- Biomedical Research (2010) Volume 21, Issue 4
Evaluation of serum ascorbic acid levels in acute falciparum malaria
Adil Raza1*, Haris M. Khan1, Fatima Shujatullah1, Trivendra Tripathi2, Mohd. Ashraf Malik3, Mohd. Shahid1, Abbas A. Mahdi41Department of Microbiology, J.N. Medical College & Hospital, AMU, Aligarh-202002, UP, India
2Department of Biochemistry, J.N. Medical College & Hospital, AMU, Aligarh-202002, UP, India
3Department of Paediatrics, J.N. Medical College & Hospital, AMU, Aligarh-202002, UP, India
4Department of Biochemistry, Chhatrapati Shahuji Maharaj Medical University (Formerly King George’s Medical Uni-versity), Lucknow, UP, India.
- *Corresponding Author:
- Adil Raza
Department of Microbiology J.N. Medical College & Hospital
Aligarh Muslim University
Aligarh 202002
India.
E.mail: dradil786_786@yahoo.co.in
Accepted date: March 17 2010
Abstract
Vitamin C (Ascorbic acid) is a water soluble vitamin which is an antioxidant and has a wide variety of biological functions for growth and development of the human body. It is very es-sential for immune enhancement in human beings. Vitamin C-deficiency and falciparum malaria are two major public health problems in developing countries. Falciparum malaria is associated with significant destruction of erythrocytes and leading to severe anaemia. The present study was delineated to estimate the serum ascorbic acid concentration in 150 acute falciparum-malaria patients (aged two to ten years). Serum ascorbate level concentrations of 20 healthy volunteers (aged two to ten years) were included as controls. The mean serum ascorbic acid concentration of healthy controls was 1.163 ± 0.059 mg/dL and that of diseased cohort was 0.685±0.0145 mg/dL. The mean parasitemia was 1239.2±33.609 per μL. The dis-eased cohort demonstrated significant reduction in concentrations of ascorbic acid in com-parison to healthy controls (p<0.001) and there was inverse relationship (coefficient of cor-relation r = -0.98) between parasitemia and serum ascorbic acid concentration.
Keywords
Falciparum malaria, serum ascorbic acid, parasitemia, Vitamin C, Indian children.
Introduction
Malaria has proved to be a formidable deterrent to the cultural and socioeconomic progress of mankind through-out the globe especially in the tropical, subtropical and monsoon prone regions [1]. Malaria inflicts socioeco-nomic burden on humanity with six other diseases such as diarrhoea, HIV/AIDS, tuberculosis, measles, hepatitis-B and pneumonia accounts for 85% of global infectious dis-ease burden [1,2,3]. Malaria afflicts more than ninety countries and territories in the tropical and subtropical regions and almost one half of them are in Africa and South of Sahara. About 36% of world population is ex-posed to the risk of contacting malaria. The World Health Organization (WHO) calculated approximately 300-500 million malaria cases annually with 90% of this burden in Africa [1,4]. Additionally, the approximated annual mor-tality attributed to malaria ranges from 0.7 - 2.7 million globally and > 75% of the total morbidity accounts in children and expectant mothers [1,5,6] In the South-Eastern Asian Region of WHO, ~ 1.4 billion people living in 11 countries, 1.2 billion are exposed to the risk of ma-laria, and majority of them are from India [4,7]. However, Southeast Asia contributed 76% of the total cases [1]. Malaria is mostly contributed in India by Orissa state. Although Orissa has a population of 36.7 million (3.5% of India), it contributed 25% of a total of 1.5-2.0 million reported malaria cases annually, 39.5% of Plasmodium falciparum malaria and 30% of deaths caused by malaria in India [1,8]. Uttar Pradesh (UP), one of the biggest states of India, contributes only 5% of total cases [8]. However, documented evidences have highlighted that malaria cases and their investigations have been increased in UP zone of India. Falciparum malaria, which has been an important cause of acute renal failure in certain highly endemic zones of India, is showing an increasing preva-lence in other parts such as Eastern UP due to an imbal-ance between the increasing population and inadequate sanitary facilities, which further worsen during floods [9]. In recent community based study conducted in Solana Village, Meerut district, Uttar Pradesh, India the preva-lence of falciparum malaria was found to be 79%, vivax malaria was of 18.7% and that of mixed infection was 2.3% [10], which reflects the prevalence of malaria in western Uttar Pradesh [10].
