- Biomedical Research (2011) Volume 22, Issue 1
Evaluation of Plasma Malondialdehyde as a Biomarker in Patients with Carcinoma of stomach
Aparna R. Bitla1*, E. Prabhakar Reddy1, K.Sambasivaih2, M.M. Suchitra1, V. Seshadri Reddy1, P.V.L.N. Srinivasa Rao12Department of Medical Oncology, Sri Venkateswara Institute of Medical Sciences, Tirupati-517 507, A.P, India
- Corresponding Author:
- Aparna R. Bitla
Department of Biochemistry
S V Institute of Medical Sciences
Tirupati. (AP)-517507
India.
Accepted Date: October 13 2010
Abstract
Carcinoma of stomach is the second most fatal malignancy. Tumor markers like carcinoem-bryonic antigen (CEA) are of little benefit in early diagnosis and monitoring the progress in these patients due to their low specificity and sensitivity. Hence there is a need to look for bet-ter biochemical markers. We conducted a case control study involving thirty healthy indi-viduals and twenty two patients with stomach carcinoma. All of the control group members had normal blood chemistry, ECG, Chest X-ray, blood counts apart from a normal clinical ex-amination. All the patients included in the study were untreated cases and had a confirmed histo-logical diagnosis. The parameters studied included Malondialdehyde (MDA), and Carcinoem-bryonic antigen [CEA]. The mean age for patients and controls was 51 and 45 years, respectively. There was an increase in the levels CEA (p<0.0001) and MDA (p<0.0001) whereas the levels of vi-tamin E decreased (p<0.05) in the patient group compared to controls. The ROC analysis showed significant diagnostic accuracy of MDA as tumor marker. Logistic regression studies revealed a significant association of MDA with carcinoma stomach. As a biomarker for carcinoma stomach, MDA had significant diagnostic value independent of CEA levels. The sensitivity and specificity of MDA was more than that of CEA. Combined use of MDA & CEA was found to yield better diagnostic information than individual use of CEA
Keywords
Carcinoma stomach, Malondialdehyde, Biomarker.
Introduction
Carcinoma of stomach is the second most fatal malignancy in the world and is the cause of more than 7,50,000 deaths annually [1]. The high mortality rate from carcinoma of stomach arises from its late detection and surgical resection at an advanced stage of the disease [2]. Efforts directing at prevention, early detection and intensive therapy will go a long way in saving these patients. CEA was identified as early as 1969 in the sera of patients with large bowel cancer [3]. This discovery led to further work on identification of other tumor associated antigens (TAA) that could be useful for the early detection of cancer.
Tumor markers should be specific and sensitive to a given cancer type for early diagnosis. The markers studied till now include enzymes, hormones, antigens and proteins that are present in higher concentration in blood, other body fluids or tissues from cancer patients and propor-tional to the tumor burden but are not found in the non-tumor bearing hosts.[4]. Unfortunately, different markers produced by different tumors are neither specific nor sen-sitive enough for screening as well as monitoring the prognosis. CEA, a marker elevated in the variety of can-cers is not recommended for screening because of the false-positive results associated with benign and false-negative results due to CEA-nonproducing tumors [5]. Its sensitivity is only 30% to 40% for early stage tumors of the colon [6] and only 17% for other gastrointestinal malignan-cies [7]. Other tumor markers like carbohydrate antigen 72-4 (CA 72-4) and carbohydrate antigen 19-9 (CA19-9) have been evaluated for gastric cancer, but with little positive outcome [8].
Therefore, the scope for identification of newer markers opened up other molecular interactions like oxidative dam-age, immune mechanisms, and cytokines for clinical use in the management of malignancies either individually or in combination with the existing ones. Also, as most of the tumour markers lack in specificity, using a combination of markers always has a chance to increase the diagnostic abil-ity of the tumour markers.
