5]. For many NTMs, especially the rapid growth of nontuberculous mycobacterium (RGM), they have not only high drug resistance rate but also extensive drug resistance, leading to limited alternative drugs and difficulties in clinical treatment. Therefore, searching for new drugs or exploring conventional drugs in new use becomes the urgent task for current treatment of NTM disease.
Linezolid is an oxazolidinone antibiotic used for the treatment of gram-positive cocci infection, which was found in recent research that it has satisfactory antibacterial activity against multidrug-resistant tuberculosis and Mycobacterium avium complex (MAC) [6,7]. Moxifloxacin is a new class of quinolones, which has certain curative effect on MAC disease [8]. However, there are few reports on research about the drug sensitivity of different NTMs to linezolid and moxifloxacin in vitro in China. To explore the possibilities of using linezolid and moxifloxacin drugs for the treatment of NTM disease, we have collected 98 NTM clinical isolates to detect and compare the minimal inhibitory concentration (MIC) of the two antiinfective drugs.
Materials and Methods
Materials
Strain source: 98 NTM strains were collected from clinical tuberculosis patients in Microbiology Laboratory, College of Medicine, the First Affiliated Hospital of Zhejiang University (China) from January 2012 to June 2014.
Strain identification: Jingxin Mycobacteria Identification Diagnostic Kit (Capital Bo Corporation, China) was used for Mycobacteria strains identification. According to the identification results, there were 32 strains of Mycobacterium intracellulare, 26 strains of Mycobacterium avium, 26 strains of Mycobacterium abscessus, 6 strains of Mycobacterium Gordonae, 2 strains of Mycobacterium chelonae, 2 strains of Mycobacterium kansasii, 2 strains of Mycobacterium fortuilum, 1 strain of Mycobacterium toad and 1 strain of Mycobacterium smegmatis.
Antimicrobial agents: linezolid (Sigma Company, the U.S.) and moxifloxacin (Dalian Merro Pharm, China).
Quality-control strains: Mycobacterium intracellulare ATCC 700898 and Staphylococcus aureus ATCC 29213.
Methods
Preparation of inoculated strain: Inoculate the retained strains in 7H9 liquid medium (containing 10% OADC addictive), culture them at 37 for about 7 days (for slow growing Mycobacterium) or 3 days (for rapid growing Mycobacterium). Until the strains grow to a certain degree of turbidity, and during their logarithmic growth phase, perform drug sensitive test.
Drug preparation: Linezolid and moxifloxacin were dissolved with sterile deionized water, with the parent solution concentration of 1280 mg/L. Diluted them by 7H9 broth under doubling dilution after 10 fold dilution , with the concentration ranging from 0.5~128 μg/mL; added 100 μL to each well.
Inoculation, incubation and result: Adjust the strains to be tested to the turbidity of 0.5 McFarland with 0.85% normal saline, then dilute the bacterial suspension for 100 times by 7H9 medium, and add 10 μL to each well. Place the reaction plate in a sealed bag containing wetted cotton to prevent evaporation of the culture medium from the 96-well culture plate. Place the sealed bag in a 37 incubator and culture it for 7 days or 3 days, and observe the growth of bacteria in the 96- well culture plate. Record the minimal inhibitory concentration (MIC) of drugs against bacteria when the bacteria in the positive control well grow well and on significant bacterial growth was observed in the negative control well.
Interpretation of drug sensitivity breakpoint: Refer to the interpretation standard for NTM drug sensitivity test of the United States Clinical and Laboratory Standards Institute (CLSI M24-A2 2011) (Table 1) [9].
| Mycobacterium Strains | Drugs | MIC (µg/mL) Interpretation Point |
||
|---|---|---|---|---|
| S | I | R | ||
| Mycobacterium avium complex | moxifloxacin | ≤1 | 2 | ≥4 |
| linezolid | ≤8 | 16 | ≥32 | |
| Mycobacterium kansasii | moxifloxacin | >2 | ||
| linezolid | >16 | |||
| Rapid Growing Mycobacterium | moxifloxacin | ≤1 | 2 | ≥4 |
| linezolid | ≤8 | 16 | ≥32 | |
Table 1. The MIC Drug Sensitivity Interpretation Breakpoint of Mycobacterium.
