- Biomedical Research (2012) Volume 23, Issue 1
Evaluation of thalidomide against indomethacin-induced small intestinal damage and systemic toxicity in rats
Silva MA1, Rao VS1,2, Souza, CM1, Neves JCS2, Menezes DB3, Santos FA1,2, Andrade GM1,2*
1Post-Graduate Programme in Medical Sciences, Department of Clinical Medicine, Faculty of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
2Institute of Brazilian Semi-arid, Post-Graduate Programme in Pharmacology, Department of Physiology and Pharmacology, Faculty of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
3Department of Pathology and Legal Medicine, Faculty of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
- Corresponding Author:
- Geanne Matos de Andrade
Department of Physiology and Pharmacology, Faculty of Medicine Federal University of Ceará Rua Cel Nunes de Melo, 1127, Rodolfo Teófilo CEP 60430-270, Fortaleza, Ceará, Brasil
Accepted date: November 24 2011
Abstract
Clinical use of indomethacin although efficacious in suppressing pain, fever and inflammation is frequently associated with deleterious effects on gastrointestinal, hematological and renal systems that limit its therapeutic use. This study examined in rats whether thalidomide, a known antiinflammatory agent with TNF-α inhibitory, immunomodulatory and anti-angiogenic properties could ameliorate indomethacin-induced toxicity that includes lethality, hematological and biochemical changes in blood, as well as the small intestinal damage. Wistar male rats in groups were treated orally with indomethacin (5, 10, and 20 mg/kg), thalidomide (100 and 200 mg/kg, either alone or in combination with indomethacin 5 mg/kg) once daily during 5 days. Lethality was assessed during this period and on day-5 blood samples were collected to examine the hematological and biochemical changes. The animals were then sacrificed and the small intestine removed for histological analysis. Results demonstrated that treatment with thalidomide did not improve the survival rate of indomethacin-treated rats. However, indomethacin-associated leucopenia, decrease in red blood cells, hemoglobin, and hematocrit as well as the elevation in plasma fibrinogen, serum AST and ALP, small intestinal lesion score, and the peritoneal liquid myeloperoxidase level were significantly suppressed by thalidomide treatment. In conclusion, the study suggests that thalidomide has the potential of ameliorating the toxic effects of indomethacin to a large extent, possibly by virtue of its anti-inflammatory properties.
Keywords
Indomethacin toxicity, thalidomide, plasma fibrinogen, small intestinal damage.
Introduction
The clinical use of non-steroidal anti-inflammatory drugs (NSAIDs) including indomethacin is associated with potentially life-threatening deleterious effects of gastrointestinal ulceration, bleeding and nephropathy [1,2,3]. Also, there were reports of injury to liver and bone marrow with the use of NSAIDs [4,5]. There have been many strategies aimed at attempting to decrease the toxicity of NSAIDs, but none have completely solved. Inhibition of cyclooxygenase, mitochondrial dysfunction, oxidative stress, an increase in pro-inflammatory cytokine TNF-α, nitric oxide (NO), and enhanced intestinal permeability seem to contribute to the pathogenesis of NSAID induced gastrointestinal damage [6,7,8,9,10]. These events are attributable to the ability of these drugs to suppress prostaglandin and thromboxane synthesis. Indomethacin reduces PGE2 production by inhibition of the cyclooxygenases (COXs), COX-1 and COX-2 [11]. There is evidence regarding a possible risk for cardiovascular thromboembolic events with COX-2 selective inhibitors and a risk for gastrointestinal bleeding with COX-1 inhibitors. Literature reports describe the gastrointestinal bleeding effect of indomethacin but not on cardiovascular thromboembolic event.
Thalidomide is a glutamic acid derivative that exerts antiinflammatory, immunomodulatory and anti-angiogenic activities and its proposed mechanisms include inhibition of tumor necrosis factor alpha (TNF-α) release and inhibition of angiogenesis [12,13]. Despite the high risk of venous thromboembolism, the anti-angiogenic activity of thalidomide suggested its use for the treatment of refractory multiple myelomas and in the control of gastrointes tinal bleeding in patients with Crohn’s disease and angiodysplasias [14,15,16]. We hypothesize that thalidomide by its anti-inflammatory, anti-angiogenic properties would abrogate indomethacin associated intestinal bleeding (consequence of COX-1 inhibition), but on the other side it might intensify thromboembolism. Thus the present study aims to investigate the potential beneficial effects of thalidomide on indomethacin-induced toxicity in relation to lethality, intestinal damage, hematological, biochemical and histological alterations in a rat model.
Materials and Methods
Chemicals
Thalidome (Talidomida- Funed®-100mg) was purchased from Ezequiel Dias Foundation – Brazil, indomethacin (Indocid®- 50mg) from Merck Sharp & Dohme - USA and ampicilin (500mg) from EMS /SA- Brazil. All other drugs or reagents were of analytical grade.