Moreover, several studies have showed the decrease of vitamin-C levels in malarial patients [6,7,8]. However, oxidative stress has been demonstrated an important role in the development of malarial anemia [11,12]. Malarial infection activates the immune system of the body and thereby leads to release of reactive oxygen species (ROS). The malarial parasite itself generates large quantities of ROS and also through its interaction with phagocytes [13-15]. Vitamin-C is known as an antioxidant because by donating its electrons and prevents other compounds from getting oxidized [16]. Ascorbic acid is also an strong im-mune modulator [17,18].
Thus, keeping in mind the existing studies showing in-creasing prevalence of malaria in the UP zone of India, we have designed our study to delineate the therapeutic importance of antioxidant (Vitamin C) by analyzing the serum ascorbate level in acute falciparum malaria in chil-dren and their relation with parasitemia that is fragmen-tary in reported literature.
Materials and Methods
Study population
The study was conducted on in- and out-patients of J. N. Medical College and Hospital, Aligarh Muslim Univer-sity, Aligarh, India during a period from August 2006 to July 2007. The study population was comprised of 150 children with the age range of two to ten years. Twenty age and sex matched, population-based healthy volunteers were also included as controls. The cases were presented with fever with chills and rigors, prostration, headache, nausea and vomiting, abdominal pain, splenomegaly etc, and the study was approved by Institutional Ethical Committee, J. N. Medical College and Hospital, A.M.U., Aligarh, India.
Diagnostic methods
Diagnosis was made by demonstration of parasite in Giemsa-stained thin and thick blood smear and Quantita-tive Buffy Coat (QBC) or by rapid malaria antigen detec-tion test, DIAGNOS MALARIA STIX (Biomed indus-tries, India).
Thick and thin Giemsa-stained blood films were screened for the presence of Plasmodium species. The parasite count (parasites/μL) was done by counting 200 white blood cells (WBCs) and the number expressed on the ba-sis of 8000 WBCs/μL [19,20].
Following formula was used to calculate Parasitemia as shown by Raza et al. [1].
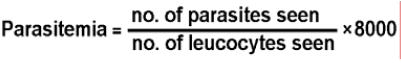
Estimation of ascorbic acid levels
Ascorbic acid levels were determined by the method of Natelson [21]. Briefly, 0.5 mL of serum was treated with 10% trichloro acetic acid (TCA), centrifuged at 10000 g, and 0.2 mL of protein free supernatant was collected for the detection of ascorbic acid. The collected supernatant was mixed with 0.2 mL of 3-[4,5 dimethyl thizolyl-2]-2,5-diphenyl tetrazolium bromide (MTT), and 0.2 mL of citrate phosphate buffer and incubated at 37°C for 30 minutes after incubation 0.5 mL of acetic acid was added to stop the reaction. The amount of formazon formed was measured at 578 nm (double beam UV Spectrophotometer 2203, Sistronics) against the reference vitamin-C tube run simultaneously.
Data analysis
Student’s t-test was used to compare the difference be-tween mean plasma ascorbate level in patients and their healthy controls. Statistical significance was concluded at probability p< 0.05. However, p< 0.001 were considered highly significant. Analyses were performed on SPSS for windows (version 12.0, Inc., Chicago, IL).
Results
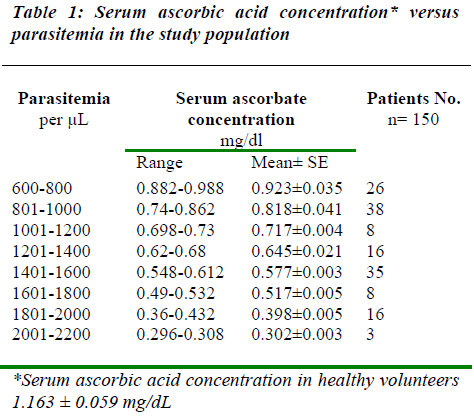
The malarial patients were diagnosed as acute falciparum malaria on laboratory investigations and clinical features, and age ranging of this group was 2 to 10 years. In our study, the male female ratio in malarial patients (n = 150) and healthy control (n = 20) was randomized 5:3. The mean parasitemia in patients belonging to acute falcipa-rum malaria was 1239.2±33.61 parasites/μL. The mean serum ascorbate level was 0.685±0.0145 mg/dL, refer to Table 1.