Statistical analysis
Statistical analysis was performed using SPSS17.0 software, and chi-square test or Fisher test was used to compare the rates. P <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
The demographic and clinical features of 98 NTM cases were showed in Table 2. Among the 98 cases, pulmonary NTM (PNTM) infections accounted for 87.8% 86/98, and 37.2% (32/86) of PNTM patients had chronic lung comorbidities. Chronic lung disease and diabetes mellitus were the two major comorbidities, and a total of 18 patients had more than two comorbidities.
| Variable | Patients (n=98), n (%) |
| Age, y, median (range) | 59 (26-90) |
| Sex, male: female | 70:28 |
| Fever | 82 (83.7) |
| Comobidity diseases | 88 (89.8) |
| Chronic lung disease | 32 (32.7) |
| Diabetes mellitus | 17 (17.3) |
| Chronic liver disease | 15 (15.3) |
| Hypertension | 12 (12.2) |
| Malignancy | 12 (12.2) |
| Transplantation | 5 (5.1) |
| HIV-infected | 5 (5.1) |
| Chronic kidney disease | 4 (4.1) |
| Syhilis | 4 (4.1) |
| Limb paralysis | 1 (1.0) |
| Infection site | |
| Lung | 86 (87.8) |
| Cervical lymph node | 4 (4.1) |
| Intracalvarium | 2 (2.0) |
| Urinary system | 2 (2.0) |
| Antrum auris | 1 (1.0) |
| Lumbar vertebrae | 1 (1.0) |
| Wound | 2 (2.0) |
| Positive acid-fast bacillus (n=68) | 68 (69.4) |
Table 2. Demographic and clinical features of 98 NTM cases.
Antibacterial effect in vitro of moxifloxacin and linezolid
The MIC of moxifloxacin and linezolid to 98 NTM isolates were showed in Table 3. The MIC90 of moxifloxacin to the clinical common strains Mycobacterium intracellulare, Mycobacterium avium and Mycobacterium abscesses were 4 μg/mL, 1 μg/mL and 8 μg/mL respectively; while the MIC90 of linezolid to the above three kinds of strains were 64 μg/mL, 32 μg/mL and 16 μg/mL. The sensitivity of moxifloxacin to Mycobacterium avium complex was 84.5%, which was significantly higher than that of Mycobacterium abscesses (11.5%) (χ2=40.505, P=0.000). Among Mycobacterium avium complex, the sensitivity of Moxifloxacin to Mycobacterium avium (96.2%) was higher than that of Mycobacterium intracellulare (75%), the difference was statistically significant (Fisher test, P=0.033). The sensitivity of linezolid to Mycobacterium avium complex was 65.5%, which was lower than that of Mycobacterium abscess (88.5%), and the difference was statistically significant (χ2=4.753, P=0.029). Among Mycobacterium avium complex, the sensitivity of linezolid to Mycobacterium avium and Mycobacterium intracellulare were 69.2% and 62.5% respectively, and the difference was not statistically significant (χ2=0.288P=0.592).