Animals
Male Wistar rats weighing 180-200 g obtained from the Central Animal House of Federal University of Ceará were used. They were maintained in polypropylene cages under controlled temperature, humidity and a light/dark (12/12 h) cycle, and allowed free access to laboratory rat chow (Purina Rat Chow, Sao Paulo, Brazil) and filtered water. Experiments were performed according to the Guide of Care and Use of Laboratory Animals from the US Health and Human Services Department. The study was approved by the local Institutional Ethics Committee
Experimental protocol
Rats were treated orally with indomethacin (5, 10, and 20 mg/kg), thalidomide (100 and 200 mg/kg,) [17], or in their combination (indomethacin 5 mg/kg + thalidomide 100 or 200 mg/kg) once daily during 5 days. Control animals received 5% sodium bicarbonate (indomethacin vehicle) followed 1 h later for 2% DMSO (thalidomide vehicle) by gavage in a volume of 10 mL/kg. Lethality was assessed during this period and on day-5 blood was collected from live animals for hematological and biochemical analysis. The animals were then sacrificed under excess anesthesia to aspirate peritoneal liquid and remove small intestine for histological analysis.
Hematological and coagulation studies
Blood samples were collected from orbital plexus in collecting tubes containing EDTA (blood cell and platelet counts, hematocrit and hemoglobin) and 3.8 % sodium citrate (coagulation studies). The samples were centrifuged at 3000 × g at 4°C for 10 min to obtain plasma samples. Prothrombin time, activated thromboplastin, fibrinogen and antithrombin were measured with a coagulometer (Sysmex® CA-1500-Siemens- Healthcare Diagnostics). Additionally, peritoneal liquid was collected and examined for leukocyte counts (Cell Dyn-1800- Abbot Diagnostic-USA)
Biochemical studies
For biochemical assays, blood samples were centrifuged at 3000 × g at 4°C for 10 min. The alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ- glutamyltransferase (GGT), alkaline phosphatase (ALP), urea, creatinine, glucose, and albumin were determined by routine colorimetric methods using the commercial kits (Wiener lab- Rosario, Argentina) and quantified on clinical biochemistry autoanalyser (Wiener lab, BTS 3000 Plus- Rosario-Argentina).
Myeloperoxidase (MPO) assay
MPO activity, a marker for neutrophils infiltration, was quantified in peritoneal fluid according to Bradley et al. with adaptations [18]. Briefly, samples of peritoneal fluid (0.1 ml) were sonicated and then vigorously mixed with 0.9 ml from 0.5% HTAB in potassium phosphate buffer solution, and aliquots of 30 μl was added into 96 wells plate containing 200 μl of 0.9% hydrogen peroxide and 0.52 mM o–dianisidine solution. The reaction between H2O2 and o–dianisidine is catalyzed by MPO that generates a color compound that is quantified by measuring the absorbance at 460 nm. One unit of MPO activity was defined as that degrading 1 mmol of peroxide per min at 25°C. The MPO activity was expressed in units per ml.
Histological examinations
Samples of ileal tissues were fixed in 10% buffered formalin solution, embedded in paraffin using standard methods, cut into 5-μm sections, stained with hematoxylin- eosin, and evaluated under light microscopy by a pathologist blinded to experimental groups who scored the histological alterations according Chiu et al. with modifications: 0 = Absence of inflammatory reaction, ulceration or tissue destruction; 1 = slight inflammatory reaction but no ulceration or tissue destruction; 2 = moderate inflammatory infiltration with mild tissue destruction of villous, crypts but no ulceration; 3 = intense inflammatory reaction, presence of ulceration and extensive tissue destruction [19].
Statistical analysis
Statistical analysis were performed using one-way analysis of variance (ANOVA) followed by Kruskal Wallis or Student Newman Keul as post-hoc tests. The non parametric data were expressed as median (with low and high ranges), and parametric data as mean ± S.E.M. For sur vival curve the comparisons between curves were made by log-rank test and Bonferroni for multiple comparisons. Differences were considered significant at P < 0.05.
