Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2021) Volume 11, Issue 83
Evaluation of reprogramming mscs by lentivirus harboring ngn3 to produce insulin-secreting cells.
Samira Talebi1, Fathollah Ahmadpour2, Teresa May B. Bandiola3*
1Department of Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
2Trauma Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
3Department of Pharmacy, National University, Manila City, Phillippines
- Corresponding Author:
- Bandiola TMB
Department of Pharmacy,
National University,
Manila City, Phillippines
E-mail: bandiolateresamay@gmail.com
Accepted date: October 11, 2021
Abstract
Neurogenin 3 (NGN3) is critical transcription factor that expressed by a population of progenitor cells for developing pancreas and it is alone sufficient to differentiation of pancreatic epithelial cells to islet cells. One of the multipotent stem cell of mesodermal origin is Mesenchymal Stem Cells (MSCs) that can be converted into different cell types; Therefore Reprogramming of these cells can be created insulin-producing cells. NGN3 protein is transiently expressed by exocrine cells undergoing reprogramming to an endocrine cell fate. One of the Major tools for gene delivery in mammalian cells is Lentiviral vectors that have stable expression and ability to mediate potent transduction in mammalian cells both in vitro and in vivo. In this study, lentivirus harboring NGN3 has been used for persistence gene expression in MSC. The idea of this research was to evaluate the expression of the NGN3 gene in MSCs in order to investigate the unique role of NGN3 and the lentivirus to introduce to produce insulin-secreting cells and apply it for gene therapy of hyperglycemia in diabetic rats. MSCs were transducted by lentivirus contain NGN3 gene and expressions of neurogenin3 and insulin gene were confirmed by RT-PCR and western blotting. NGN3 as a master regulator expressed in little percentage of cells within pancreas development functions as a primary activator which directly enhances the expression of the transcription factors involved differentiation of the endocrine progenitor cells into each of the endocrine cell subtypes. The MSCs transfected by lentivirus contain NGN3 gene were secreted insulin 1.1-fold higher in the high-glucose medium than the low-glucose medium. These cells implanted into diabetic rats then the blood glucose level was measured at 0 and 3 days. The results demonstrated a decrease in blood glucose level; therefore, the MSCs transfected with lentivirus contain NGN3 gene can be used as a cell-based gene therapy method for treatment of type 1 diabetes.
Keywords
Neurogenin 3, Mesenchymal stem cells, Lentivirus, Diabetic.
Introduction
The diabetes is growing rapidly worldwide and several pressures are used to treat it. Recently a lot of researches have been done on the stem cell technology and applied for treatment of diseases including insulin-dependent diabetes [1-3]. A large number of genes orderly activate differentiation of MSCs into the different exocrine and endocrine cell types during development and formation of the pancreas. Many of these genes act as transcription factors involved in releasing of insulin such as Neurogenin3 (NGN3), Nkx2.2, Neurod1 and Pancreatic Duodenum Homeobox Protein-1 (PDX1). Moreover, these genes have critical role for beta-like cell differentiation [4-6].
One of these transcription factors belongs to basic helix-loop-helix is Neurogenin 3 (NGN3) which expressed by progenitor cells during rodent development, it is crucial and enough for endocrine specification. The NGN3 doesn't have an apparent role in adult pancreas and has not been detected in the rodent pancreas but its function established on the adult islet [7-10]. During early pancreatic development, expression of neurogenin 3 is done by Ipf-1/PDX promoter in the development differentiation of pancreatic forerunner cells into endocrine cells, so that neurogenin 3 is required to produce any pancreatic endocrine cells in mice. Many numbers of class B bHLH transcription factors expressed in the developing pancreas including scleraxis, NeuroD1, math3, NeuroD4 and Neurogenin 3 [10].
Mesenchymal Stem Cells (MSCs) are multipotent stem cells derived from bone marrow. MSCs can be obtained from different adult tissues such as adipose, spleen, bone marrow, skin, salivary gland. The variety of cell types (osteoblasts, chondrocytes, myocyte, adipocytes, Cells) derived from them furthermore the in vitro development of insulin-producing cells can be done through them.
Gene therapy delivers DNA into cells by two main classes of methods including recombinant viruses (viral vectors) and naked DNA or DNA complexes (non-viral methods). Viruses have used to delivery nucleic acid into specific cell types efficiently, while evading from the infected host's immune system. Therefore, viruses are attractive gene-delivery vectors for gene therapy. Numerous types of viruses, including retrovirus, herpes simplex virus, Adeno-Associated Virus (AAV) and adenovirus have been used in gene therapy. Retroviral vectors efficiently integrate into the genome of the host cell, but host cell should be a mitotic cell for transduction. Although dividing and nondividing cell types can be infected efficiently by Adenoviral vectors, host's immune system often restricts gene expression in vivo; AAV despite low DNA capacity can infect dividing and nondividing host cells. Herpes simplex virus despite cytotoxicity can deliver large amounts of exogenous DNA into host cells. Each of these vectors has advantages and disadvantages. So, the appropriate vector is selected according to the type of transformation.