| Mycobacterium Strains | Quantity | The Quantity of inhibited strains by different concentration of antibacterial drugs (µg/mL) | MIC50 | MIC90 | S% | I% | R% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | ≥128 | |||||||
| Mycobacteriumintracellulare | |||||||||||||||
| Moxifloxacin | 32 | 21 | 3 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0.5 | 4 | 75 | 12.5 | 12.5 |
| Linezolid | 32 | 1 | 2 | 5 | 6 | 6 | 2 | 4 | 4 | 2 | 8 | 64 | 62.5 | 6.3 | 31.2 |
| Mycobacterium avium | |||||||||||||||
| moxifloxacin | 26 | 18 | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.5 | 1 | 96.2 | 0 | 3.8 |
| linezolid | 26 | 6 | 2 | 2 | 2 | 6 | 4 | 2 | 2 | 0 | 8 | 32 | 69.2 | 15.4 | 15.4 |
| Mycobacterium abscessus | |||||||||||||||
| Moxifloxacin | 26 | 0 | 3 | 9 | 11 | 1 | 1 | 1 | 0 | 0 | 4 | 8 | 11.5 | 34.6 | 53.9 |
| Linezolid | 26 | 0 | 1 | 4 | 13 | 5 | 3 | 0 | 0 | 0 | 4 | 16 | 88.5 | 11.5 | 0 |
| Mycobacterium Gordonae |
|||||||||||||||
| Moxifloxacin | 6 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.5 | 4 | - | - | - |
| Linezolid | 6 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 64 | - | - | - |
| Mycobacterium chelonae |
|||||||||||||||
| Moxifloxacin | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| Linezolid | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| Mycobacterium kansasii |
|||||||||||||||
| moxifloxacin | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| linezolid | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - |
| Mycobacterium fortuilum |
|||||||||||||||
| moxifloxacin | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| linezolid | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | - | - | - | - |
| Mycobacterium toad | |||||||||||||||
| moxifloxacin | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| linezolid | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| Mycobacterium megmatis |
|||||||||||||||
| moxifloxacin | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| linezolid | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - |
| Slow GrowingMycobacterium | |||||||||||||||
| moxifloxacin | 67 | 46 | 10 | 5 | 6 | 0 | 0 | 0 | 0 | 0 | 0.5 | 2 | - | - | - |
| linezolid | 67 | 10 | 6 | 7 | 9 | 12 | 7 | 6 | 7 | 3 | 8 | 64 | - | - | - |
| Rapid GrowingMycobacterium | |||||||||||||||
| moxifloxacin | 31 | 2 | 4 | 10 | 11 | 2 | 1 | 1 | 0 | 0 | 2 | 8 | 19.3 | 32.3 | 48.4 |
| linezolid | 31 | 1 | 2 | 5 | 14 | 5 | 3 | 1 | 0 | 0 | 4 | 16 | 87.1 | 9.7 | 3.2 |
Table 3. MIC Strain Distribution of Antibacterial Drugs to NTMs.
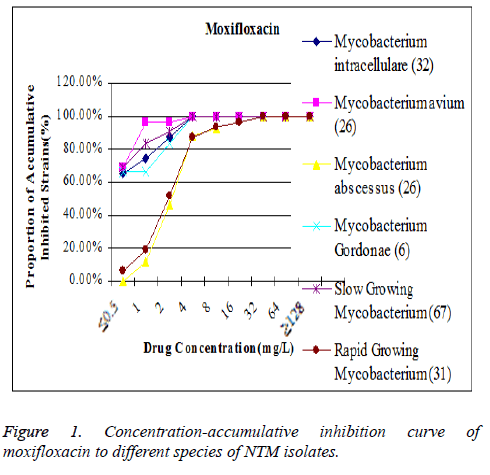
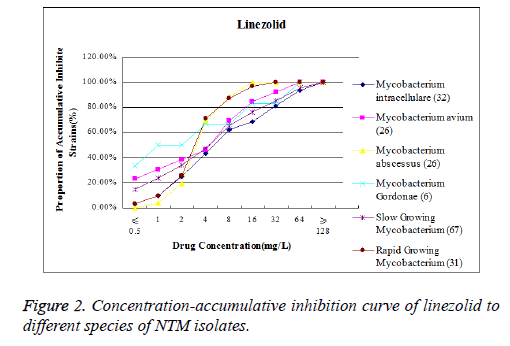
The MIC90 of moxifloxacin to Mycobacterium avium was lower that its position on the concentration-accumulative inhibition curve was inclined to the left, while MIC 90 of moxifloxacin to Mycobacterium abscesses was higher that its position on the concentration-accumulative inhibition curve was inclined to the right (Figure 1). The MIC90 of linezolid to Mycobacterium abscesses was lower that its position on the concentration-accumulative inhibition curve is inclined to the left, while the MIC90 of linezolid to Mycobacterium intracellulare is higher that its position on concentrationaccumulative inhibition curve is inclined to the right (Figure 2).
Discussion
Currently, there are more than 150 species of NTM strains have been found, some of which have the capability to cause human opportunistic infection. The result showed that prevalent NTM strains differed in different regions, and different species of NTM also differed in their pathogenic types, which were closely related to the local climate factors, geographical location and the patients’ personal working and living conditions [10]. Each NTM isolate has its unique drug susceptibility profile, which requires different antimicrobial agents for treatment, and thus it is necessary to identify the strain types and perform NTM drug sensitivity test in vitro before the treatment.