Results
Effect of thalidomide on indomethacin-induced lethality
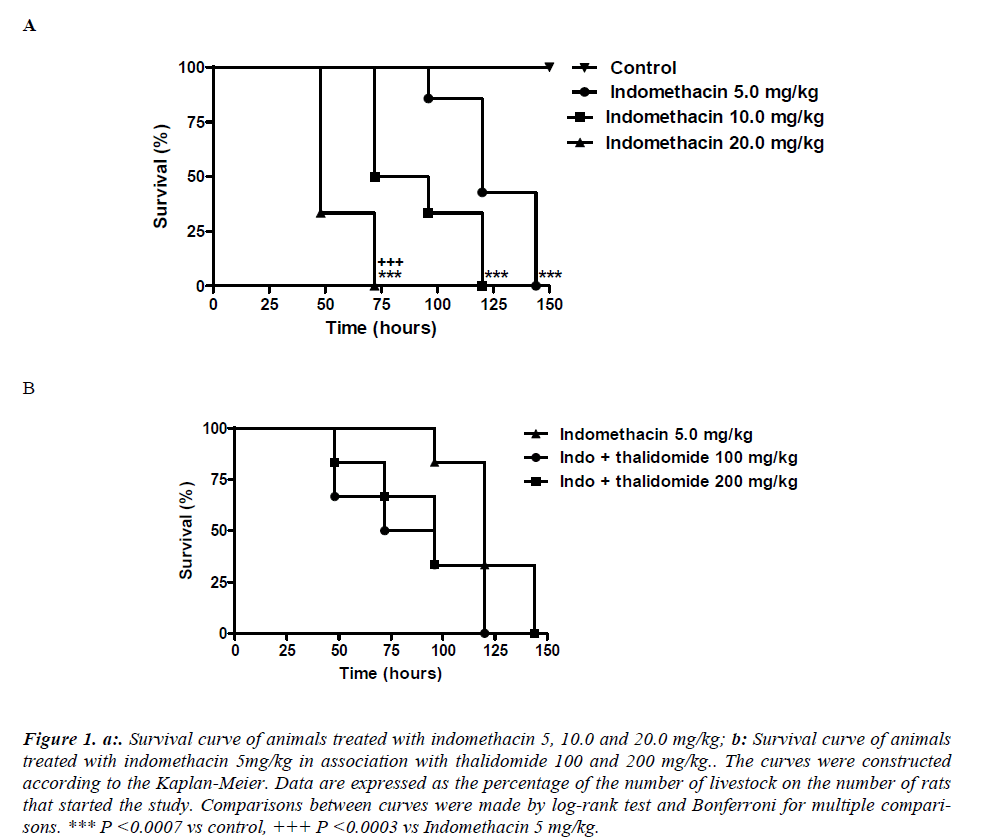
Indomethacin given by gastric gavage induced a doserelated lethality. The lethality with 5 mg/kg was significantly less compared to 20 mg/kg. It was found that the survival curve on the group treated with indomethacin 20 mg/kg was significantly (p< 0.0003) different from that corresponding to 5 mg/kg. The median survival in the groups treated with indomethacin 5, 10 and 20 mg/kg were respectively equal to 120, 84 and 48 hours. Survival in the 150th hour of the experiment was 100% in control group and 0% in groups treated with indomethacin at all doses (Figure 1a). The protective effect of thalidomide were analyzed in animals receiving a dose of 5mg/kg indomethacin administered for 5 days, although thalidomide has reduced the mortality, the difference between the indomethacin group was not significantly different. Comparisons between curves were made by log-rank test that showed no statistically significant differences (p = 0.3079) between them. The median survival in group indomethacin 5 mg/kg was 120 hours while in the groups treated with combination of indomethacin + thalidomide 100 mg/kg and indomethacin + thalidomide 200 mg/kg the median survival were respectively equal to 84 and 96 hours. Survival in the 144th hour of the experiment was 0% in all groups (Figure 1b).
Figure 1. a:. Survival curve of animals treated with indomethacin 5, 10.0 and 20.0 mg/kg; b: Survival curve of animals treated with indomethacin 5mg/kg in association with thalidomide 100 and 200 mg/kg.. The curves were constructed according to the Kaplan-Meier. Data are expressed as the percentage of the number of livestock on the number of rats that started the study. Comparisons between curves were made by log-rank test and Bonferroni for multiple comparisons. *** P <0.0007 vs control, +++ P <0.0003 vs Indomethacin 5 mg/kg.