In this study, the Lentivirus Harboring Ngn3 (LV-Ngn3) was evaluated for reprogramming MSCs by gene delivery system, also, the necessity and sufficiency of Ngn3 was investigated for endocrine differentiation during pancreatic development.
Materials and Methods
Isolation and characterization of MSCs
MSCs were collected from the thigh and shin bones of Sprague-Dawley rats. Bone marrows were suspended in DMEM medium and then were cultured in the presence of 20% Fetal Bovine Serum (FBS) and antibiotics (100 U/ml penicillin and 100 U/ml streptomycin) on tissue treated culture plates at 37˚C in a humidified atmosphere containing 5% CO2 for 72 h. The plates were washed away twice with Phosphate-Buffered Saline (PBS) in order to remove non adhered cells. When the cells were filled 80% of the plate surface, they were harvested using 0.25% trypsin and 0.02% EDTA for 1-2 min at 37˚C. The pure cells were achieved after multi-round plastic-adherence selection approximately in 10–14 days. Then, MSCs were identified by flow cytometry. In order to cytometry, cells were stained with fluorescent (isothiocyanate) conjugated antibodies against rat CD 29, CD45, CD 90 (Serotec, Raleigh,NC, USA). Isotype-matched control antibody was used as controls.
Construction of the recombinant lentiviral vector
The Ngn3 gene was amplified by PCR with the specific premiers, then the PCR product was purified and cloned into the shuttle plasmid pCDH and named pcDH-Ngn3 lentivirus vector. Using calcium phosphate precipitation method on the first day, 5 × 106 HEK-293T cells (lentivirus producing cells) were seeded in a 10 cm2 plate in DMEM with 10% FBS (Gibco, USA). On the second day, three lentiviral plasmids, including pcDH-Ngn3 (lentiviral expression plasmid), psPAX2 (packaging plasmid) and pMD2G (pseudotyping plasmid) (GeneCopia, Rockville, MD, USA) were transfected in HEK293T cells and were mixed with calcium chloride, added to 2 × HBS (HEPES-buffered saline) and vortexed. Then, this mixture was added to HEK-293 cells. Two days after transfection, active viral vectors were extracted from the supernatant through 0.22 μm filter (Millipore, Billerica, MA, USA) and were stored at -70˚C for subsequent experiments. Then, these active viral vectors were used for MSCs transduction.
MSCs transduction
MSCs (2 × 105 cells/ml) seeded in a 24-well plate in medium supplemented with 10% FBS for 48 h and incubated with recombinant lentivirus-Ngn3 for 12 h. To obtain stable transduction, the transduced MSCs were exposed to 2.5 μg/ml puromycin for 2 days. The selection was continued for 3 weeks with 2 μg/ml puromycin until Ngn3-expressing single colonies appeared.
RT-PCR analysis for Ngn3 detection in HEK-293T and transduced MSCs
Total RNA from transduced MSCs and HEK 293 were extracted by High Pure RNA Isolation Kit (Roche, Germany). cDNA was synthesized using cDNA synthesis kit (Fermentase). PCR was performed with specific primer in a DNA thermal cycler according to a standard protocol (Techne Flexigen; USA). PCR amplification was carried out as 5 min of initial denaturation at 94˚C, 35 cycles each at 95˚C for the 30 s, 60˚C for 30 s and 72˚C for 30 s and a final extension at 72˚C for 10 min. Agarose gel electrophoresis was used for analysis of PCR products.
In vitro development of MSCs into insulin-producing cells
To establish of the Ngn3 differentiation effect on the transduced MSCs, in vitro development of MSC into insulin-producing cells was performed, then induction was done with HG-DMEM (containing 25 mM glucose) and 10% FBS for 21 days. The untransduced MSCs were identified as control cells under same treatment.
Analysis gene expressions of insulin, glucagon, Ngn3 and by real-time RT-PCR
The gene expression levels of insulin, Ngn3, glucagon, and p48 as pancreatic marker genes, after 21 days from induction in transduced MSCs were monitored by real-time PCR. The rat pancreatic cells were used as positive control.
Real-time RT-PCR was performed using SYBR Green RT-PCR Kit (Roche) and Roche Light Cycler PCR (version 5.3) according to the manufacturer’s instructions. The Gapdh gene was used as an internal control of real-time RT-PCR. The data of Real-time RT-PCR were analyzed using 2-∆∆Ct method.