Reactive oxygen species [ROS] have been known to play an important role in the initiation and promotion of carcinogene-sis and have been implicated in carcinogenesis in human as well as animal models [9]. They have been shown to be as-sociated with the different steps of carcinogenesis either through structural DNA damage or interaction with onco-genes or tumor suppressor genes or immunological mecha-nisms [10]. MDA, the end product of lipid peroxidation, ow-ing to its high cytotoxicity has been suggested to act as a tumor promoter and a co-carcinogenic agent [11]. Free radical in-termediates of xenobiotic chemicals as well as oxygen radi-cal production by chemical carcinogens have been related to environmental carcinogenesis [12,13]. Infection with H. py-lori has been shown to be a major predisposing factor for the development of malignancies in stomach and oxidative stress has been demonstrated in gastritis [1]. Also, oxidative stress has been shown to occur throughout the clinical history of any malignancy. The oxidative stress during the progression of disease is believed to be due to altered metabolic pathways in tumor cells, macrophageal intrusion of tumor tissue, and therapeutic interventions. Ionizing radiation yields a variety of reactive products, including free radicals that can damage macromolecules, including DNA [14]. It has also been shown that oxidative stress will come down with reduc-tion in tumor mass after treatment [15]. An increase in se-rum/plasma MDA concentrations has been widely re-ported in various cancers [1,16-21]. The increase in MDA levels were found even in early stages of cancer [17]. In-creased amounts of lipid peroxidation end products have been demonstrated in tumor tissue itself [19-22] clearly pointing to the source of increased MDA levels in cancer patients.
Recently plasma MDA levels have been tested and found to be useful as tumor marker with predictive values as good as established tumor markers in gastrointestinal malignancies [22,23]. However, to our knowledge there are no such stud-ies on carcinoma of the stomach patients. Therefore, a case control study was undertaken in an attempt to establish the clinical use of plasma MDA as biomarker either inde-pendently or in combination with CEA in patients with stomach carcinoma.
Materials and Methods
Twenty two patients with Carcinoma of stomach attending medical oncology out patient clinic of S.V. Institute of Medi-cal Sciences, Tirupati were recruited into the study along with 30 healthy individuals who served as controls. The control group was recruited from the people attending the master health checkup program of the hospital and staff of the department. All of them had normal blood chemistry, ECG, Chest X-ray, blood counts apart from a normal clini-cal examination. All the patients included in the study were untreated cases and had a confirmed histological diagnosis. None of patients and controls was smokers or alcoholics at the time of study. Persons with diabetes and or hypertension, renal failure and active infection were not included in the study. All the members were recruited with informed con-sent. The patient group consisted of 15 males and 7 females with mean age of 51±12 years. The control group consisted of 18 males and 12 females with mean age of 45±13 years. The two groups were found to be matching with respect to age.
Five ml of heparinised blood was collected from patients and controls. Plasma was separated and analyzed immedi-ately or stored at - 80°C until further analysis. Vitamin E was analyzed using HPLC [24]. Plasma MDA was estimated spectrophotometrically as thiobarbituric acid reactive sub-stances (TBARS) [25]. CEA was estimated by ELISA us-ing commercial kits (United Biotech Inc, USA). Urea levels were estimated by photometric method using com-mercial kits on Beckman CX9 Random access clinical chem-istry analyzer.
The data analysis included comparison between groups, cor-relation and association studies. Mann Whitney U test was used to compare the differences in parameters between the patient and control groups. Spearman's Rank correlation was used to assess the association between parameters in the pa-tient group. A scatter plot was plotted for MDA levels in the patients and controls. Receiver-operating characteristic (ROC) curves were constructed for these parameters and the areas under the curve (AUC) values were determined.
A p value of < 0.05 was considered to be significant. The logistic regression model was established with the parameters as predictor variables and cancer status (the individual having cancer or not) as binary outcome variable. Analysis was per-formed using Microsoft excel spread sheets, SPSS for Win-dows Version 11.5.