Effect of thalidomide on indomethacin-induced changes in hematological and coagulation parameters
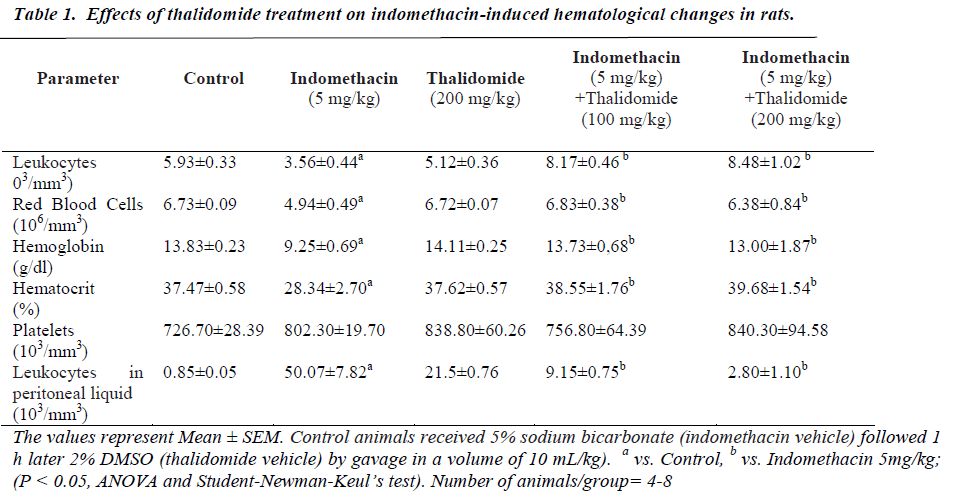
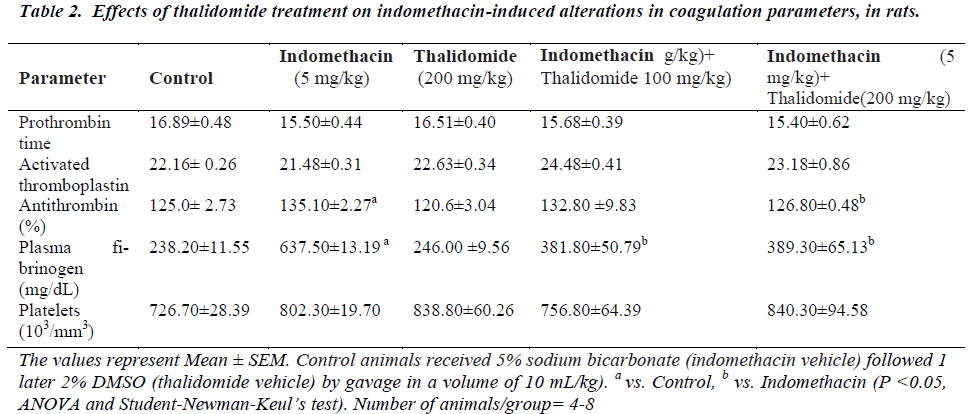
The five day treatment with indomethacin caused significant leucopenia, decreases in RBC count, hemoglobin, and hematocrit values when compared to normal controls (Table 1). However, there was no change in platelet numbers. The significant reductions produced by indomethacin were mitigated by thalidomide without showing a dose-dependence. On coagulation parameters, an increase in plasma fibrinogen and antithrombin levels were evidenced in indomethacin treated group. These increases were effectively counteracted by thalidomide (200 mg/kg)-pretreatment, almost to the levels seen in normal controls. In relation to prothrombin time and activated thromboplastin, no significant influence of indomethacin was observed (Table 2).
Effect of thalidomide on indomethacin-induced biochemical alterations
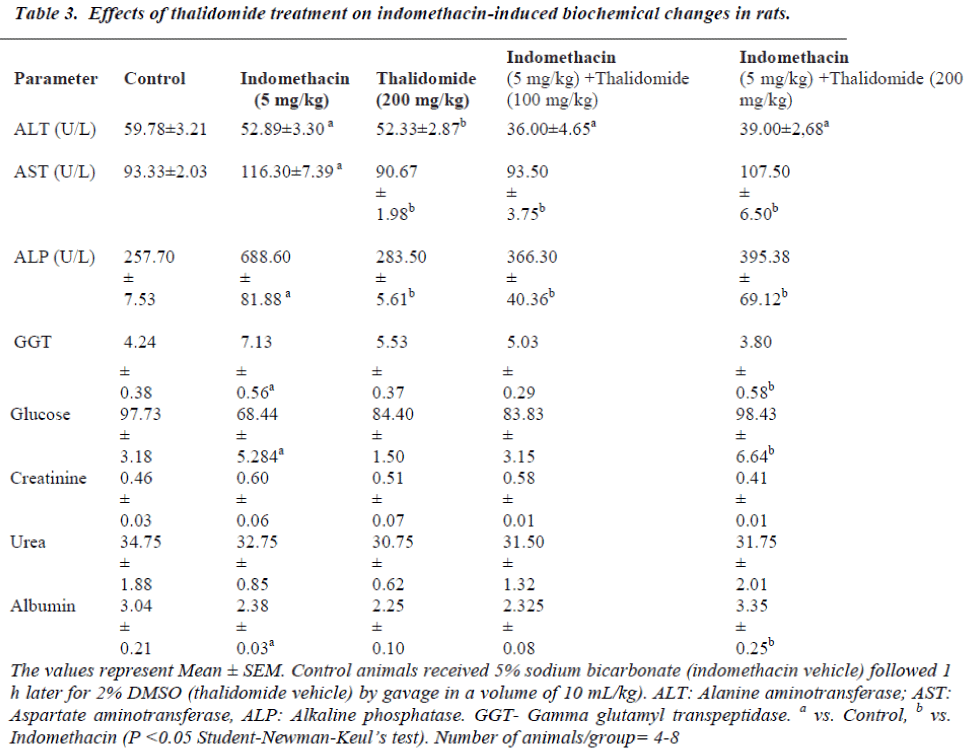
The effects of indomethacin and thalidomide treatments are shown in Table 3. Compared to controls, a five day treatment with indomethacin (5 mg/kg) significantly enhanced serum AST, ALP and GGT activities, and decreased glucose and albumin, and no alteration on urea and creatinine concentration. These increases or decreases were respectively diminished by thalidomide treatment. At the doses employed, there were no significant changes from vehicle treated controls.