In this study, the MIC value of moxifloxacin against NTM was detected by micro-broth dilution method, and the result showed that the MIC50 and MIC90 of moxifloxacin against Mycobacterium intracellulare were 0.5 μg/mL and 4 μg/mL respectively, and the drug resistance rate was 12.5%; the MIC50 and MIC90 of moxifloxacin against Mycobacterium avium was 0.5 μg/mL and 1 μg/mL, and the drug resistance rate was 3.8%. Duan et al. have detected the isolated strains from Japanese patients of MAC disease, the result was that the MIC50 and MIC90 of moxifloxacin against Mycobacterium intracellulare was 2 μg/mL and 8 μg/mL respectively; the MIC50 and MIC90 of moxifloxacin against Mycobacterium avium was 2 μg/mL and 8 μg/mL respectively [11]. Li et al. have collected strains from 4 specialized tuberculosis hospitals in China, the drug sensitivity result showed that, the MIC50 and MIC90 of moxifloxacin against Mycobacterium intracellulare was 0.5 μg/mL and 1 μg/mL, and the drug resistance rate was 1.6%; the MIC50 and MIC90 of moxifloxacin against Mycobacterium avium was 0.5 μg/mL and 4 μg/mL, and the drug resistance rate was 10.8% [12]. According to Li et al. the drug resistance rate of moxifloxacin against Mycobacterium avium was significantly higher than that against Mycobacterium intracellulare, while our result showed that the sensitivity of moxifloxacin to Mycobacterium avium was higher than that to Mycobacterium intracellulare. In China, the reports on research applying micro-broth dilution method to detect the MIC of moxifloxacin against Mycobacterium abscesses are very rare. A Singapore study showed that the sensitivity of moxifloxacin to 124 strains of Mycobacterium abscesses was 3%, and the drug resistance rate was 93% [13]. The data from Taiwan showed that the drug resistance rate of moxifloxacin to 13 strains of Mycobacterium abscesses was 100%, and the MIC90 was 16 μg/mL (3 days) and 32 μg/mL (5 days) [14]. While our result indicated that the sensitivity of moxifloxacin to 26 strains of Mycobacterium abscesses was 11.5%. Currently, the use of moxifloxacin in the treatment of NTM infection is still lack of sufficient research data; however, in drug resistance test in vitro, it revealed a relatively strong antibacterial ability to Mycobacterium avium complex and weak antibacterial ability to Mycobacterium abscesses, and a better ability in removing MAC in animal models [15], which suggesting that moxifloxacin may have potential clinical value for the effective treatment of MAC infection. Certainly, this conclusion requires more in-depth study for confirmation.
In this study, the MIC50 and MIC90 of linezolid to Mycobacterium intracellulare were 8 μg/mL and 64 μg/mL respectively, with the drug resistance rate of 31.2%; while the MIC50 and MIC90 of linezolid against Mycobacterium avium were 8 μg/mL and 32 μg/mL respectively, with the drug resistance rate of 15.4%. Duan et al. showed that the MIC of linezolid to 76 strains of Mycobacterium intracellulare was 64 μg/ml, and the drug resistance rate was 44.7% [16]. Li et al. presented that the MIC50 and MIC90 of linezolid to Mycobacterium intracellulare were 8 μg/mL and 16 μg/mL respectively, with the drug resistance rate of 8.5%; while the MIC50 and MIC90 of linezolid to Mycobacterium avium were 16 μg/mL and 32 μg/mL respectively, with the drug resistance rate of 40% [12]. Huang et al. mentioned in their research that the MIC value of linezolid to 15 strains of Mycobacterium intracellulare was all less than 8 μg/mL [17]. The antibacterial activity of linezolid on NTM strains differed with different strains. In this study, the MIC50 and MIC90 of linezolid to Mycobacterium abscesses were 4 μg/mL and 16 μg/mL respectively, and the sensitivity rate was 88.5%, without nonsensitive strains. Similar to the result of this study, Chen et al. mentioned in their research report that the sensitivity of linezolid to Mycobacterium abscesses was 78.5% and the drug resistance rate was 3.5% [18]. The difference appeared in Singapore’s research that the sensitivity of linezolid to 306 strains of Mycobacterium abscess was 42% and the drug resistance rate was 16% [13]. The reason of the difference in research results may be due to the differences of the strains’ coming time, region and the sample size.