Results
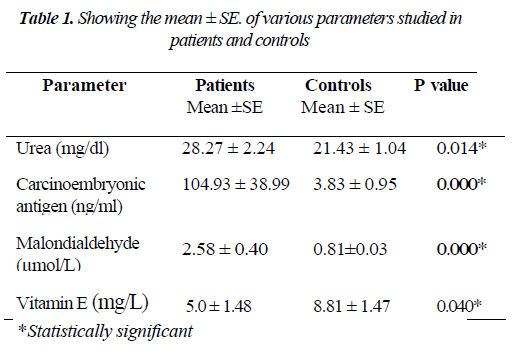
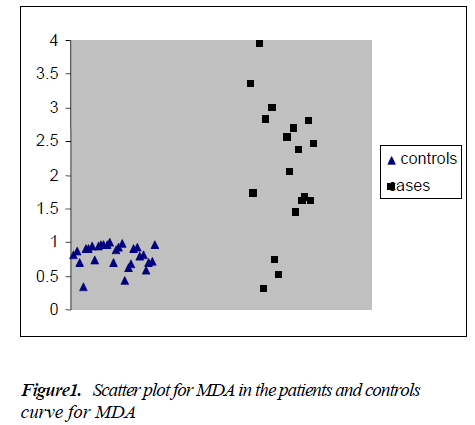
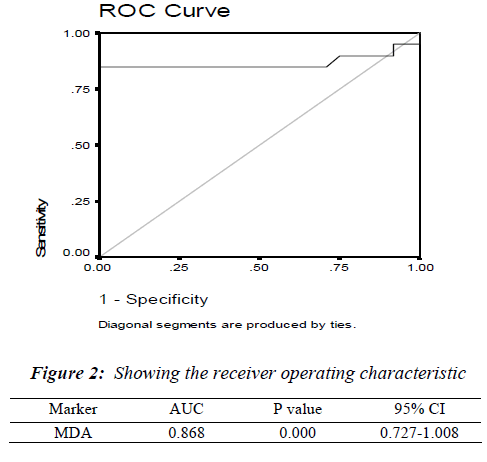
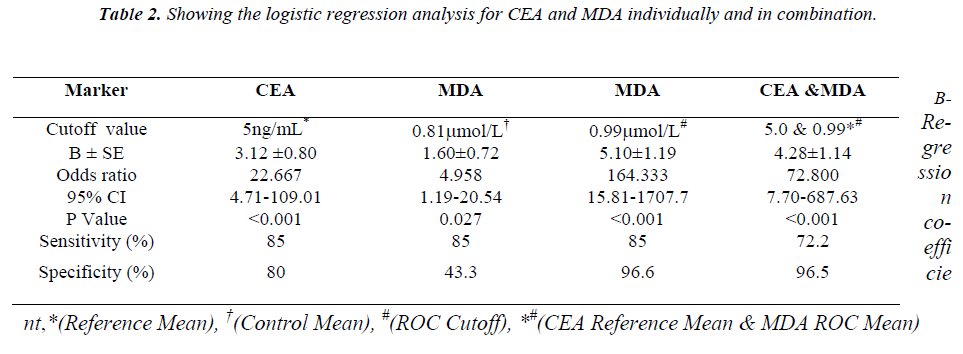
The mean ± S.E. of various parameters studied in patients and controls along with the significance of difference be-tween the two groups are presented in Table - I. There was an increase in the levels of urea, CEA and MDA whereas the levels of vitamin E decreased in the patient group compared to controls. As shown in Figure: 1 the scatter plot shows the distinct distribution of MDA levels in the patients and controls. The patient group had visibly higher values compared to the control group. The Receiver operating characteristic (ROC) analysis was performed to test the diagnostic accuracy of MDA as tumor marker (Fig.2). It gave an area under the curve of 0.868 which was statistically significant. The predictive power of CEA and MDA as tumor markers was assessed with logistic regression by entering the parameters both individually and combined together. The cut off value for plasma MDA was obtained from ROC curves with the maximum sensitivity and specificity. The upper normal limit provided by the manufacturer was used as cut off for CEA. The odds ratios were significant for both MDA and CEA when introduced into the test either individually or together (Table 2).