Effect of thalidomide on myeloperoxidase activity in the peritoneal fluids
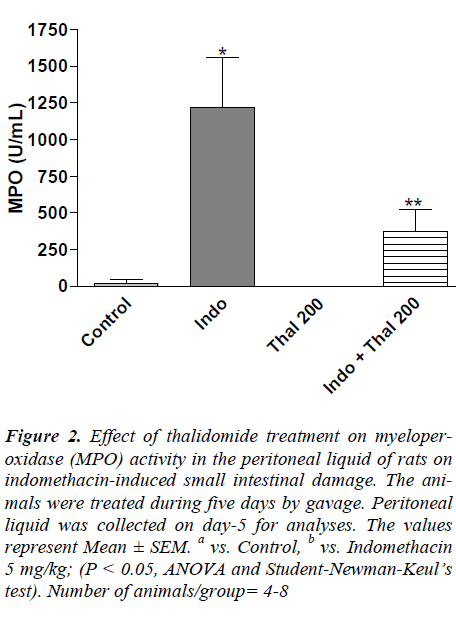
In the indomethacin-treated rats, MPO activity in peritoneal liquid that represents the neutrophil infiltration was significantly elevated when compared to control group (Figure 2). Treatment with thalidomide (100 and 200 mg/kg) significantly (P < 0.05) lowered the indomethacin- evoked increases in MPO activity.
Figure 2. Effect of thalidomide treatment on myeloperoxidase (MPO) activity in the peritoneal liquid of rats on indomethacin-induced small intestinal damage. The animals were treated during five days by gavage. Peritoneal liquid was collected on day-5 for analyses. The values represent Mean ± SEM. a vs. Control, b vs. Indomethacin 5 mg/kg; (P < 0.05, ANOVA and Student-Newman-Keul’s test). Number of animals/group= 4-8
Histological findings on the effect of thalidomide on indomethacin-induced small intestinal damage
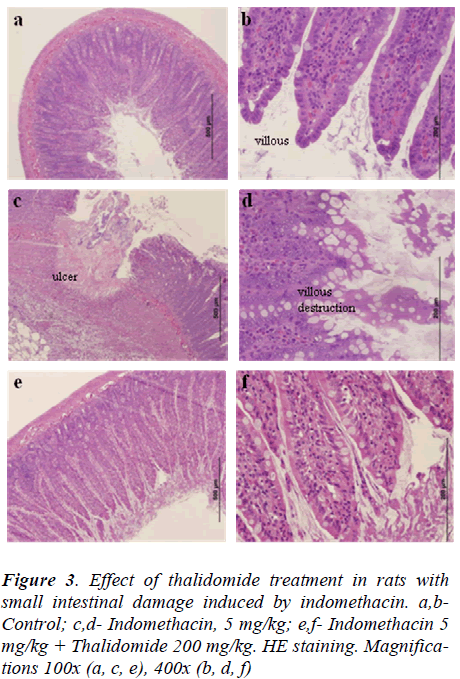
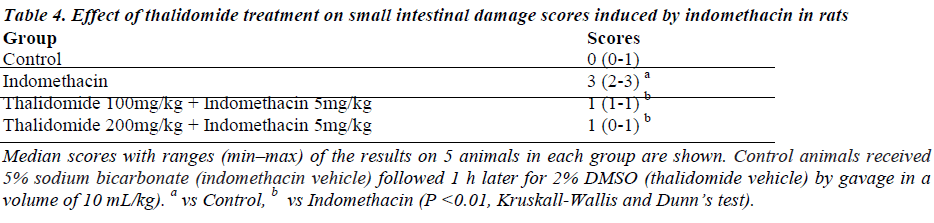
Indomethacin induced numerous small ulcerations that appeared in a punctuate pattern with destruction of underlying tissues (data not shown). Few rats had perforations or adhesions of the small intestine. Almost all villi were injured at their top, as compared to normal controls (Fig. 3a, b, c, and d). Treatment with thalidomide ameliorated the intestinal damage induced by indomethacin as evidenced by reduced indices of histological scores (Table 4) and injury to small intestinal villi, when compared to indomethacin-treated rats (Fig 3e and f).
Discussion
The non-steroidal anti-inflammatory drugs like indomethacin cause inflammation in the small bowel in more than 70% of patients receiving these drugs over the long term. The severity of this damage is reflected by the fact that patients bleed from the small bowel. These events are largely attributable to the ability of these drugs to inhibit cyclooxygenase, mitochondrial dysfunction and oxidative stress [6,7,8]. Damage to enterocytes and mucosa by indomethacin may lead to increased intestinal permeability and inflammation, which may then progress to erosions and ulcers. The intestinal lesions induced by indomethacin in rats occur in the small intestine, mainly in the jejunum and ileum regions, the lesions initially consist of white nodes and hemorrhagic palpable segments of the serous surface. Subsequently there occurs massive adherence of the abdominal viscera in response to intestinal perforation culminating in death from peritonitis and sepsis [20,21]. Corroborating the findings of other authors, our study showed that treatment for 5 days with indomethacin (5mg/kg) orally, caused hemorrhagic lesions in duodenal level in some animals, adhesions between different segments of the bowel with fibrin deposits covering the entire intestine, with the presence of large amounts of enterobacteria, confirming an intestinal perforation and intestinal damage. Moreover, indomethacin- treated group evidenced an increase in MPO activity possibly associated with an increased neutrophil numbers to the peritoneal fluid (transmigration), as a consequence of increased intestinal permeability (Fig 2) and this might be the reason for the observed leucopenia (Table 1). In contrast to indomethacin, thalidomide treated group presented not only suppression of macroscopic and microscopic tissue lesions but also corrected the indomethacininduced leucopenia as well as the increased MPO activity, a finding consistent with the reported anti-inflammatory properties of thalidomide in mitigating granulocytemediated tissue injury [22]. Thalidomide although moderately reduced the indomethacin-associated lethality, its protection did not attain the statistical significance. We believe that inhibition of cyclooxygenase (COX) which leads to depletion of endogenous prostaglandins (PGs) could be a major factor in the pathogenesis of these lesions, since PGs supplementation prevents the damage to gastrointestinal tract, in response to indomethacin [23,24]. However, there is a controversy on the involvement of PGs in this pathogenesis, and some studies have shown a role for bacterial flora in the intestinal damage effect produced by indomethacin and in this context, it has been demonstrated that germ-free animals or animals treated with antibiotics did not develop these lesions [21,25,26]. Furthermore, Evans et al. described a role for nitric oxide synthase (iNOS) and nitric oxide (NO) to the microvasculature damage in jejunum of rats treated with indomethacin [27]. These earlier observations suggest that invasion of enterobacteria in the mucosa is the first event responsible for the appearance of intestinal lesions induced by indomethacin. It is interesting to note that thalidomide treatment effectively reduced the transmigration of enterobacteria and neutrophils into the peritoneal fluid, possibly through reduced intestinal permeability, which however, needs to be confirmed in a future study.