Western blotting for insulin expression analysis
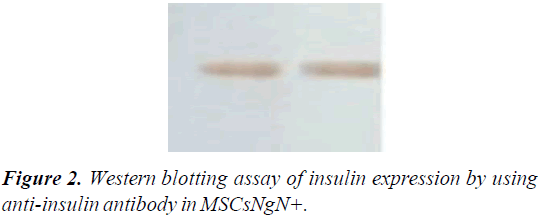
The protein expression assessment in untransduced MSCs and transduced MSCs were analyzed after 14 days by western blotting. The untransduced and transduced MSCs cultures were washed twice with ice-cold PBS and then lysed by lysis buffer (40 μl) (Promega) and 1 μl of cocktail proteinase inhibitor were added. The product of total cell lysate was electrophoresed using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS–PAGE). Then, proteins were blotted to PVDF membrane (Millipore). The membranes were blocked using mouse insulin antibody (1:200; Abcam) and incubated overnight at 4˚C. Alkaline phosphatase-conjugated goat antimouse IgG (1:250; Abcam) was used as secondary antibodies for detection.
In vivo MSC transplantation in rat
Alloxan Intraperitoneal (IP) injection was used to induce diabetes in twenty adult male rats according to the procedure mentioned. Bioethics laws were met in all experiments according to the principles by National Academy of Science for the care and use of laboratory animals. Blood glucose level of rats was measured before and after alloxan injection. When blood-glucose levels were more than 400 mg/dl and it was constant, they were considered hyperglycemic. Glucose oxidase method was used for measuring of Blood-glucose levels twice a week by ACCU-CHEK Active strips (Roche Diagnostics, Germany).
Results
Isolation and characterization of MSCs
Mononuclear cells were obtained from rat bone marrow and seeded into the plastic flask. The MSCs adhered and non-adherent cells were removed through passaging. Morphological observation of MSCs exhibited after 7-10 days. Then, Flow cytometry analysis confirmed isolation of MSCs cells.
Construction of the recombinant lentiviral vector and MSC transduction
The pcDH-Ngn3 lentivirus vector was contracted through calcium phosphate precipitation method. HEK-293T cells were transfected with three lentiviral plasmids, including pcDH-Ngn3, psPAX2, and pMD2G. Two days after transfection, active viral vectors were obtained. Then, the transducted MSCs cells were produced. The stable transducted MSCs cells were confirmed in the present of puromycin for 21 days. Therefore, the Ngn3-expressing colonies appeared after 3 weeks.
Evaluation of Ngn3 expression in HEK-293T and transduced MSCs by RT-PCR analysis
To Evaluation of Ngn3 expression in HEK-293T and transduced MSCs, total RNA from transduced MSCs and HEK 293 were extracted and cDNA was synthesized, then PCR was performed. Agarose gel electrophoresis of the PCR products showed Ngn3 RNA expression in HEK-293T and transduced MSCs cells. Therefore Ngn3 gene was cloned correctly and the lentiviral particles were constructed accurately (Figure 1).
Results of gene expressions by real-time RT-PCR and western bolt
The gene expression levels of insulin, Ngn3, glucagon, and p48 were analyzed by real-time RT-PCR. These results indicated that transduced MSCs have not been differentiated into exocrine lineage. Insulin expression was also detected at the protein level by western blotting assay using anti-insulin antibody in MSCsNgN+ and un-transduced MSCs. Expression of insulin demonstrated that ectopic expression of Ngn3 can activate the endogenous insulin-production (Figure 2).
Treatment of diabetic rat by MSCsNgN+ in rat
The effect of differentiated MSCsNgN+ to treatment diabetic male rats was studied. The blood glucose level of all rats was increased from 116 ± 10 mg/dl to 400-556 mg/dl and their weight was decreased about 23% one week after injection of alloxan. The rats divided into three group containing 6 diabetic rats, the first group of diabetic rats were considered as control. The second and third groups were injected with 2 × 106 MSCsNgN+ and MSCs, respectively. The results of transplantation with MSCsNgN+ were promising and demonstrated reduction in blood glucose levels to 136-161 mg/dl five day after injection. The MSC-transplanted rats showed different results in which a decrease in blood glucose levels were happened after 12-14 days of transplantation. It was evaluated tumor formation in the all of the transplanted rats and was not observed in any of them. Control nontransplanted diabetic rats remained hyperglycemic and died within 21 days.