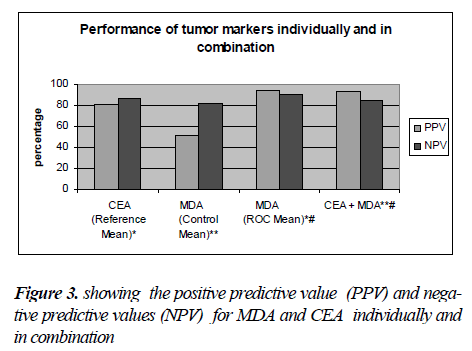
Similarly, the diagnostic relevance of CEA and MDA at these cut off values was assessed in terms of their sensitiv-ity and specificity, Positive predictive value (PPV) and Negative predictive value (NPV) (Fig.3). The logistic re-gression analysis showed that both CEA and MDA were comparable as predictors of disease. Individual use of MDA was found to be more diagnostic with the best com-bination of sensitivity, specificity, PPV and NPV. Com-bined use of CEA and MDA was also found to be useful as diagnostic markers for carcinoma stomach. The results of the study point to the possibility of using plasma MDA levels as an additional tumour marker.
Discussion
Carcinoma of stomach is a multifactorial process. Factors such as infection with H, pylori, cigarette smoking and chronic gastritis have been implicated in the pathogenesis. Of these H. pylori is believed to be a major etiological agent that causes chronic gastritis [1]. One characteristic event in gastritis is an infiltration of the sub-epithelial gas-tric lamina propria by phagocytes, mainly neutrophils and macrophages which produce large amounts of reactive oxygen species. These ROS have been shown to cause pro-teome changes in the gastric mucosa and DNA damage [26]. CEA has been shown to be a membrane constituent [27]. From the cell surface, CEA may be released into the interstitial spaces and thence into the circulation of the pa-tient. CEA levels in serum have proven to be of little use in the early diagnosis of gastric cancer.
In the present study levels of MDA were found to be sig-nificantly elevated in patients with carcinoma of stomach (P=0.027) as depicted in Fig: 1. This finding is in line with previous reports [1,28,29]. The renal and liver function tests were evaluated and found to be normal pointing to the absence of a source of ROS from these organs. The mild in-crease in urea levels (P<0.01) in patients might as well be due to pre-renal causes like dehydration.
The antioxidant defense in plasma mainly lies with chain breaking antioxidant vitamins i.e. A, C and E. Vitamin E be-ing lipid soluble and associated with lipoprotein fraction of plasma will be the one which is more likely to be involved first. In the present study, there was a significant decrease in the vitamin E levels corrected for cholesterol (P<0.05) in the patient group pointing towards consumption of vitamin E. Vitamin E levels corrected for cholesterol take care of the changes in the lipid profile and confirm its decrease in plasma as a result of oxidative stress. Increased plasma MDA levels along with decreased antioxidant defense in the form of decreased Vitamin E levels and lack of evidence of other sources of oxygen radical production put together point towards continuous oxidative stress in stomach cancer pa-tients. Our findings are supported by the reports that showed that serum lipid peroxides observed in carcinoma stomach patients originate in the cancer tissue [29,30].
The diagnostic accuracy of MDA as a biomarker was assessed by receiver operating characteristic (ROC) analysis which showed statistically significant area under the curve (Fig.2). The diagnostic relevance was then assessed using logistic re-gression analysis, Sensitivity, and specificity (Table-II), and the positive predictive value and negative predictive value analysis (Fig.2). The odds ratio was much higher (164.33) when the cut off value obtained from ROC curve was used when compared to the mean of the control group (4.958).This indicates that a properly identified cut off value will be very much useful as a diagnostic tool in stomach carcinoma. Com-binations of markers have been studied previously by various authors in carcinoma of stomach, both in the serum [30] as well as in gastric juice [31]. These combinations apart from being of diagnostic and prognostic significance also increase the sensitivity compared to their individual use [32,33]. Though there was a decrease in sensitivity (85%vs 72.2%) with use of combination of markers. there was an improve-ment in the specificity (80% vs 96.5%) and PPV (80.9% vs 92.8%) Spearman rank correlation test did not reveal any correlation between the CEA and MDA. This could possibly be due to different mechanisms of the production of these markers which further supports the use of these two markers in combination.