Indomethacin is metabolized by the liver and converted to active metabolites and there have been some reports on the incidence of hepatitis and jaundice, as well as hematopoietic alterations such as neutropenia, thrombocytopenia, and rarely aplastic anemia in clinical settings [28- 30]. So as to verify such possible effects, we examined the effects of indomethacin on liver and blood cells. Treatment for 5 days with indomethacin (5 mg/kg) alone resulted in leucopenia, decreases in RBC count, hemoglobin, and hematocrit, which were mitigated by thalidomide treatment. Indomethacin is known to cause an uncoupling of cellular oxidative phosphorylation, inhibition of platelet aggregation, and prevent the formation of thromboxane A2, and these actions may largely explain the tendency of indomethacin to prolong the bleeding time. But this study points out no significant alterations in the coagulation cascade or fibrinolysis by indomethacin treatment. The results show normal prothrombin time, activated partial thromboplastin time, platelet numbers, levels of factor XIII and fibrin degradation products.
It is well established that different types of tissue injury causes an increased synthesis of plasma fibrinogen [31]. Little is known about the signal that the damaged tissue produces an increase in plasma fibrinogen. Studies using adrenalectomized rats have shown a decrease in plasma fibrinogen, implicating the sympathetic stimulation and a role for epinephrine [32,33]. Another reason for this increase in plasma fibrinogen may be a result of its increased synthesis in liver, after suffering the stimulatory action of interleukins TNF-α, IL-1 and IL-6, released by the inflammatory process. Additionally, fibrinogen degradation products (FDP) also enhance fibrinogen synthesis in the liver, which seems to play a regulatory role [34]. The FDP stimulates IL-6 production by prostaglandinindependent way, besides it also cause metabolic changes in the liver [35]. It has been suggested that this metabolic effect of LPS is mediated by eicosanoids (PGD2) and (PGE2) produced by Kupffer cells [36]. Thus the cause of increase in plasma fibrinogen by indomethacin seems to be multifactorial. Since the increase in fibrinogen levels by indomethacin was reversed by thalidomide, known for its TNF-α inhibitory and anti-inflammatory potential, we assume that thalidomide could be a liver protectant against tissue damage induced by NSAIDs.
A variety of abnormal coagulation profiles have been described during thalidomide or lenalidomide treatments such as acquired resistance to activated protein C (APCR) and increased plasma levels of factor VIII, von Willebrand factor antigen, fibrin, D-dimer, homocysteine, and soluble thrombomodulin as well as a reduction in have all been described [37-39]. However, our results show that neither thalidomide alone nor its combination with indomethacin failed to alter coagulation parameters.
Fibrinogen, as well as several other homeostasis factors, belongs to the group of interleukin-6 (IL-6)-stimulated positive acute-phase proteins, their blood levels being elevated following bacterial endotoxin (LPS) treatment [40,41]. Nandi et al. [42], have shown that indomethacin can induce jejunoileitis, and an increase in mucosal MPO activity, and these findings were associated with significantly increased production of serum and tissue levels of TNF-α, IL-1β, and nitric oxide. Other factors may be important in the pathogenesis of indomethacin-induced intestinal lesions. For instance, enterobacteria and their products contribute to an inflammatory response, evidenced by attenuated acute indomethacin-induced small intestinal ulceration in germ-free animals [43]. The gradual rise in serum IL-1β and iNOS-derived NO levels suggests that TNF-α may up-regulate other cytokines and pro-inflammatory mediators, thereby contributing to intestinal damage, and thus may in part explain the protective effect of thalidomide that possess TNF-α, and iNOSinhibitory properties.