Discussion
Different methods are presented for treatment of complex clinical diseases and efforts are being still progressed, the current studies demonstrated that gene therapy can be generally defined as the approach for the treatment of diseases or medical disorders. This approach introduces therapeutic genes into the cellular targets. The deleterious gene mutations can be corrected and the cell functions reprogrammed by therapeutic genes to overcome a disease. When the therapeutic genes introduced into the target cells efficiently, specifically, and stable. The gene therapy will be successful, on the other hand, stem cells are applicable tools for treatment of clinical diseases, and these cells can be differentiated into the cell type of many tissues. It is important the selection of suitable stem cells for regenerative of damage cells. The main idea of this study is the use of gene therapy and cell therapy for the treatment of diseases. Using of Bone Marrow-Derived Mesenchymal Stem Cells (BMMSCs) as adult stem cells for the cure of disorders is studied extensively. The different cell types can be derived from MSCs, because they have regenerative potential and immune modulatory properties which make them suitable and also, they can overcome GVHD. Therefore these cells are powerful tools for clinical applications in order to gene therapy. In this study, using gene therapy is applied for regenerating of MSCs into insulin-secreting cells and apply it for gene therapy of hyperglycemia in diabetic rats. The results demonstrated the Bone Marrow-Derived Mesenchymal Stem Cells (BMMSCs) reprogram to pancreatic cells as previously reported. These cells can be efficiently reprogrammed and produced suitable tools for gene therapy.
Viruses have used to delivery nucleic acid into specific cell type and they become highly efficient tools in gene delivery to while evading from the infected host's immune system. Therefore viruses are attractive gene-delivery vectors for gene therapy. Lentivirus is one of the functional viruses for gene therapy; generally, lentivector particles are produced by co-transfection of 3 plasmids including packaging, transfer, and envelope-encoding plasmids in HEK 293T cells. In the current study, the lentivirus harboring Ngn3 was evaluated for reprogramming of MSCs by gene delivery system. The results of this research confirmed the suitable capacity of the lentiviral particles for gene therapy in MSCs.
Conclusion
In the presented study, the feasibility of BMMSCs differentiating investigated with its transduction by lentivirus harboring NGN3 in order to creation islet-like cells. Then transduced cells implanted into model diabetic rats. The necessary and sufficient role of Ngn3 gene is endocrine differentiation during pancreatic development.
The MSCsNgN+ secreted insulin in vitro (culture medium) and in vivo (diabetic rat model). The RT-PCR and western blotting confirmed the ability of MSCsNgN+ for insulin synthesis and secretion. The results showed the MSCs transduced by lentivirus contain NGN3 gene secreted insulin 1.1-fold higher than control MSCs. The results of transplantation with MSCsNgN+ were promising and demonstrated a decrease in blood glucose levels to 136–161 mg/dl one day after injection.
One of these transcription factors belongs to a family of basic helix-loop-helix is Neurogenin 3 (NGN3) which expressed by progenitor cells during rodent development, it is crucial and enough for endocrine specification. The Ngn3 have function during the embryonic development of the pancreas, and endocrine cell neogenesis in the adult. It reported expression of Ngn3 using viral vector is sufficient to differentiation of endocrine cell in adult primary pancreatic duct cells, therefore the adult tissue is prepared to respond to Ngn3.
The Ngn3 expression causes differentiation of the four distinct endocrine cell lineages, α, β, PP and δ cells. These cell lineages express insulin, glucagon, pancreatic polypeptide and somatostatin. Ngn3 is activated after injury of the adult mouse pancreas in duct progenitor cells and then they transform into α and β-cells. The β-cells express insulin. Therefore Ngn3 is a master regulator of endocrine pancreas development which can be a therapeutic approach for diabetic disorders in order to increase β-cells mass and subsequently boost express of insulin.
References
- Zhu Y, Liu Q, Zhou Z, et al. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res Ther. 2017; 8(1): 240.
- Van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011; 91: 79-118.
- Conrad E, Stein R, Hunter CS. Revealing transcription factors during human pancreatic beta cell development. Trends Endocrinol Metab. 2014; 25(8): 407-14.
- Yoshihara E, Wei Z, Lin CS, et al. ERRγ Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells. Cell Metab. 2016; 23(4): 622-34.
- Miki R, Yoshida T, Murata K, et al. Fate maps of ventral and dorsal pancreatic progenitor cells in early somite stage mouse embryos. Mech Dev. 2012; 128(11): 597-609.
- Jennings RE, Berry AA, Kirkwood-Wilson R, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013; 62(10): 3514-22.
- Stoffers DA, Zinkin NT, Stanojevic V, et al. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997; 15(1): 106-10.
- Holland AM, Hale MA, Kagami H, et al. Experimental control of pancreatic development and maintenance. Proc Natl Acad Sci U S A. 2002; 99(19): 12236-41.
- Li SW, Koya V, Li Y, et al. Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes. Lab Invest. 2010; 90(1): 31-39.
- Rukstalis JM, Habener JF. Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets. 2009; 1(3): 177-84.