High mortality rate from carcinoma of stomach arises from its late detection. At present, early detection of carcinoma of stomach is difficult without interventional procedures. The proportion of cancers diagnosed as early gastric car-cinoma clearly depending on the discovery of the sensitive diagnostic markers useful in screening. This naturally directs medical research on identification of sensitive markers that can be used for early detection. In light of no currently recom-mended markers for the early diagnosis of carcinoma of the stomach, our positive result points towards the presence of other markers which indeed can be used as markers for early diagnosis. Also, as our understanding of oxidative stress with respect to cause and consequence of disease increases, the ef-forts to make use of oxidative stress as biomarker will be the natural flow of events in medical research.
In conclusion, the elevated levels of MDA in stomach carcinoma could be used as an important parameter in patients at risk for this disease mainly due to its dual role as a mutagen and a tumor promoter. MDA used alone or in combination with CEA was found to provide signifi-cant diagnostic information for the diagnosis of carci-noma of the stomach. Our results suggest that combined use of these two markers can increase the diagnostic accu-racy as against their individual use for diagnosis of the stomach carcinoma.
References
- Ebubekir B, Seyithan T, Fevzi P, Sabahattin D, Zuhal U, Nuri B and Metehan G. Nitric Oxide Levels and Lipid Peroxidation in Plasma of Patients with Gastric Cancer. Japanese Journal of Clinical Oncology 2002; 32: 162-166.
- Doglietto GB, Pacelli F, Caprino P, Sgadari A, and Crucitti F. Surgery: independent prognostic factor in curable and far advanced carcinoma of stomach. World J Surg 2000; 24: 459-463.
- Thomson DMP, Krupey J, Freedman SD, and Gold P. The radioimmunoassay of circulating carcinoembry-onic antigen of the human digestive system. PNAS 1969; 64: 161-167.
- Singal AK, Agarwala S. Tumor markers in pediatric solid tumors. J Indian Assoc Pediatr Surg 2005 ; l 10 (3): 183:190.
- Daniel WC, Stewart S. Tumor markers: In: Carl A. Burtis, Edward R. Ashwood, eds. Tietz Fundamentals of Clinical Chemistry. 2nd ed. Saunders Co., Philadel-phia 2001; pp 390 - 413.
- Fletcher RH. Carcinoembryonic antigen. Ann Intern Med 1986; 104: 66-73
- Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9levels in gastric cancer. J Clin Gastroenterol 2001; 32: 41-44.
- Mattar R, Alves de Andrade CR, DiFavero GM, Gama-Rodrigues JJ, Laudanna AA. et al. Preoperative serum levels of CA 72-4 in patients with gastric adenocarci-noma. Hepato Gastroenterology 1997; 44: 866-871.
- Chen X, Ding YW, Yang G, Bondoc F, Lee MJ and Yang CS.Oxidative damage in an esophageal adeno-carcinoma model with rats. Carcinogenesis 2000; 21(2); 257-263.
- Carla B, Patrizia F, Sergio AT, Carla V, Francesco R and Antonia F. Vitamin E serum levels and gastric cancer; results from a cohort of patients in Tuscany, It-aly. Cancer Lett 2000; 151: 15-8.
- Seven A, Civelek S, Inci E, Korkut N, and Burcak G. Evaluation of oxidative stress parameters in blood of patients with laryngeal carcinoma. Clin Biochem 1999; 32: 369-73.
- Nicholas S Brown, and Roy Bicknell. Hypoxia and oxidative stress in breast cancer. Oxidative stress - its effects on the growth, metastatic potential and re-sponse to therapy of breast cancer. Breast Cancer Res 2001; 3: 323-327.
- Toyokuni S, Okamoto K, Yodoi J, and Hiai H. Persis-tent oxidative stress in cancer. FEES Lett 1995; 358: 1-3.
- Ward JF, Evans JW, Limoli CL, Calabro-Jones PM. Radiation and hydrogen peroxide induced free radical damage to DNA. Br J Cancer 1987;55:105?12.