In the present work, indomethacin although enhanced the serum levels of AST and GGT activities, lowered the plasma glucose and albumin concentrations with no apparent changes in blood urea and creatinin levels, but did not evidence any significant hepatic or renal tissue damage. These increases or decreases were respectively diminished by thalidomide treatment. The effects on hepatic enzymes might have been partly a consequence of the intestinal damage [44].
In summary, a 5-day treatment of rats with indomethacin induced small intestinal damage, increased plasma fibrinogen, and antithrombin and caused leucopenia. Thalidomide treatment protected intestinal tissue to a large extent from indomethacin lesion as well as the associated hematological, biochemical and coagulation parameters. Also, thalidomide reduced but not significantly the indomethacin- associated lethality. We conclude that thalidomide ameliorates the intestinal tissue damage induced by indomethacin possibly by acting as an anti-inflammatory agent without the risk of thromboembolism.
Acknowledgements
We wish to thank the Brazilian National Research and Development Council (CNPq), and the Research Support Foundation of Ceará (FUNCAP) for financial support in the form of grants and fellowship awards. We would like to thank Dr Francisco Vagnaldo Fechine Jamacaru for statistical analysis and Paulo Ricardo Sousa da Silva for technical assistance.
Competing interests
The authors declare that they have no competing interests.
References
- Davies NM. Review article: non-steroidal antiinflammatory drug-induced gastrointestinal permeability. Alimentary Pharmacology & Therapeutics 1998; 12: 303-320.
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. New England Journal of Medicine (NEJM) 1999; 340: 1888- 1899.
- Davies NM, Jamali F. COX-2 selective inhibitors cardiac toxicity: getting to the heart of the matter. Journal of Pharmacy & Pharmaceutical Sciences 2004; 7: 332- 336.
- Rabinovitz M, Van Thiel DH. Hepatotoxicity of nonsteroidal anti-inflammatory drugs. American Journal of Gastroenterology 1992; 87: 1696-1704.
- Strom BL, Carson JL, Schinnar R, Snyder ES, Shaw M, Lundin FE Jr. Nonsteroidal anti-inflammatory drugs and neutropenia. Archives of Internal Medicine 1993; 153: 2119-2124.
- Whittle BJ, Vane JR. A biochemical basis for the gastrointestinal toxicity of non-steroid antirheumatoid drugs. Archives of toxicology Supplement 1984; 7: 315-322.
- Basivireddy J, Vasudevan A, Jacob M, Balasubramanian KA. Indomethacin-induced mitochondrial dysfunction and oxidative stress in villus enterocytes. Biochemical Pharmacology 2002; 64: 339-349.
- Fukumoto K, Naito Y, Takagi T, Yamada S, Horie R, Inoue K, Harusato A, Hirata I, Omatsu T, Mizushima K, Hirai Y, Yoshida N, Uchiyama K, Ishikawa T, Handa O, Konishi H, Wakabayashi N, Yagi N, Kokura S, Ichikawa H, Kita M, Yoshikawa T. Role of tumor necrosis factor-α in the pathogenesis of indomethacininduced small intestinal injury in mice. International Journal of Molecular Medicine 2011, 27:353-359.
- Nandi J, Saud B, Zinkievich JM, Yang ZJ, Levine RA. Over-expression of GTP-cyclohydrolase 1 feedback regulatory protein attenuates LPS and cytokine-stimulated nitric oxide production. Vascular Medicine 2008; 13: 29-36.
- Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. Journal of Gastroenterology 2009; 19: 23-29.
- Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. International Immunopharmacology 2002; 2: 603-630.
- D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proceedings of the National Academy of Sciences 1994; 91: 4082- 4085.
- Majumdar S, Rajaram M, Reddy HS, Tamilarasan KP, Kolluru GK, Sinha S, Siamwala JH, Ilavarasan R, Venkataraman S, Sivakumar KC, Anishetty S, Kumar PG, Chatterjee S. Thalidomide attenuates nitric oxidedriven angiogenesis by interacting with soluble guanylyl cyclase. British Journal of Pharmacology 2009; 158: 1720.
- Bauditz J, Schachschal G, Wedel S, Lochs. Thalidomide for treatment of severe intestinal bleeding. Gut 2004; 53: 609-612.
- Bauditz J, Lochs H. Angiogenesis and vascular malformations: antiangiogenic drugs for treatment of gastrointestinal bleeding. World Journal of Gastroenterology 2007; 13: 5979-5984.
- Zangari M, Elice F, Fink L, Tricot G. Thrombosis in multiple myeloma. Expert Review of Anticancer Therapy 2007; 7: 307-315.
- Chaves DN, Alberti LR, Petroianu A. Assessment of immunosuppression induced by thalidomide, cyclosporine and diclofenac on skin allograft survival in rabbits. Revista da Associação Médica Brasileira 2008; 54: 42- 47.
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology 1982; 78: 206-209.
- Chiu CJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Archives of Surgery 1970; 101: 478-483.
- Koyluoglu G, Bagcivan I, Karadas B, Guney C, Durmus N, Altun A, Kaya T. Alterations in spontaneous contractions of rat ileum and jejunum after peritonitis. European Journal of Pharmacology 2008; 580: 250- 255.