A distinct downward trend of serum ascorbate level was noticed as the parasitaemia increased (Table 1) which shows inverse relation of parasitaemia with serum ascor-bate level and this was found to be statistically significant (p< 0.001). The mean ascorbate level of healthy control group was 1.163 ± 0.059 mg/dL. Also Pearson coefficient of correlation between parasitemia and serum ascorbate level was -0.98, which shows an inverse relationship.
Discussion
Ascorbate plays a pivotal role in protecting plasma lipids from reactive oxygen attack. However it is rapidly oxi-dized when challenged by oxidant released from activated polymorphs [22]. Moreover, reduced ascorbic acid levels have been reported in Plasmodium falciparum infection [23-25].
Plasmodia cells accumulate protective enzymes (catalase, glutathione peroxidase and superoxiade dimutase) that are depleted in the red blood cells of the host. Enhanced production of hydrogen peroxide (H2O2) and free oxygen radicals and a decrease in antioxidant enzymes have been observed in parasitised erythrocytes [13,25]. Moreover, reduced antioxidant enzymes defense in the RBCs of P. falciparum infected patients may be responsible for higher levels of lipid peroxidation and oxidative stress in children with moderate and high parasitaemia. Thus, the enhanced oxidative stress to erythrocytes in children with moderate and high parasiteamia was endogenously stimu-lated by malaria parasites during their consumption of haemoglobin and further leading to anemia [25]. Ascorbic acid plays an important role in conserving plasma lipids from ROS. However, it is rapidly oxidised when chal-lenged by oxidants released from activated polymor-phonuclear neutrophils [22,25]. Ascorbic acid level was significantly reduced by P. falciparum infection and this coincided with enhanced level of MDA. Once ascorbic acid has been used up, there is initiation of lipid peroxida-tion [25].
Furthermore, ascorbic acid is also known for the conver-sion of folic acid to folinic acid and for the regulation of respiratory cycle in mitochondria and microsomes [26], absorption of iron through reduction of ferric to ferrous form [27], correction of anemia and maturation of RBCs [28], and in removal of iron from ferritin, particularly in the reticuloendothelial cells of the liver, spleen and bone marrow [29]. Clinical observation of a number of infec-tions accompanied by fever shows decreased blood levels of ascorbic acid, indicating an increased need for this vi-tamin [30].
In nutshell plasma ascorbate levels in, acute falciparum malaria was lower in comparison to healthy controls and there was inverse relation between parasitaemia and se-rum ascorbate levels. This is among the premier reports evaluating the serum ascorbate levels and their relation to falciparum malaria. We therefore feel that Vitamin C supplementation could be of therapeutic help in clinical outcome of the patients and thus suggest Vitamin C sup-plementation in malaria therapy. However, further re-searches with large study population are required to estab-lish the firmness of this fact.
References
- Raza A, Khan HM, Malik MA, Mehdi AA, Shahid M, Shujatullah F. Serum retinol concentration in patients with acute falciparum malaria in Aligarh, India. J Infect Dev Ctries 2009;3:865-868.
- Murray CJL, Lopez AD. Evidence-based health policy lessons from the Global Burden of Disease Study. Sci-ence 1996; 274:740-743.
- Murray CJL, Lopez AD. The Global Burden of Dis-ease 1990-2020: alternative projections of mortality and disability by cause for eight regions. Lancet 1999; 349: 1498-1504.
- Kumar A, Valecha N, Jain T, Dash AP. Burden of Ma-laria in India: Retrospective and Prospective View. Am J Trop Med Hyg 2007; 77 (Suppl6): 69-78.
- Bremen JG. The ears of the hippopotamus: manifesta-tions, determinants and estimation of the malaria bur-den. Am J Trop Med Hyg 2001; 64 (Suppl 1): 1-11.
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmo-dium falciparum malaria. Nature 2005; 434: 214-217.