- D. Hristozov, V. Gadjeva, T. Vlaykova and G. Dimi-trov. Evaluation of Oxidative Stress in Patients with Cancer. Archives Of Physiology And Biochemistry 2001; 109(4): 331-336
- Sander CS, Hamm F, Elsner PM, and Thiele JJ. Oxida-tive stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol 2003;148: 913-22.
- Akbulut H, Akbulut KG, Icli F, and Buyukceli K. Daily variations of plasma malondialdehyde levels in patients with early breast cancer. A Cancer Detect Prev 2003; 27: 122-6.
- Yalcin O, Karatas F, Erulas FA, and Ozdemir E. The levels of glutathione peroxidase, vitamin A, E, C and lipid peroxidation in patients with transitional cell car-cinoma of the bladder. BJU Int 2004; 93: 863-6.
- Skrzydiewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga KL, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastro-enterol 2005; 11(3):403-406.
- Inayama M, Hashimoto N, Tokoro T, and Shiozaki H. Involvement of oxidative stress in experimentally in-duced reflux esophagitis and esophageal cancer. Hepa-togastroenterology 2007; 54: 761-5.
- Yilmaz YF, Akbiyik F, Tuncel U, and Unal A. Lipid peroxidation and antioxidant levels in patients with la-ryngeal carcinoma. Kulak Burun Bogaz Ihtis Derg 2007; 17: 81-84.
- Lauschke H, Tolba R, Burger B, Minor T, and Hirner A. Lipid peroxidation as additional marker in patients with colorecta! cancer. Results of a preliminary study. Eur Surg Res 2002; 34: 346-50.
- Peter A Southorn, and Garth Powis. Free Radicals in Medicine II. Involvement in Human disease. Mayo Clin Proc 1988; 63: 381-389.
- Catignani GL, and Bieri JG. Simultaneous determina-tion of retinol and a- tocopherol in serum or plasma by liquid chromatography. Clin Chem 1983; 29: 708-712.
- Sangeetha P, Das UN, Koratkar R, and Suryaprabha P. Increase in free radical generation and lipid peroxida-tion following chemotherapy in patients with cancer. Free Radic Biol Med 1990; 8:15.
- Baek HY, Lim JW, Kim HY, Kim JM, Kim JS, Jung HC and Kim KH. Oxidative-stress-related proteome changes in Helicobacter pylori-infected human gastric mucosa. Biochem J 2004; 379:291-299.
- Gold P, Krupey J, and Ansari H. Position of the carci-noembryonic antigen of the human digestive system in ultrastructure of tumor cell surface. Journal of the Na-tional Cancer Institute 1970; 45: 219-225.
- Batcioglu K, Mehmet N, Ozturk 1C, Yilmaz M, Ay-dogdu N, Erguvan R, Uyumlu B et al. Lipid peroxida-tion and antioxidant status in stomach cancer. Cancer Invest.2006Feb;24(l): 18-21.
- Nevin I, Necip J, Yavuz I, Handan A, and Mehmet K. C-reactive protein, procalcitonin, interleukin-6, vascu-lar endothelial growth factor and oxidative metabolites in diagnosis of infection and staging with carcinoma of stomach. World J Gastroenterol 2004; 10: 1115-1120.
- Marreli D, Pinto E, De Stefano A, Farnetani M, Garosi L and Roviello F. Clinical utility of CEA, CA 19-9 and CA 72-4 in the follow-up of patients with re-sectable gastric cancer. Am J Surg 2001; 181: 16-9.
- Harrison JD, Stanley J, and Morris DL. CEA and CA-19.9 in gastric juice and serum: an aid in the diagnosis of gastric cancer? Eur J Surg Oncol 1989; 15: 253-7.
- Ychou M, Duffour J, Kramer A, Gourgou, and Grenier J. Clinical significance and prognostic value of CA 72-4 compared with CEA and CA 19-9 in patients with gastric cancer. Dis markers 2000; 16: 105-110.
- Lai IR, Lee WJ, Huang MT, and Lin HH. Comparison of serum CA 72-4, CEA, TPA, CA19-9 and CA125 le-vels in gastric cancer patients and correlation with re-currence. Hepatogastroenterology 2002; 49: 1157-60.