- Lanas A, Scarpignato C. Microbial flora in NSAIDinduced intestinal damage: a role for antibiotics? Digestion 2006; 73: 136-150.
- Yasui K, Kobayashi N, Yamazaki T, Agematsu K. Thalidomide as an immunotherapeutic agent: the effects on neutrophil-mediated inflammation. Current Pharmaceutical Design 2005; 11: 395-401.
- Hatazawa R, Ohno R, Tanigami M, Tanaka A, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacininduced small intestinal lesions in rats. Journal of Pharmacology and Experimental Therapeutics 2006; 318: 691-699.
- Takeuchi K, Tanaka A, Kato S, Amagase K, Satoh H. Roles of COX inhibition in pathogenesis of NSAIDinduced small intestinal damage. Clinica Chimica Acta. 2010; 411: 459-466.
- Kim JW. NSAID-induced gastroenteropathy. Korean Journal of Gastroenterology 2008; 52: 134-141.
- Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, Takeuchi K. Stimulation of duodenal HCO3 secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiology 2011; 201: 117–126.
- Evans SM, Whittle BJ. Role of bacteria and inducible nitric oxide synthase activity in the systemic inflammatory microvascular response provoked by indomethacin in the rat. European Journal of Pharmacology 2003; 461: 63-71.
- Bengtsson BO, Milstein JM, Sherman MP. Indomethacin- associated neutropenia with subsequent Gramnegative sepsis in a preterm infant. Cause or coincidence? Journal of Perinatology 2006; 26: 381-383.
- LaFramboise WA, Bombach KL, Pogozelski AR, Cullen RF, Muha N, Lyons-Weiler J, Spear SJ, Dhir RJ, Guthrie RD, Magovern JA. Hepatic gene expression response to acute indomethacin exposure. Molecular Diagnosis and Therapy 2006; 10: 187-196.
- Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. Journal of Gastroenterology 2009; 44: 879-888.
- Carlson TH, Wentland SH, Leonard BD, Ruder MA, Reeve EB. Effects of prostaglandin E2, analogs, fatty acids, and indomethacin on fibrinogen level. American Journal of Physiology 1978; 235: 223-230.
- Palma J A, Enders J, Oliva P. Effects of epinephrine on plasma fibrinogen levels in rats submitted to tissue injury. Experientia 1981; 37: 780-781.
- Kessler CM, Bell WR. Stimulation of fibrinogen synthesis: a possible functional role of fibrinogen degradation products. Blood 1980; 55: 40-47.
- Csala M, Léránt I, Bánhegyi G, Kardon T, Puskás F, Mucha I, Machovich R, Falus A, Mandl J. Prostaglandin- independent stimulation of interleukin-6 production by fibrinogen degradation product D in perfused murine liver. Scandinavian Journal of Immunology 1998; 48: 269-71.
- Mandl J, Wall C, Lerant I, Falus A, Machovich R, Thurman RG. Endotoxin and fibrinogen degradation product-D have different actions on carbohydrate metabolism: role of Kupffer cells. FEBS Letters 1995; 376: 65-66.
- Zangari M, Saghafifar F, Anaissie E, Badros A, Desikan R, Fassas A, Mehta P, Morris C, Toor A, Whitfield D, Siegel E, Barlogie B, Fink L, Tricot G. Activated protein C resistance in the absence of factor V Leiden mutation is a common finding in multiple myeloma and is associated with an increased risk of thrombotic complications. Blood Coagulation & Fibrinolysis 2002; 13: 187-192.
- Minnema MC, Fijnheer R, De Groot PG, Lokhorst HM. Extremely high levels of von Willebrand factor antigen and of procoagulant factor VIII found in multiple myeloma patients are associated with activity status but not with thalidomide treatment. Journal of Thrombosis and Haemostasis 2003; 1: 445-449.
- Streetly M, Hunt BJ, Parmar K, Jones R, Zeldis J, Schey S. Markers of endothelial and haemostatic function in the treatment of relapsed myeloma with the immunomodulatory agent Actimid (CC-4047) and their relationship with venous thrombosis. European Journal of Haematology 2005; 74: 293-296, 2005.
- Koj A. Initiation of acute phase response and synthesis of cytokines. Biochimica Biophysica Acta 1996; 1317: 84-94.
- Handley DA, Hughes TE. Pharmacological approaches and strategies for therapeutic modulation of fibrinogen. Thrombosis Research 1997; 87: 1-36.
- Nandi J, Saud B, Zinkievich JM, Yang ZJ, Levine RA. TNF-alpha modulates iNOS expression in an experimental rat model of indomethacin-induced jejunoileitis. Molecular and Cellular Biochemistry 2010; 336: 17-24.
- Robert A, Asano T. Resistance of germ free rats to indomethacin-induced intestinal lesions. Prostaglandins 1977; 14: 333–341
- Whiting PH, Barnard N, Neilsch A, Simpson JG, Burke MD. Interactions between cyclosporin A, indomethacin and 16,16-dimethyl prostaglandin E2: effects on renal, hepatic and gastrointestinal toxicity in the rat. British Journal of Experimental Pathology 1987; 68: 777-786.