- Kondrachine AV. Malaria in WHO Southeast Asia Region. Indian J Malariol 1992; 29: 129-160.
- NVBDCP, Drug Resistance Status in India: An Update 2002. Delhi: Directorate of National Vector Borne Dis-ease Control Programme.
- Prakash J, Gupta A, Kumar O, Rout SB, Malhotra V, Srivastava PK. Acute renal failure in Falciparum ma-laria-increasing prevalence in some areas of India-a need for awareness. Nephrol Dial Transplant 1996; 11: 2414-2416.
- Mya MM, Saxena RK, Roy A. Seroepidemiological study of prevalence of malaria in village Solana, Uttar Pradesh, India. Afr J Clin Exp Microbiol 2004; 5: 2-14.
- Kremsner PG, Greve B, Lell B, Luckner D, Schmid D. Malarial anemia in African children associated with high oxygen radical production. Lancet 2000; 355: 40-41.
- Clark IA, Hunt NH. Evidence for reactive oxygen in-termediates causing hemolysis and parasite death in malaria. Infection and Immunity 1983; 39 (1): 1-6.
- Mohan K, Dubey ML, Ganguly NK, Mahajan RC. Plasmodium falciparum induced perturbations of the erythrocyte antioxidant system. Clin. Chim. Acta 1992; 209: 19-26.
- Kalpan JM, Weidanz WP. Malaria host responses to infection. In: Stevenson M.M. ed. Florida CRC Press 1989 Inc., pp 38-63.
- Mohan K, Ganguly NK, Dubey ML, Mahajan RC. Oxidative damage of erythrocytes infected with plas-modium falciparum an in-vitro study. Annals of Hemo-tology 1992a; 65: 131-134.
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon U, Lee JH et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J Am Coll Nutr 2003; 22(1): 18-35.
- Hughes DA. Effects of carotenoids on human immune function. Proc. Nutr. Soc. 1999; 58: 713-718.
- Meydani SN, Beharka AA. Recent development in vi-tamin E and immune response. Nutr Rev 1998; 56: 49-58.
- Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epi-demiological investigations. Trans R Soc Troop Med Hyg 1985; 79 (2): 181-84.
- WHO. Basic laboratory methods in medical parasito-logy. Special techniques for plasmodia; WHO 1991 Geneva, pp 48.
- Natelson S. Microtechnics of Clinical Chemistry. Charles Thomas, Springfield, Illinois. 1963, pp 121.
- Frei B, Stocker R, Ames MN. Antioxide defenses and lipid peroxidation in human blood plasma. Proceedings of National Academy of Sciences,USA 1988; 85: 9748-9752.
- Njoku OU, Ononogbu IC, Nwachukwu DE. Plasma cholesterol, B-carotene and ascorbic acid changes in human malaria. J. Commun Dis 1995; 27 (3): 186-190.
- Isamah GK, Asagba SO. The effect of Plasmodium falciparum infection on the levels of malondialdehyde [MDA] and ascorbic acid on Nigerian children. J. App. Sci. Environmental Management 2003; 7 (2): 59-61
- Egwunyenga AO, Isamah G, Nmorsi OP. Lipid peroxi-dation and ascorbic acid levels in Nigeria children with acute falciparum malaria. Afr. J. Biotech 2004; 3 (10) :560-563.
- Anderson L, Dribble MV, Mitchell HS, Rynbergen HK. Nutrition in nursing, Philadelphia; J.B. Lippincott Compan 1972.
- Whitney EA, Hamilton EMN. Understanding nutrition 3rd ed. Minnesota: West Publishing Company, 1984.
- Sharma DC, Mathur R. Correction of anemia and iron deficiency in vegetarians by administration of ascorbic acid. Ind. J. Physiology Pharmacology 1995; 39: 403-406.
- Williams SR. Nutrition and diet therapy, 3rd ed St. Louis Missourie; CV Mosby Company, 1977.
- Schorah CJ, Tormey WP, Brooks GH, Robertshaw AM, Young GA et al. The effect of vitamin C. supple-ments on the body weight, serum proteins and general, health of an elderly population. Am. J. Clinical nutri-tion 1981; 34: 871-876.