The result of this study suggests that, moxifloxacin and linezolid have relatively strong antibacterial ability on the clinical common Mycobacterium intracellulare and Mycobacterium avium, and have certain clinical value in the treatment of MAC infection. For Mycobacterium abscesses, linezolid has a relatively strong antibacterial ability, which could be used as a selective drug, while the use of moxifloxacin should be further studied. In summary, different species of NTM differed in their drug susceptibility profiles, therefore, it is necessary to identify and perform drug sensitivity test in the diagnosis and treatment of NTM diseases.
Acknowledgements
This paper was supported by a grant from Zhejiang Provincial Department of Education (Y201327731)
References
- Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881-886.
- Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis 2010; 16: 294-296.
- Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis (Seoul) 2012; 72: 409-415.
- National Technical Steering Group for Epidemiological Sampling Survey of Tuberculosis. The Fourth National Epidemiological Survey of Tuberculosis. Chinese J Tuberculosis Resp Dis 2002; 25: 3-7.
- Technical Guidance Group of the Fifth National TB Epidemiological Survey, The Office of the Fifth National TB Epidemiological Survey. The Fifth National Epidemiological Survey of Tuberculosis in 2010. Chinese Antitubercul Asso 2012; 34: 485-508.
- Schecter GF, Scott C, True L, Raftery A, Flood J. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2010; 50: 49-55.
- Nannini EC, Keating M, Binstoek P, Samonis G, Kontoyiannis DP. Successful treatment of refractory disseminated Mycobacterium avium complex infection with the addition of linezolid and mefloquine. J Infect 2002, 44: 201-203.
- Sano C, Tatano Y, Shimizu T, Yamabe S, Sato K, Tomioka H. Comparative in vitro and in vivo antimicrobial activities of sitafloxacin, gatifloxacin and moxifloxacin against Mycobacterium avium. Int J Antimicrob Agents 2011; 37: 296-301.
- M24-A, Susceptibility testing of Mycobacteria, Nocardiae, and other Aerobic Actinomycetes; Approved standard-second edition. Pennsylvania: Clinical and Laboratory Standards Institute, 2011.
- Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009; 49: 124-129.
- Duan HF, Doi N, Li Q, Ma Y, Chen XY. Susceptibility test of the Mycobacterium avium complex to sixteen anti-infective agents. Zhonghua Jie He He Hu Xi Za Zhi 2010; 33: 359-362.
- Li YM, Tong XL, Pang Y, Cohen C, Zhao Y, Liu C. Drug susceptibility profiles and clinical characteristics of Mycobacterium avium complex. Chin J Prey Phthisiol 2015; 37: 622-627.
- Tang SS, Lye DC, Jureen R, Sng LH, Hsu LY. Rapidly growing mycobacteria in Singapore, 2006-2011. Clin Microbiol Infect 2015; 21: 236-241.
- Chu HS, Chang SC, Shen EP, Hu FR. Nontuberculous mycobacterial ocular infections--comparing the clinical and microbiological characteristics between Mycobacterium abscessus and Mycobacterium massiliense. PLoS One 2015; 10: e0116236.
- Sano C, Tatano Y, Shimizu T, Yamabe S, Sato K, Tomioka H. Comparative in vitro and in vivo antimicrobial activities of sitafloxacin, gatifloxacin and moxifloxacin against Mycobacterium avium. Int J Antimicrob Agents 2011; 37: 296-301.
- Duan HF, Liang Q, Chu NH, Huang H. Macrolide and linezolid susceptibility testing for Mycobacterium intracellulare isolates. Chin J of Tuberculosis Resp Dis 2014; 37: 266-269.
- Huang HR, Yu X, Jiang GL. Evaluation of the mycobactericidal efficacy of linezolid in vitro. Chin J of Tuberculosis Resp Dis 2011; 34: 575-578.
- Chen PR, Cai XS, Xiao F. MIC method of pathogenic fast-growing non-tuberculosis mycobacteria and analysis of drug susceptibility result. Guangdong MedJ 2012; 33: 3620-3623.